

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM9157 (2S)-4-(1-benzofuran-2-ylmethyl)-1-[(2S,4R)-2-hydroxy-4-{[(3S,4S)-3-hydroxy-3,4-dihydro-2H-1-benzopyran-4-yl]carbamoyl}-4-(pyridin-3-ylmethyl)butyl]-N-(2,2,2-trifluoroethyl)piperazine-2-carboxamide::P3 benzofuran analog 7
SMILES: O[C@@H](C[C@@H](Cc1cccnc1)C(=O)N[C@@H]1[C@H](O)COc2ccccc12)CN1CCN(Cc2cc3ccccc3o2)C[C@H]1C(=O)NCC(F)(F)F
InChI Key: InChIKey=LLRKKARNQCFJDO-NKBKYVEPSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM9157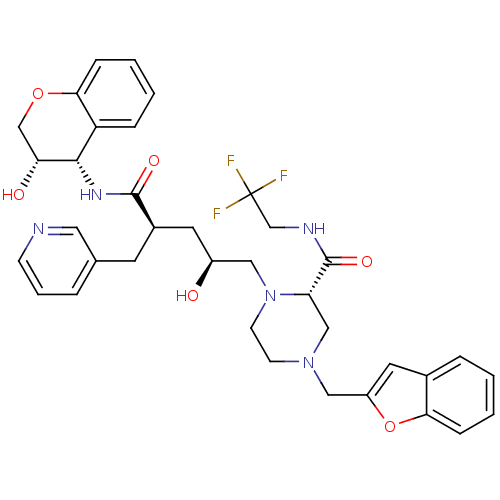 ((2S)-4-(1-benzofuran-2-ylmethyl)-1-[(2S,4R)-2-hydr...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease Mutant Q-60C (Human immunodeficiency virus type 1) | BDBM9157 ((2S)-4-(1-benzofuran-2-ylmethyl)-1-[(2S,4R)-2-hydr...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease Mutant V-18C (Human immunodeficiency virus type 1) | BDBM9157 ((2S)-4-(1-benzofuran-2-ylmethyl)-1-[(2S,4R)-2-hydr...) | MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 Protease Mutant K-60C (Human immunodeficiency virus type 1) | BDBM9157 ((2S)-4-(1-benzofuran-2-ylmethyl)-1-[(2S,4R)-2-hydr...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories | Assay Description Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... | Bioorg Med Chem Lett 13: 3323-6 (2003) Article DOI: 10.1016/s0960-894x(03)00680-2 BindingDB Entry DOI: 10.7270/Q2K072GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||