

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24969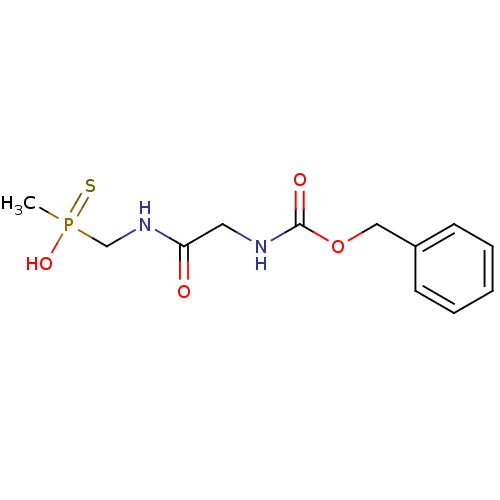 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | -39.3 | 1.80E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534783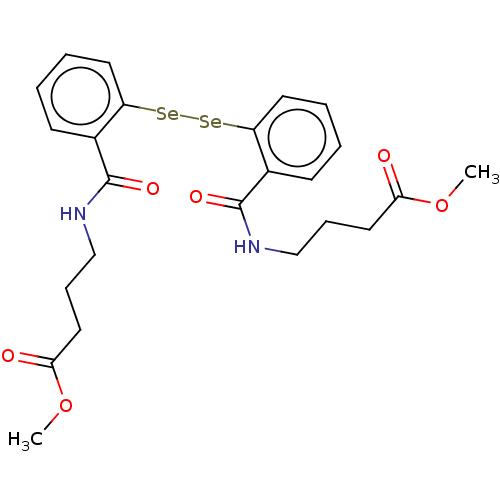 (CHEMBL4575318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 after 120 mins by Berthelot colorimetric analysis | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 after 120 mins by Berthelot colorimetric analysis | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24969 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 450 | -36.8 | 3.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50197347 (Ebselen Diselenide) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 651 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 after 120 mins by Berthelot colorimetric analysis | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534782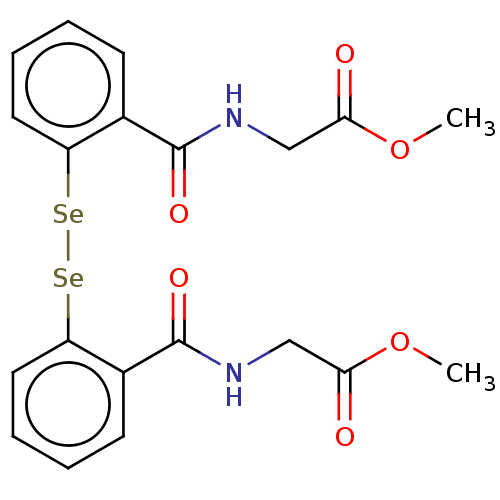 (CHEMBL4471428) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 915 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 after 120 mins by Berthelot colorimetric analysis | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534776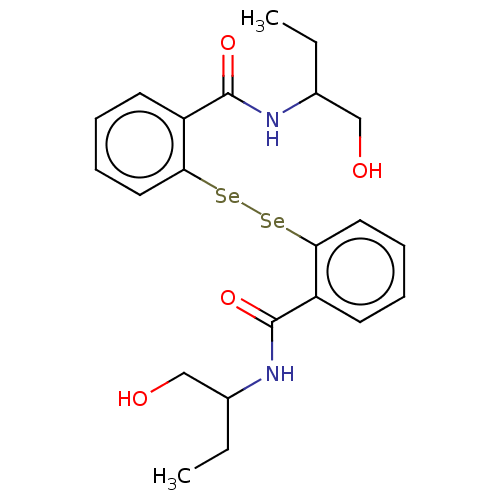 (CHEMBL4539240) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 after 120 mins by Berthelot colorimetric analysis | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24971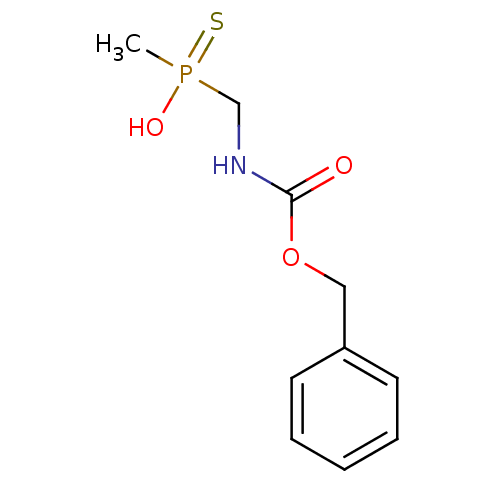 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)sulfan...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+4 | -28.2 | 1.58E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24971 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)sulfan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.75E+4 | -27.6 | 1.12E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24967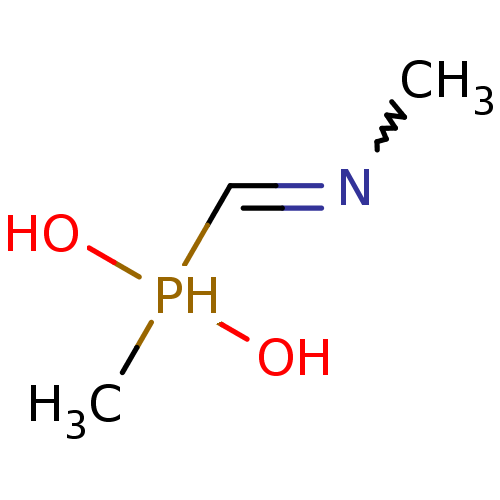 (methyl[(methylamino)methyl]phosphinic acid | organ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+4 | -27.5 | 6.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24962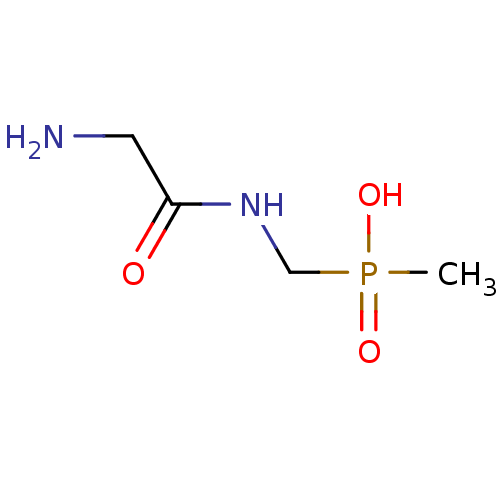 ([(2-aminoacetamido)methyl](methyl)phosphinic acid ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+4 | -27.1 | 6.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24965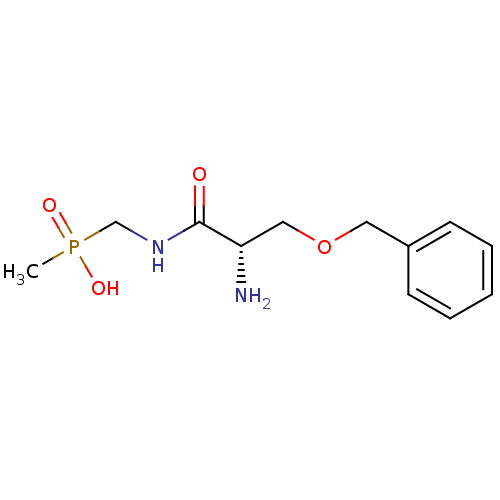 (organophosphorus derivative, 6 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.50E+4 | -26.7 | 8.00E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24967 (methyl[(methylamino)methyl]phosphinic acid | organ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+4 | -26.5 | 1.53E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24962 ([(2-aminoacetamido)methyl](methyl)phosphinic acid ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.00E+4 | -26.2 | 8.60E+4 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24965 (organophosphorus derivative, 6 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.26E+4 | -26.0 | 1.83E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24966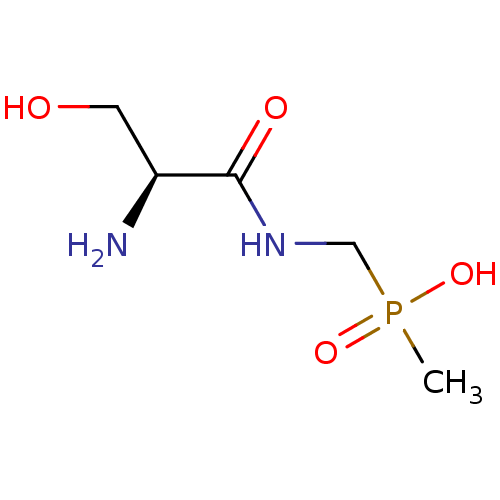 (organophosphorus derivative, 7 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+4 | -25.7 | 1.50E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24966 (organophosphorus derivative, 7 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10E+4 | -25.5 | 3.42E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24968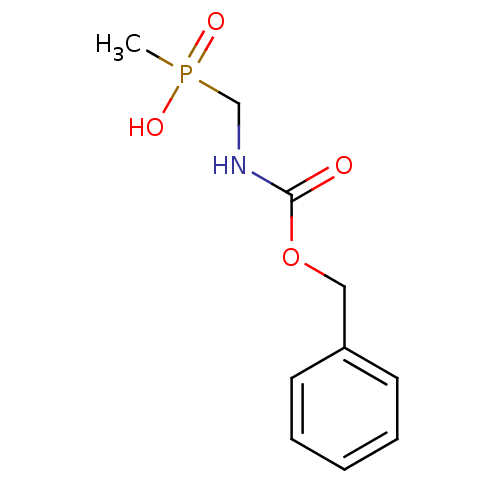 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)phosph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.30E+4 | -25.3 | 1.23E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24968 (({[(benzyloxy)carbonyl]amino}methyl)(methyl)phosph...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 6.50E+4 | -24.3 | 3.19E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50099857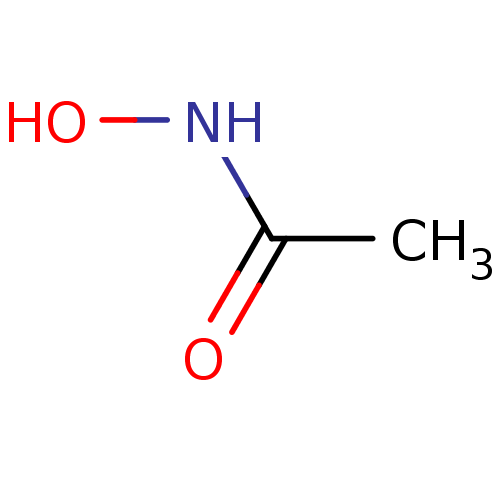 (ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 after 120 mins by Berthelot colorimetric analysis | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24963 (organophosphorus derivative, 4 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+5 | -22.8 | 4.85E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24970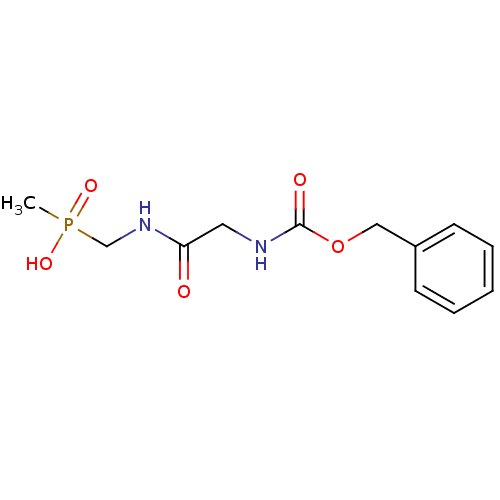 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.35E+5 | -22.5 | 4.50E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24964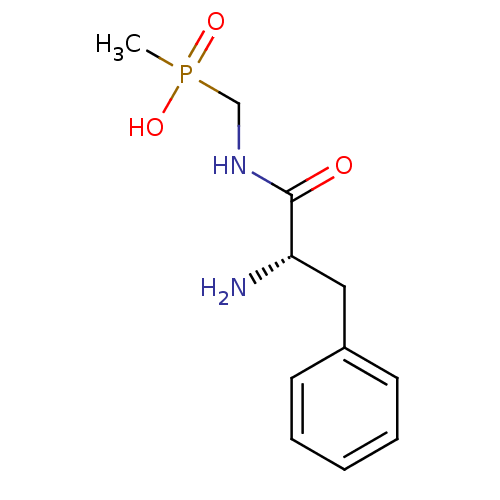 (organophosphorus derivative, 5 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.76E+5 | -21.8 | 7.54E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24970 ([(2-{[(benzyloxy)carbonyl]amino}acetamido)methyl](...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.78E+5 | -21.8 | 6.40E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24963 (organophosphorus derivative, 4 | {[(2S)-2-amino-3-...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.08E+5 | -21.4 | 6.17E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24964 (organophosphorus derivative, 5 | {[(2S)-2-amino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.15E+5 | -21.3 | 6.00E+5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha/beta/gamma (Bacillus pasteurii) | BDBM24960 ((aminomethyl)(methyl)phosphinic acid | organophosp...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 3.40E+5 | -20.1 | 1.10E+6 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit alpha [F36L]/beta/gamma (Proteus vulgaris) | BDBM24960 ((aminomethyl)(methyl)phosphinic acid | organophosp...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.25E+5 | -19.6 | 2.53E+6 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Wroclaw University of Technology | Assay Description Progress curves were obtained by initiation of urease reaction with addition of purified enzyme into assay mixtures containing increasing concentrati... | J Med Chem 51: 5736-44 (2008) Article DOI: 10.1021/jm800570q BindingDB Entry DOI: 10.7270/Q2PR7T91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50070209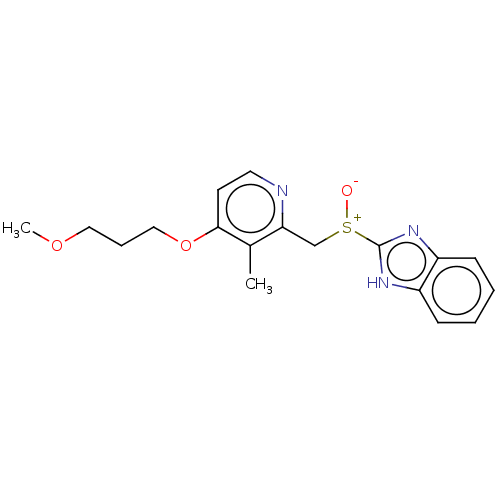 (Aciphex | CHEBI:8768 | LY-307640 | Rabeprazole) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori ATCC 43504 urease assessed as amount of ammonia after 15 mins by indophenol method | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM106944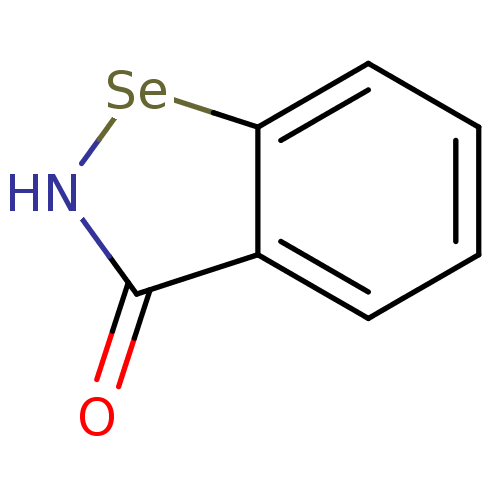 (US8592468, EbSe2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534774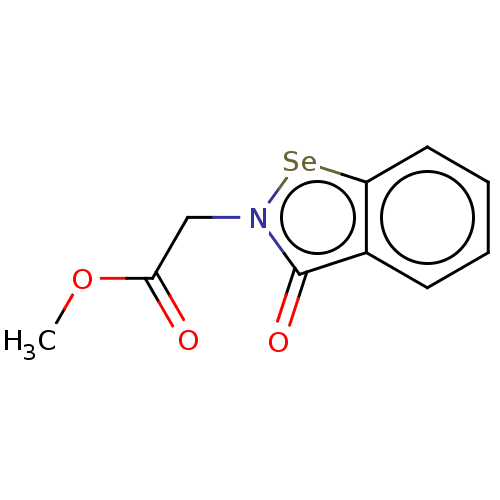 (CHEMBL4089739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534779 (CHEMBL4469463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534787 (CHEMBL4444060) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534774 (CHEMBL4089739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate after 2 hrs by Berthelot assay | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534778 (CHEMBL4563587) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534772 (CHEMBL4563580) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534780 (CHEMBL4469439) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534773 (CHEMBL4524305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50197347 (Ebselen Diselenide) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM106944 (US8592468, EbSe2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534774 (CHEMBL4089739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50197347 (Ebselen Diselenide) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534783 (CHEMBL4575318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534778 (CHEMBL4563587) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534783 (CHEMBL4575318) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534780 (CHEMBL4469439) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534773 (CHEMBL4524305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant Helicobacter pylori urease expressed in Escherichia coli rosetta DE3 using urea as substrate assessed as transformed cell u... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50534777 (CHEMBL4592440) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of urease in Helicobacter pylori J99 in Brucella broth using urea as substrate preincubated for 2 hrs followed by substrate addition measu... | J Med Chem 59: 8125-33 (2016) Article DOI: 10.1021/acs.jmedchem.6b00986 BindingDB Entry DOI: 10.7270/Q2FR0130 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |