 Found 516 hits with Last Name = 'adamo' and Initial = 'a'
Found 516 hits with Last Name = 'adamo' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600683
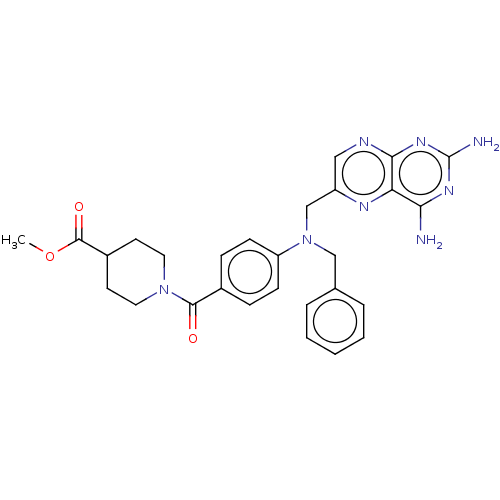
(CHEMBL5184936)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(Cc1ccccc1)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600703
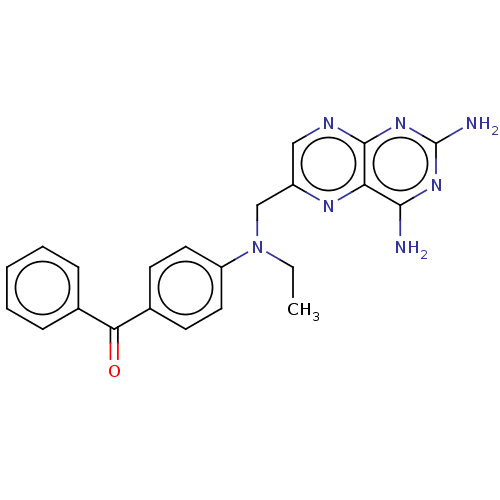
(CHEMBL5201183)Show SMILES CCN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)c1ccccc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600694

(CHEMBL5204239)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NCCc3ccccc3)cnc2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600702
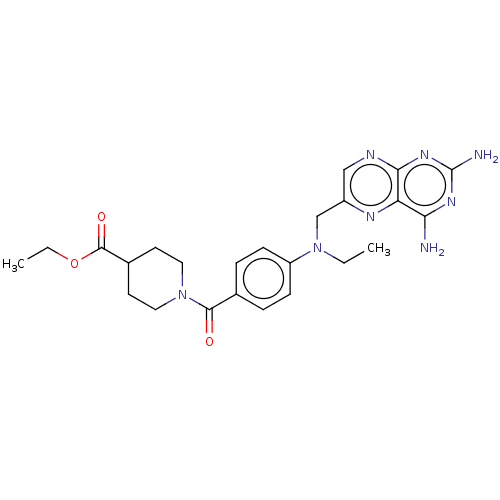
(CHEMBL5199171)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CC)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600693

(CHEMBL5175012)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)c3ccccc3)cnc2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600684

(CHEMBL5182125)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(SCc2cnc3nc(N)nc(N)c3n2)nc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600701
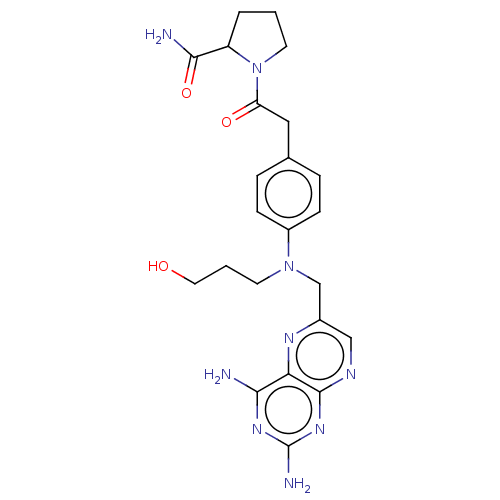
(CHEMBL5200612)Show SMILES NC(=O)C1CCCN1C(=O)Cc1ccc(cc1)N(CCCO)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50514760
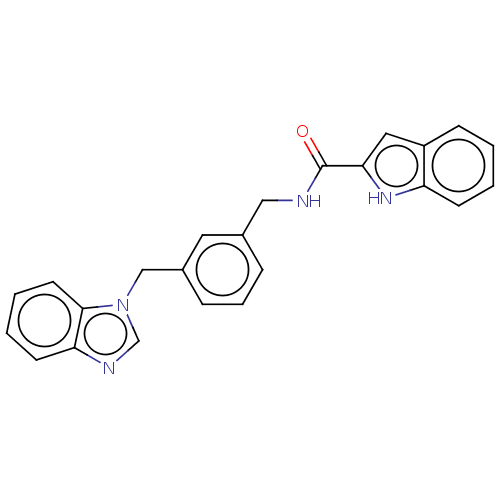
(CHEMBL4448402)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H20N4O/c29-24(22-13-19-8-1-2-9-20(19)27-22)25-14-17-6-5-7-18(12-17)15-28-16-26-21-10-3-4-11-23(21)28/h1-13,16,27H,14-15H2,(H,25,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50050426

(6-(Naphthalen-1-ylaminomethyl)-pteridine-2,4-diami...)Show InChI InChI=1S/C17H15N7/c18-15-14-16(24-17(19)23-15)21-9-11(22-14)8-20-13-7-3-5-10-4-1-2-6-12(10)13/h1-7,9,20H,8H2,(H4,18,19,21,23,24) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50050426

(6-(Naphthalen-1-ylaminomethyl)-pteridine-2,4-diami...)Show InChI InChI=1S/C17H15N7/c18-15-14-16(24-17(19)23-15)21-9-11(22-14)8-20-13-7-3-5-10-4-1-2-6-12(10)13/h1-7,9,20H,8H2,(H4,18,19,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600700

(CHEMBL5188304)Show SMILES NC(=O)C1CCN(CC1)C(=O)Cc1ccc(cc1)N(CCCO)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600692

(CHEMBL5197107)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N1CCCCC1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600689
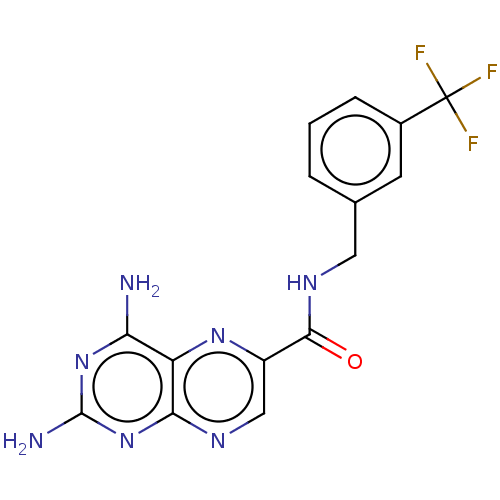
(CHEMBL5175397)Show SMILES Nc1nc(N)c2nc(cnc2n1)C(=O)NCc1cccc(c1)C(F)(F)F | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600688
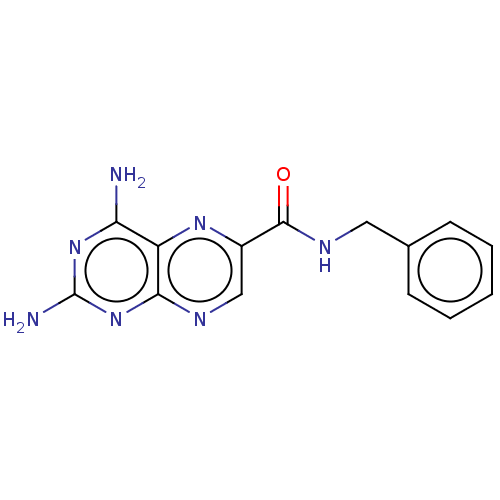
(CHEMBL5198855) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50514753

(CHEMBL4557994)Show SMILES Brc1c[nH]c(c1)C(=O)NCc1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C20H17BrN4O/c21-16-9-18(22-11-16)20(26)23-10-14-4-3-5-15(8-14)12-25-13-24-17-6-1-2-7-19(17)25/h1-9,11,13,22H,10,12H2,(H,23,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514753

(CHEMBL4557994)Show SMILES Brc1c[nH]c(c1)C(=O)NCc1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C20H17BrN4O/c21-16-9-18(22-11-16)20(26)23-10-14-4-3-5-15(8-14)12-25-13-24-17-6-1-2-7-19(17)25/h1-9,11,13,22H,10,12H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human LXF-289 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514753

(CHEMBL4557994)Show SMILES Brc1c[nH]c(c1)C(=O)NCc1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C20H17BrN4O/c21-16-9-18(22-11-16)20(26)23-10-14-4-3-5-15(8-14)12-25-13-24-17-6-1-2-7-19(17)25/h1-9,11,13,22H,10,12H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514759

(CHEMBL4571131)Show SMILES Brc1c[nH]c(c1)C(=O)NCc1cccc(Cn2cnc3ccc(Br)cc23)c1 Show InChI InChI=1S/C20H16Br2N4O/c21-15-4-5-17-19(8-15)26(12-25-17)11-14-3-1-2-13(6-14)9-24-20(27)18-7-16(22)10-23-18/h1-8,10,12,23H,9,11H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600683

(CHEMBL5184936)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(Cc1ccccc1)Cc1cnc2nc(N)nc(N)c2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514753

(CHEMBL4557994)Show SMILES Brc1c[nH]c(c1)C(=O)NCc1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C20H17BrN4O/c21-16-9-18(22-11-16)20(26)23-10-14-4-3-5-15(8-14)12-25-13-24-17-6-1-2-7-19(17)25/h1-9,11,13,22H,10,12H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600694

(CHEMBL5204239)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)NCCc3ccccc3)cnc2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600693

(CHEMBL5175012)Show SMILES Nc1nc(N)c2nc(CNc3ccc(cc3)C(=O)c3ccccc3)cnc2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600691
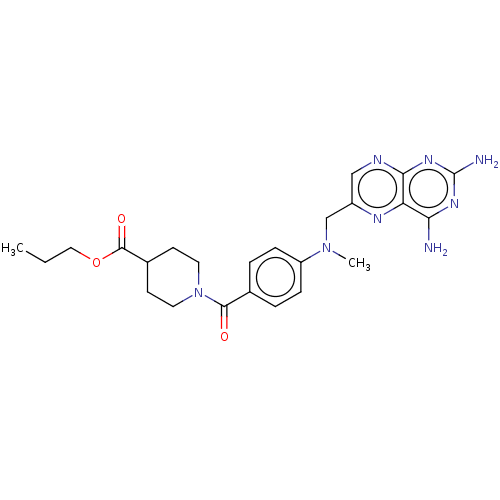
(CHEMBL5205559)Show SMILES CCCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600685

(CHEMBL5171609)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CCCO)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600682

(CHEMBL5193564)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CC#C)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600690

(CHEMBL5206994)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514753

(CHEMBL4557994)Show SMILES Brc1c[nH]c(c1)C(=O)NCc1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C20H17BrN4O/c21-16-9-18(22-11-16)20(26)23-10-14-4-3-5-15(8-14)12-25-13-24-17-6-1-2-7-19(17)25/h1-9,11,13,22H,10,12H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HepG2 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50398394

(CHEMBL1232702)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C22H26N8O3/c1-29(12-15-11-25-19-17(26-15)18(23)27-22(24)28-19)16-5-3-13(4-6-16)20(31)30-9-7-14(8-10-30)21(32)33-2/h3-6,11,14H,7-10,12H2,1-2H3,(H4,23,24,25,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600679

(CHEMBL426 | Methotrexate | TCMDC-123832 | TCMDC-12...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600703

(CHEMBL5201183)Show SMILES CCN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600684

(CHEMBL5182125)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(SCc2cnc3nc(N)nc(N)c3n2)nc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514753

(CHEMBL4557994)Show SMILES Brc1c[nH]c(c1)C(=O)NCc1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C20H17BrN4O/c21-16-9-18(22-11-16)20(26)23-10-14-4-3-5-15(8-14)12-25-13-24-17-6-1-2-7-19(17)25/h1-9,11,13,22H,10,12H2,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514760

(CHEMBL4448402)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H20N4O/c29-24(22-13-19-8-1-2-9-20(19)27-22)25-14-17-6-5-7-18(12-17)15-28-16-26-21-10-3-4-11-23(21)28/h1-13,16,27H,14-15H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514760

(CHEMBL4448402)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H20N4O/c29-24(22-13-19-8-1-2-9-20(19)27-22)25-14-17-6-5-7-18(12-17)15-28-16-26-21-10-3-4-11-23(21)28/h1-13,16,27H,14-15H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514760

(CHEMBL4448402)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H20N4O/c29-24(22-13-19-8-1-2-9-20(19)27-22)25-14-17-6-5-7-18(12-17)15-28-16-26-21-10-3-4-11-23(21)28/h1-13,16,27H,14-15H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514752
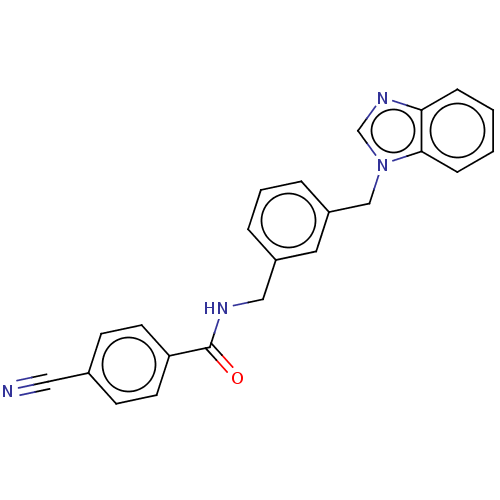
(CHEMBL4458549)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H18N4O/c24-13-17-8-10-20(11-9-17)23(28)25-14-18-4-3-5-19(12-18)15-27-16-26-21-6-1-2-7-22(21)27/h1-12,16H,14-15H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600681

(CHEMBL5180552)Show SMILES CCN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N1CCC(CC1)C(=O)OC | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514752

(CHEMBL4458549)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H18N4O/c24-13-17-8-10-20(11-9-17)23(28)25-14-18-4-3-5-19(12-18)15-27-16-26-21-6-1-2-7-22(21)27/h1-12,16H,14-15H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Pteridine reductase 1
(Leishmania major) | BDBM50600702

(CHEMBL5199171)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(CC)Cc1cnc2nc(N)nc(N)c2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514752

(CHEMBL4458549)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H18N4O/c24-13-17-8-10-20(11-9-17)23(28)25-14-18-4-3-5-19(12-18)15-27-16-26-21-6-1-2-7-22(21)27/h1-12,16H,14-15H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human MCF7 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50398395
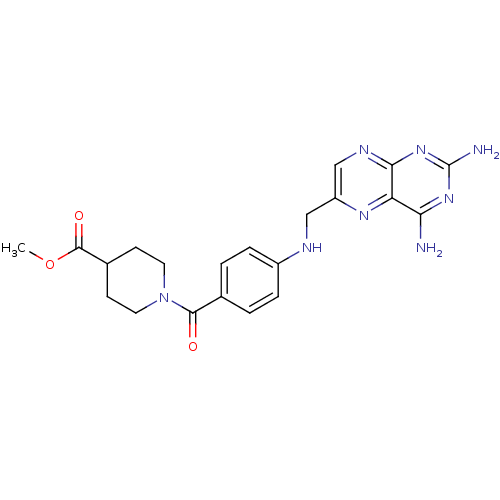
(CHEMBL1232399)Show SMILES COC(=O)C1CCN(CC1)C(=O)c1ccc(NCc2cnc3nc(N)nc(N)c3n2)cc1 Show InChI InChI=1S/C21H24N8O3/c1-32-20(31)13-6-8-29(9-7-13)19(30)12-2-4-14(5-3-12)24-10-15-11-25-18-16(26-15)17(22)27-21(23)28-18/h2-5,11,13,24H,6-10H2,1H3,(H4,22,23,25,27,28) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50514752

(CHEMBL4458549)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1ccc(cc1)C#N Show InChI InChI=1S/C23H18N4O/c24-13-17-8-10-20(11-9-17)23(28)25-14-18-4-3-5-19(12-18)15-27-16-26-21-6-1-2-7-22(21)27/h1-12,16H,14-15H2,(H,25,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of mouse IDO1 transfected in P815 cells assessed as reduction in L-Kyn level measured after 16 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Leishmania major) | BDBM50600690

(CHEMBL5206994)Show SMILES CCOC(=O)C1CCN(CC1)C(=O)c1ccc(cc1)N(C)Cc1cnc2nc(N)nc(N)c2n1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Pteridine reductase, putative
(Trypanosoma brucei brucei (strain 927/4 GUTat10.1)) | BDBM50600695

(CHEMBL5208403)Show SMILES COc1ccc(CNC(=O)c2ccc(CNCc3cnc4nc(N)nc(N)c4n3)cc2)cc1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00232
BindingDB Entry DOI: 10.7270/Q2CJ8JJC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514757
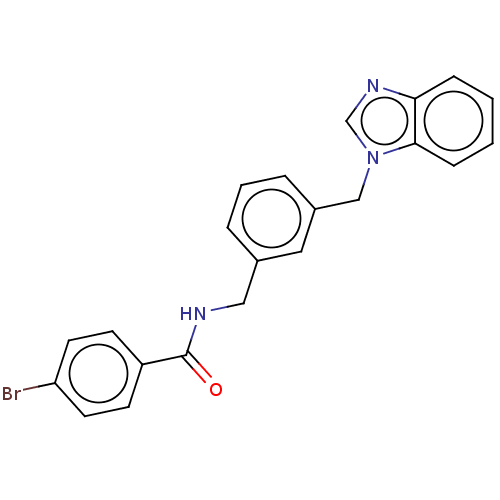
(CHEMBL4466117)Show SMILES Brc1ccc(cc1)C(=O)NCc1cccc(Cn2cnc3ccccc23)c1 Show InChI InChI=1S/C22H18BrN3O/c23-19-10-8-18(9-11-19)22(27)24-13-16-4-3-5-17(12-16)14-26-15-25-20-6-1-2-7-21(20)26/h1-12,15H,13-14H2,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human A375 cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50514760

(CHEMBL4448402)Show SMILES O=C(NCc1cccc(Cn2cnc3ccccc23)c1)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C24H20N4O/c29-24(22-13-19-8-1-2-9-20(19)27-22)25-14-17-6-5-7-18(12-17)15-28-16-26-21-10-3-4-11-23(21)28/h1-13,16,27H,14-15H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ del Piemonte Orientale
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human DAN-G cells assessed as reduction in L-Kyn level measured after 48 hrs by HPLC analysis |
J Med Chem 63: 3047-3065 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01809
BindingDB Entry DOI: 10.7270/Q29K4FKS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data