

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279520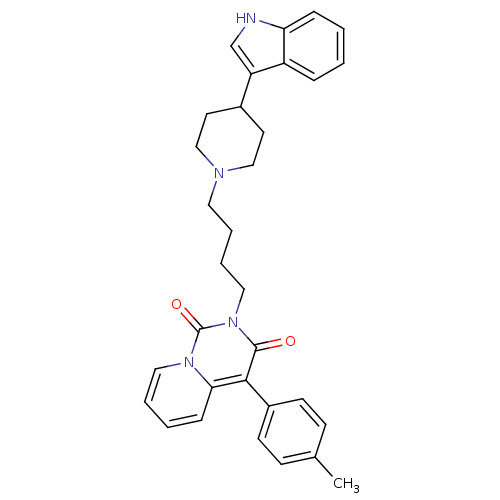 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | DrugBank Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Binding affinity to 5-HT2BR (unknown origin) | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-Ketanserin from human 5-HT2A receptor expressed in rat cortex tissue incubated for 30 mins by liquid scintillation counting meth... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM85222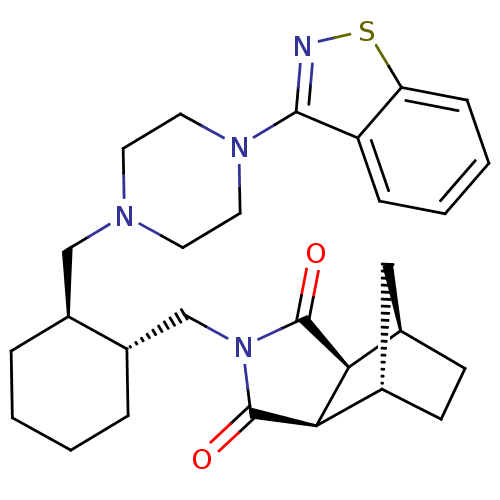 (CAS_441351-20-8 | Lurasidone | SM 13496) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279541 (2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-(2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM30708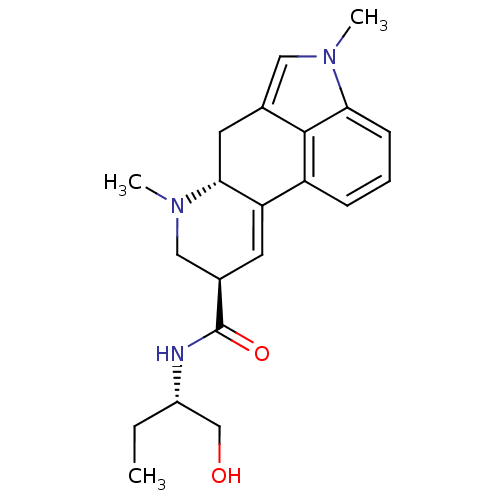 ((6aR,9R)-4,7-dimethyl-N-[(1S)-1-methylolpropyl]-6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Binding affinity to 5-HT2CR (unknown origin) | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM85222 (CAS_441351-20-8 | Lurasidone | SM 13496) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM30708 ((6aR,9R)-4,7-dimethyl-N-[(1S)-1-methylolpropyl]-6,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Binding affinity to 5-HT2BR (unknown origin) | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-imipramine from human serotonin transporter expressed in HEK293 cells membranes incubated for 30 mins by microbeta scintillation... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM85222 (CAS_441351-20-8 | Lurasidone | SM 13496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-Ketanserin from human 5-HT2A receptor expressed in rat cortex tissue incubated for 30 mins by liquid scintillation counting meth... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50504436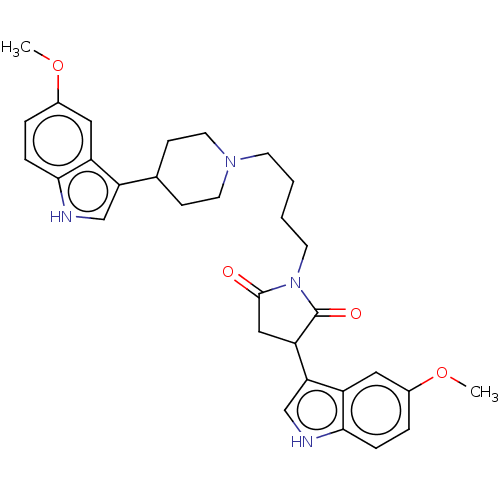 (CHEMBL4591440) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50010859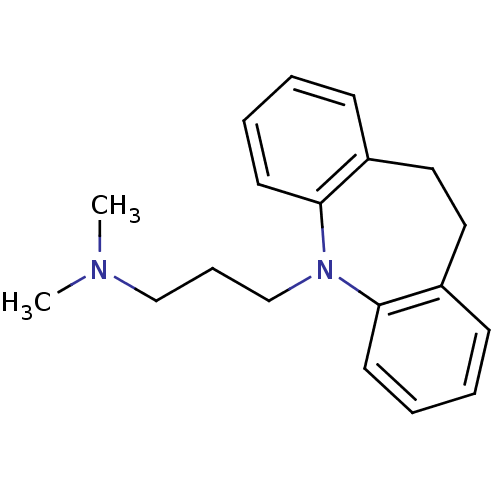 (CHEMBL11 | IMIPRAMINE HYDROCHLORIDE | IMIPRAMINE P...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-imipramine from human serotonin transporter expressed in HEK293 cells membranes incubated for 30 mins by microbeta scintillation... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50443101 (Cariprazine | RGH-188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50504435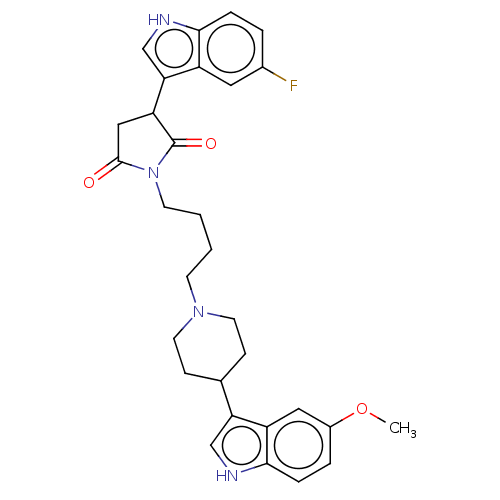 (CHEMBL4454176) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-Ketanserin from human 5-HT2A receptor expressed in rat cortex tissue incubated for 30 mins by liquid scintillation counting meth... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM194780 (7-(4-(4-(1-benzothiophen-4-yl)piperazin-1-yl)butox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-5-CT from human 5HT7 receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50062156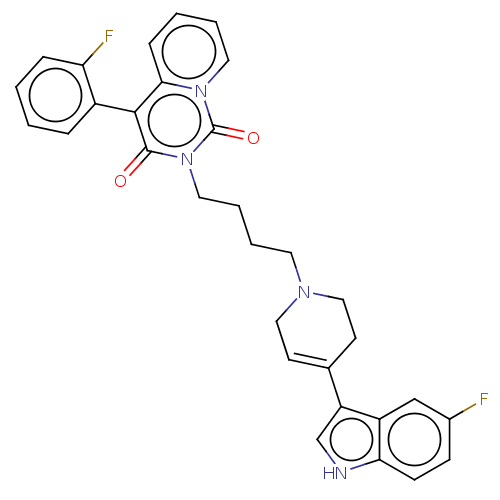 (CHEMBL3397081) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain cerebral cortex SERT by liquid scintillation counting analysis | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279541 (2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-(2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50504438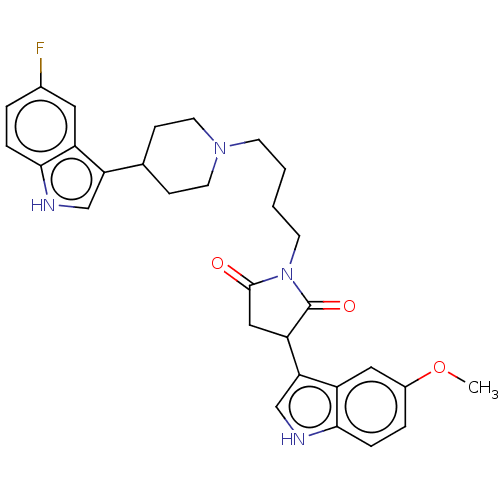 (CHEMBL4571321) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50062173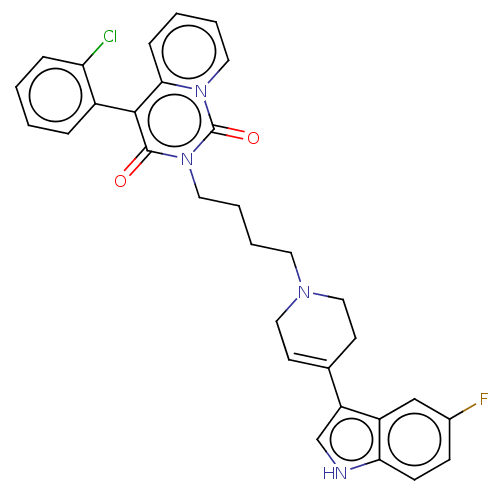 (CHEMBL3397082) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain cerebral cortex SERT by liquid scintillation counting analysis | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50062241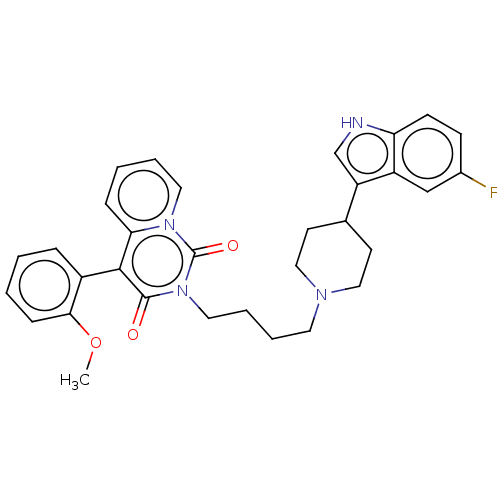 (CHEMBL3397075) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat brain hippocampus 5-HT1A receptor by radioligand binding assay | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279520 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50504431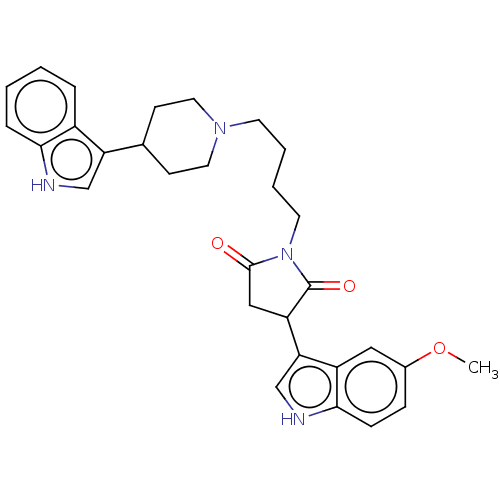 (CHEMBL4483554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM85222 (CAS_441351-20-8 | Lurasidone | SM 13496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50504439 (CHEMBL4515160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279540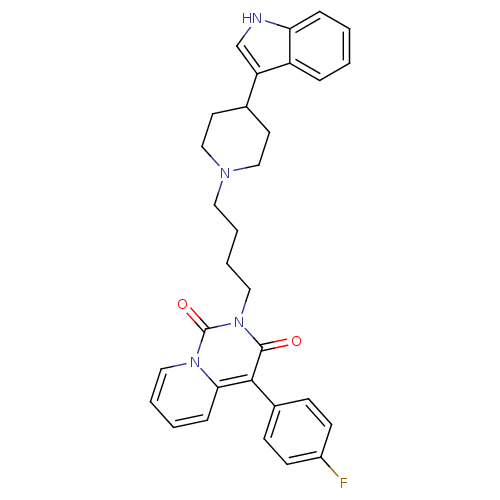 (4-(4-Fluoro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50062247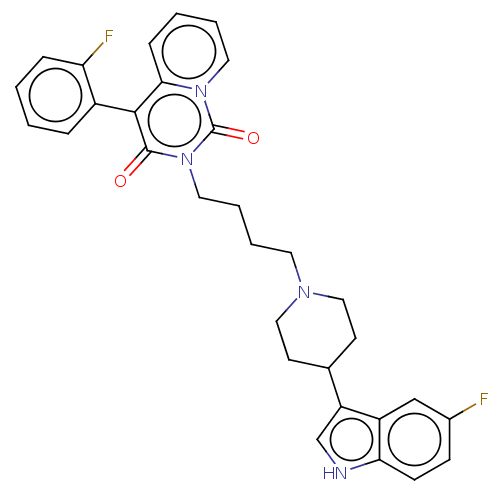 (CHEMBL3397073) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat brain hippocampus 5-HT1A receptor by radioligand binding assay | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50151982 (5-{4-[4-(5-Cyano-1H-indol-3-yl)-butyl]-piperazin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301431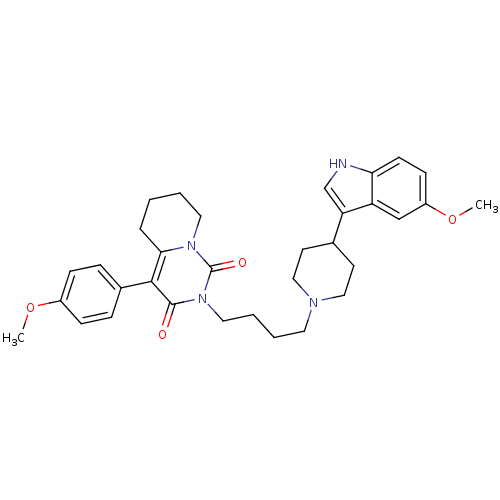 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279561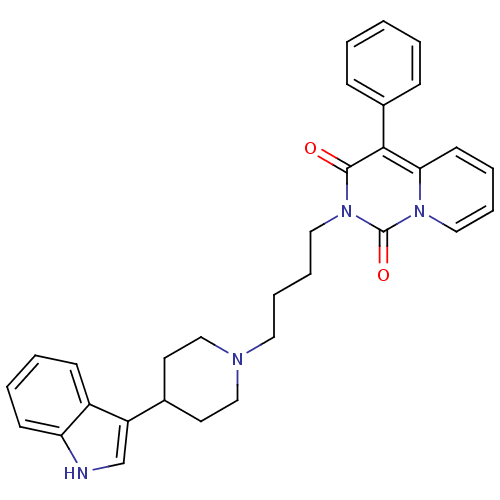 (2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50062154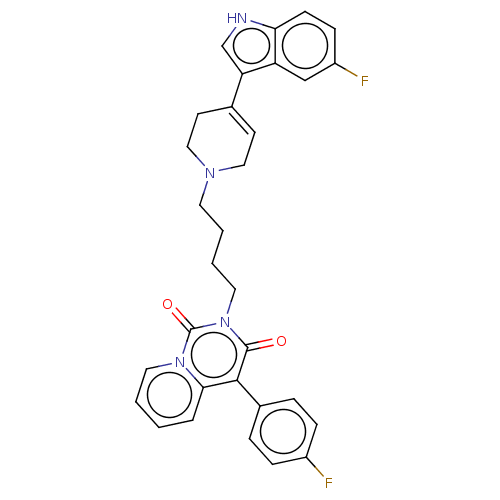 (CHEMBL3397080) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain cerebral cortex SERT by liquid scintillation counting analysis | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50062243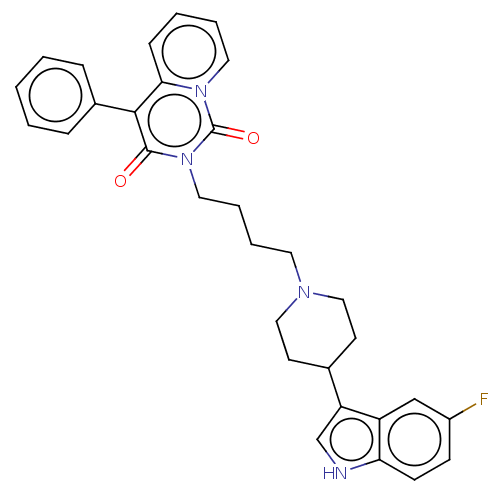 (CHEMBL3397069) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat brain hippocampus 5-HT1A receptor by radioligand binding assay | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50062246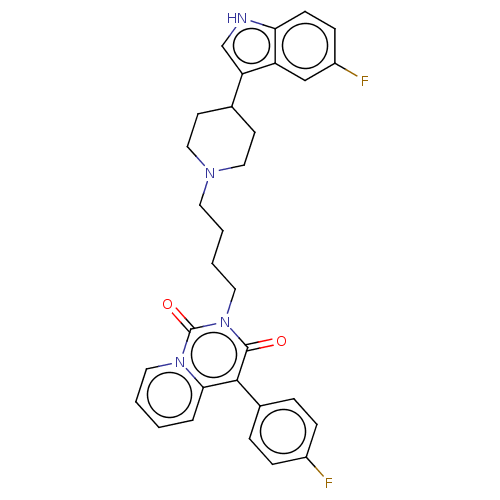 (CHEMBL3397072) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat brain hippocampus 5-HT1A receptor by radioligand binding assay | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50504432 (CHEMBL4571862) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301442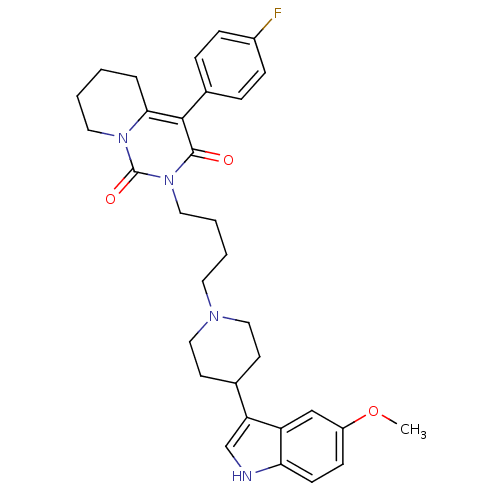 (4-(4-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50433127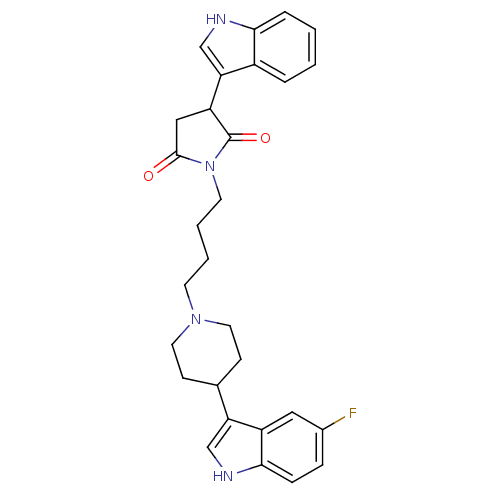 (CHEMBL2377590) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50433127 (CHEMBL2377590) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D2L receptor expressed in HEK293 cells after 1 hr | Eur J Med Chem 63: 484-500 (2013) Article DOI: 10.1016/j.ejmech.2013.02.033 BindingDB Entry DOI: 10.7270/Q2N017WF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301429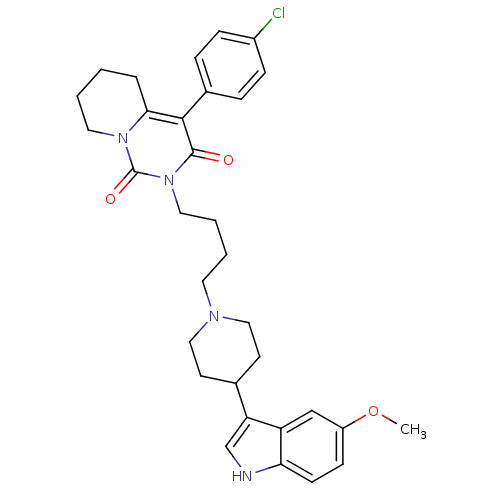 (4-(4-Chloro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50504431 (CHEMBL4483554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human D2L receptor expressed in HEK293 cells incubated for 1 hr by liquid scintillation counting method | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50062243 (CHEMBL3397069) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain cerebral cortex SERT by liquid scintillation counting analysis | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50062277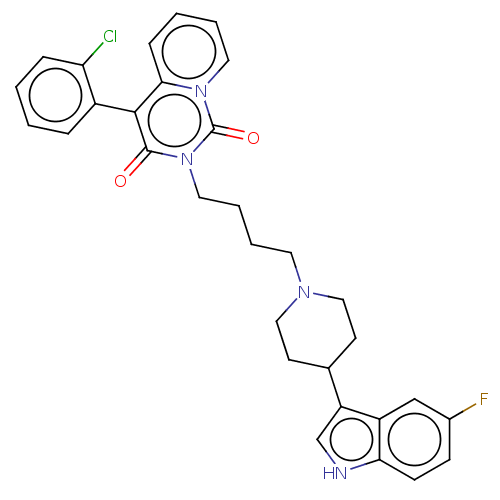 (CHEMBL3397074) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from rat brain hippocampus 5-HT1A receptor by radioligand binding assay | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50062152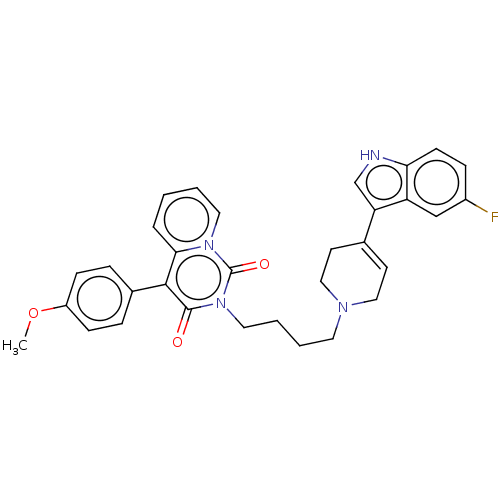 (CHEMBL3397078) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from rat brain cerebral cortex SERT by liquid scintillation counting analysis | Eur J Med Chem 90: 21-32 (2015) Article DOI: 10.1016/j.ejmech.2014.10.069 BindingDB Entry DOI: 10.7270/Q2VQ34CB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279586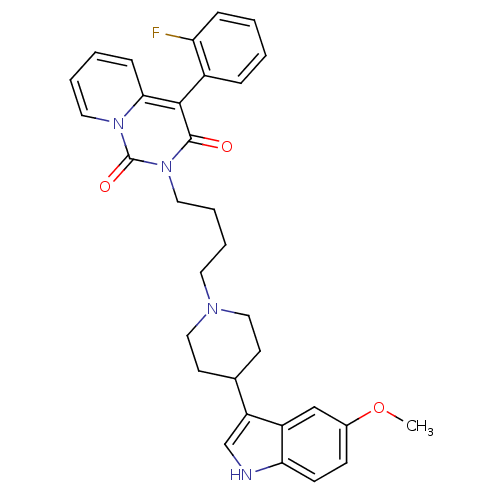 (4-(2-fluorophenyl)-2-(4-(4-(5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM30708 ((6aR,9R)-4,7-dimethyl-N-[(1S)-1-methylolpropyl]-6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from human 5HT1A receptor expressed in CHO-K1 cell membranes incubated for 60 mins by microbeta scintillation counting... | Eur J Med Chem 183: (2019) Article DOI: 10.1016/j.ejmech.2019.111736 BindingDB Entry DOI: 10.7270/Q2W0996H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 258 total ) | Next | Last >> |