 Found 93 hits with Last Name = 'horvath' and Initial = 'a'
Found 93 hits with Last Name = 'horvath' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134333
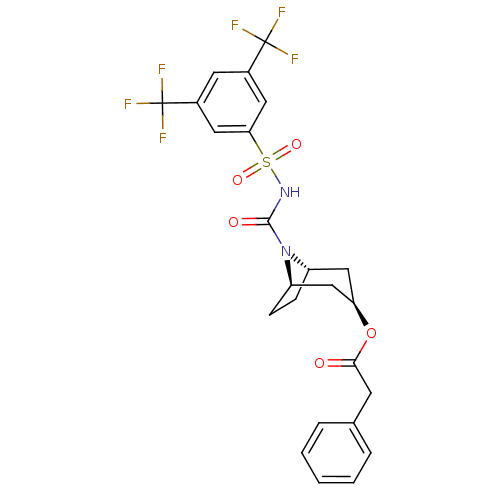
(CHEMBL331089 | Phenyl-acetic acid (1R,3R,5S)-8-(3,...)Show SMILES FC(F)(F)c1cc(cc(c1)S(=O)(=O)NC(=O)N1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)Cc1ccccc1)C(F)(F)F |THB:14:16:23.21.22:18.19| Show InChI InChI=1S/C24H22F6N2O5S/c25-23(26,27)15-9-16(24(28,29)30)11-20(10-15)38(35,36)31-22(34)32-17-6-7-18(32)13-19(12-17)37-21(33)8-14-4-2-1-3-5-14/h1-5,9-11,17-19H,6-8,12-13H2,(H,31,34)/t17-,18+,19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against Steroid sulfatase expressed in CHO cells |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50135162
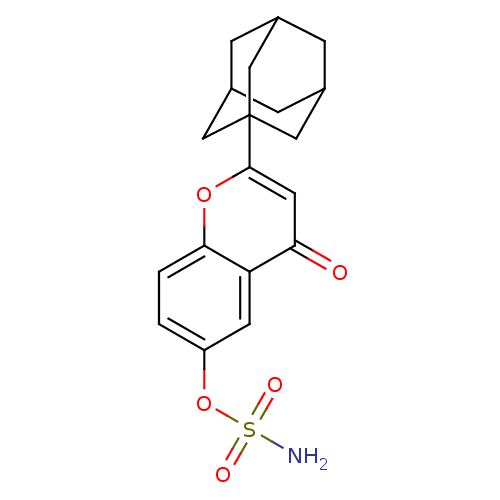
(CHEMBL147705 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:18:25:24.22.21,23:22:25:17.18.19,THB:21:20:17:24.22.23,21:22:17:25.20.19| Show InChI InChI=1S/C19H21NO5S/c20-26(22,23)25-14-1-2-17-15(6-14)16(21)7-18(24-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118560
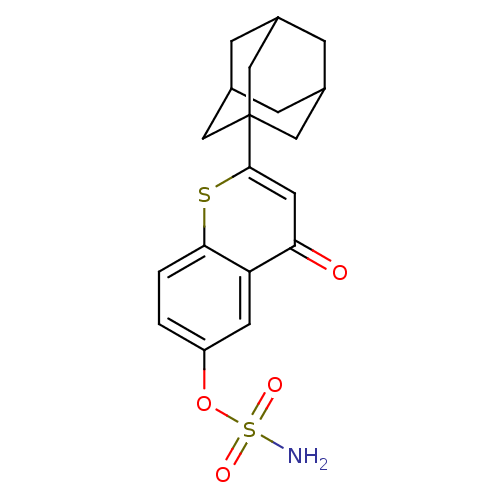
(CHEMBL262050 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2sc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,THB:21:22:17:25.20.19,21:20:17:24.22.23| Show InChI InChI=1S/C19H21NO4S2/c20-26(22,23)24-14-1-2-17-15(6-14)16(21)7-18(25-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151142
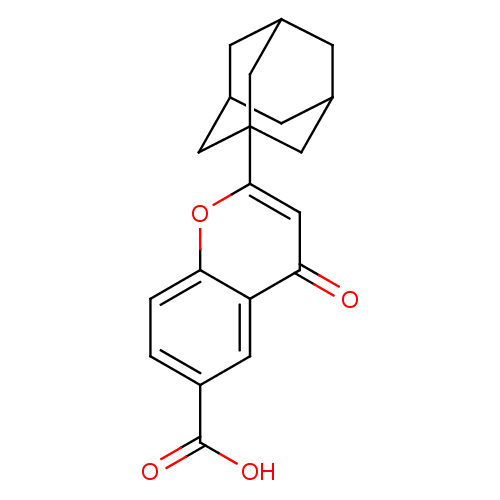
(2-Adamantan-1-yl-4-oxo-4H-chromene-6-carboxylic ac...)Show SMILES OC(=O)c1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:21:20:23:15.16.17,21:16:23:22.20.19,17:18:22:15.16.21,THB:17:16:22:23.18.19| Show InChI InChI=1S/C20H20O4/c21-16-7-18(24-17-2-1-14(19(22)23)6-15(16)17)20-8-11-3-12(9-20)5-13(4-11)10-20/h1-2,6-7,11-13H,3-5,8-10H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151137
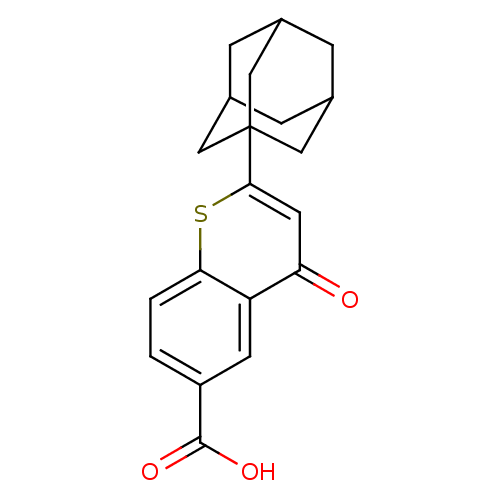
(2-Adamantan-1-yl-4-oxo-4H-thiochromene-6-carboxyli...)Show SMILES OC(=O)c1ccc2sc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:21:20:23:15.16.17,21:16:23:22.20.19,17:18:22:15.16.21,THB:17:16:22:23.18.19| Show InChI InChI=1S/C20H20O3S/c21-16-7-18(24-17-2-1-14(19(22)23)6-15(16)17)20-8-11-3-12(9-20)5-13(4-11)10-20/h1-2,6-7,11-13H,3-5,8-10H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134332
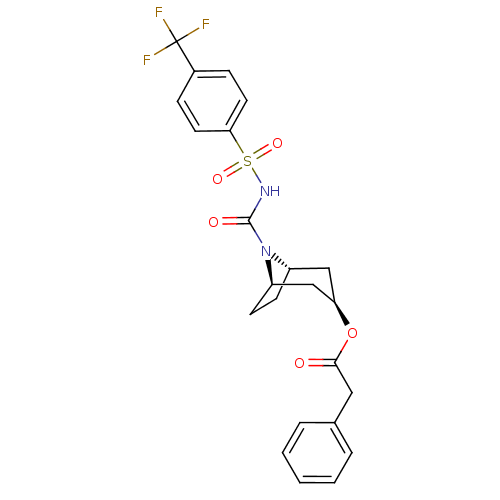
(CHEMBL332811 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)NC(=O)N1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)Cc1ccccc1 |THB:14:16:21.23.22:19.18| Show InChI InChI=1S/C23H23F3N2O5S/c24-23(25,26)16-6-10-20(11-7-16)34(31,32)27-22(30)28-17-8-9-18(28)14-19(13-17)33-21(29)12-15-4-2-1-3-5-15/h1-7,10-11,17-19H,8-9,12-14H2,(H,27,30)/t17-,18+,19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory constant against purified human Steroid sulfatase |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human Steroid sulfatase |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134335
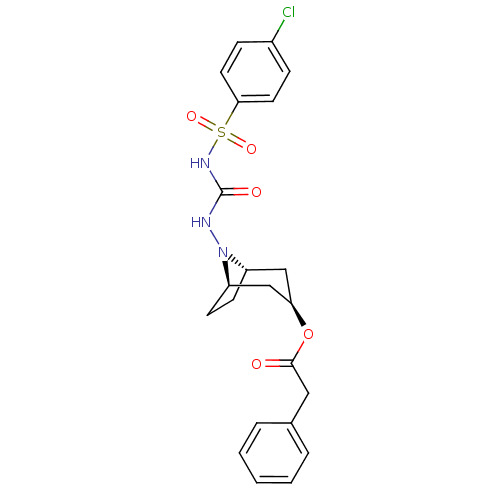
(8-[N-(4-chlorophenylsulfonamido)-carbonyl amino]-8...)Show SMILES Clc1ccc(cc1)S(=O)(=O)NC(=O)NN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)Cc1ccccc1 |THB:13:14:20.19.21:16.17| Show InChI InChI=1S/C22H24ClN3O5S/c23-16-6-10-20(11-7-16)32(29,30)25-22(28)24-26-17-8-9-18(26)14-19(13-17)31-21(27)12-15-4-2-1-3-5-15/h1-7,10-11,17-19H,8-9,12-14H2,(H2,24,25,28)/t17-,18+,19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against Steroid sulfatase expressed in CHO cells |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134325
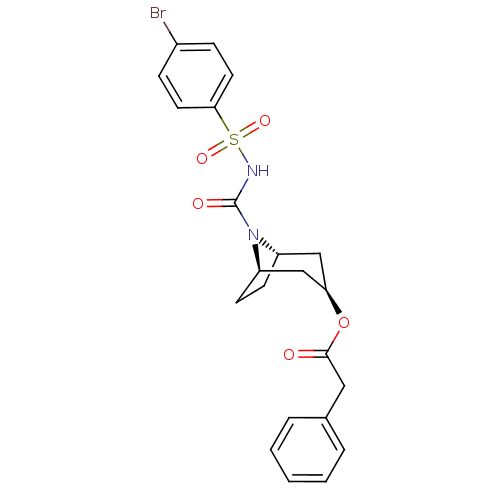
(CHEMBL440159 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...)Show SMILES Brc1ccc(cc1)S(=O)(=O)NC(=O)N1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)Cc1ccccc1 |THB:11:13:20.18.19:15.16| Show InChI InChI=1S/C22H23BrN2O5S/c23-16-6-10-20(11-7-16)31(28,29)24-22(27)25-17-8-9-18(25)14-19(13-17)30-21(26)12-15-4-2-1-3-5-15/h1-7,10-11,17-19H,8-9,12-14H2,(H,24,27)/t17-,18+,19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against Steroid sulfatase expressed in CHO cells |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151135
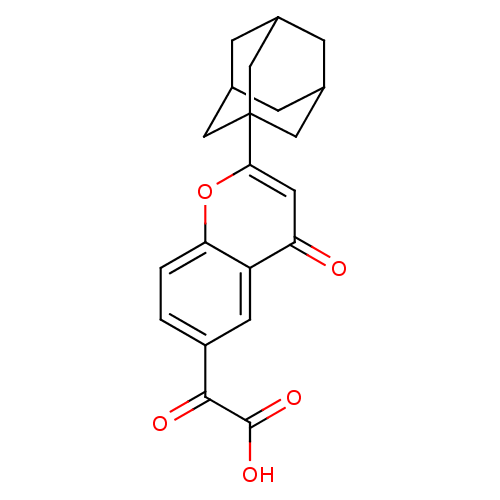
((2-Adamantan-1-yl-4-oxo-4H-chromen-6-yl)-oxo-aceti...)Show SMILES OC(=O)C(=O)c1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,19:20:24:17.18.23,THB:19:18:24:25.20.21| Show InChI InChI=1S/C21H20O5/c22-16-7-18(21-8-11-3-12(9-21)5-13(4-11)10-21)26-17-2-1-14(6-15(16)17)19(23)20(24)25/h1-2,6-7,11-13H,3-5,8-10H2,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134328
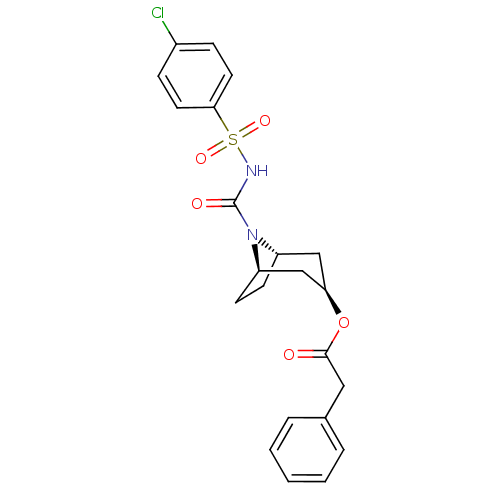
(CHEMBL124349 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...)Show SMILES Clc1ccc(cc1)S(=O)(=O)NC(=O)N1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)Cc1ccccc1 |THB:11:13:20.18.19:15.16| Show InChI InChI=1S/C22H23ClN2O5S/c23-16-6-10-20(11-7-16)31(28,29)24-22(27)25-17-8-9-18(25)14-19(13-17)30-21(26)12-15-4-2-1-3-5-15/h1-7,10-11,17-19H,8-9,12-14H2,(H,24,27)/t17-,18+,19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against Steroid sulfatase expressed in CHO cells |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151140
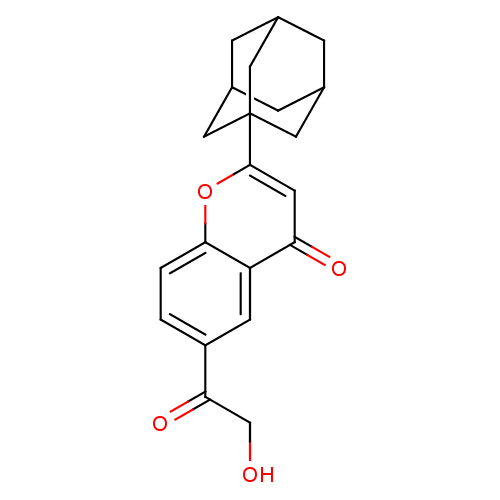
(2-Adamantan-1-yl-6-(2-hydroxy-acetyl)-chromen-4-on...)Show SMILES OCC(=O)c1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:22:21:24:16.17.18,22:17:24:23.21.20,18:19:23:16.17.22,THB:18:17:23:24.19.20| Show InChI InChI=1S/C21H22O4/c22-11-18(24)15-1-2-19-16(6-15)17(23)7-20(25-19)21-8-12-3-13(9-21)5-14(4-12)10-21/h1-2,6-7,12-14,22H,3-5,8-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134337
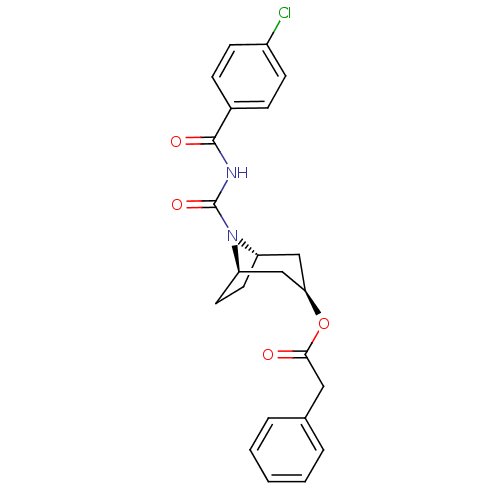
(CHEMBL121068 | Phenyl-acetic acid (1R,3R,5S)-8-(4-...)Show SMILES Clc1ccc(cc1)C(=O)NC(=O)N1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)Cc1ccccc1 |THB:10:12:19.17.18:15.14| Show InChI InChI=1S/C23H23ClN2O4/c24-17-8-6-16(7-9-17)22(28)25-23(29)26-18-10-11-19(26)14-20(13-18)30-21(27)12-15-4-2-1-3-5-15/h1-9,18-20H,10-14H2,(H,25,28,29)/t18-,19+,20+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory constant against purified human Steroid sulfatase |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151134
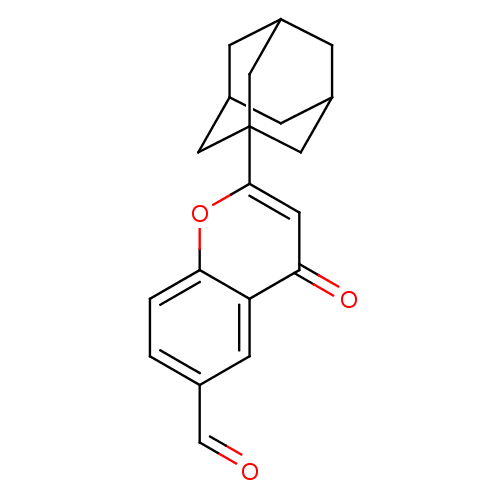
(2-Adamantan-1-yl-4-oxo-4H-chromene-6-carbaldehyde ...)Show SMILES O=Cc1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:16:17:21:14.15.20,THB:16:15:21:22.17.18,18:17:14:21.19.20,18:19:14:22.17.16| Show InChI InChI=1S/C20H20O3/c21-11-12-1-2-18-16(6-12)17(22)7-19(23-18)20-8-13-3-14(9-20)5-15(4-13)10-20/h1-2,6-7,11,13-15H,3-5,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151141
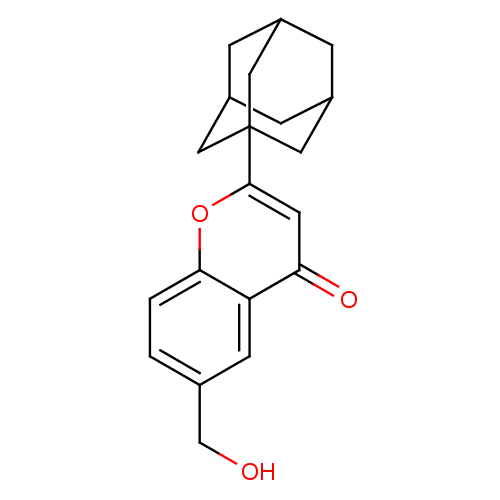
(2-Adamantan-1-yl-6-hydroxymethyl-chromen-4-one | C...)Show SMILES OCc1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:16:17:21:14.15.20,THB:16:15:21:22.17.18,18:17:14:21.19.20,18:19:14:22.17.16| Show InChI InChI=1S/C20H22O3/c21-11-12-1-2-18-16(6-12)17(22)7-19(23-18)20-8-13-3-14(9-20)5-15(4-13)10-20/h1-2,6-7,13-15,21H,3-5,8-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151136

(2-Adamantan-1-yl-4-oxo-4H-thiochromene-6-carbonitr...)Show SMILES O=c1cc(sc2ccc(cc12)C#N)C12CC3CC(CC(C3)C1)C2 |TLB:16:17:21:14.15.20,THB:16:15:21:22.17.18,18:19:14:22.17.16,18:17:14:21.19.20| Show InChI InChI=1S/C20H19NOS/c21-11-12-1-2-18-16(6-12)17(22)7-19(23-18)20-8-13-3-14(9-20)5-15(4-13)10-20/h1-2,6-7,13-15H,3-5,8-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151139

(2-Adamantan-1-yl-4-oxo-4H-chromene-6-carboxylic ac...)Show SMILES COC(=O)c1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:22:21:24:16.17.18,22:17:24:23.21.20,18:19:23:16.17.22,THB:18:17:23:24.19.20| Show InChI InChI=1S/C21H22O4/c1-24-20(23)15-2-3-18-16(7-15)17(22)8-19(25-18)21-9-12-4-13(10-21)6-14(5-12)11-21/h2-3,7-8,12-14H,4-6,9-11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151143
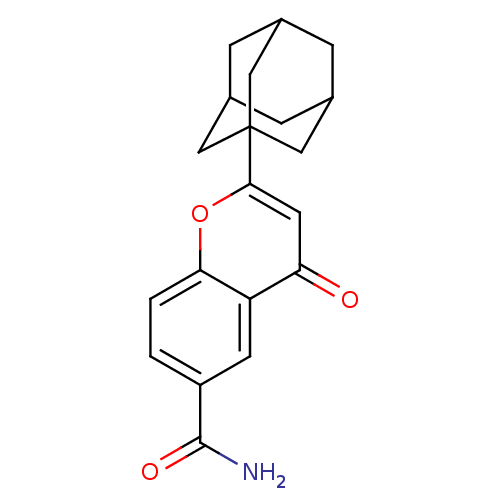
(2-Adamantan-1-yl-4-oxo-4H-chromene-6-carboxylic ac...)Show SMILES NC(=O)c1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:21:20:23:15.16.17,21:16:23:22.20.19,17:18:22:15.16.21,THB:17:16:22:23.18.19| Show InChI InChI=1S/C20H21NO3/c21-19(23)14-1-2-17-15(6-14)16(22)7-18(24-17)20-8-11-3-12(9-20)5-13(4-11)10-20/h1-2,6-7,11-13H,3-5,8-10H2,(H2,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151138
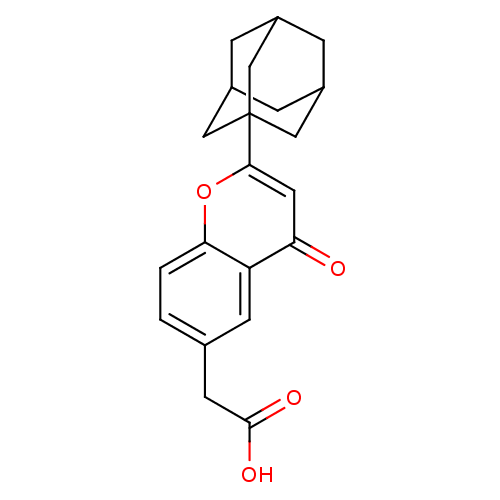
((2-Adamantan-1-yl-4-oxo-4H-chromen-6-yl)-acetic ac...)Show SMILES OC(=O)Cc1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:22:21:24:16.17.18,22:17:24:23.21.20,THB:20:19:16:23.21.22,20:21:16:24.19.18| Show InChI InChI=1S/C21H22O4/c22-17-8-19(21-9-13-3-14(10-21)5-15(4-13)11-21)25-18-2-1-12(6-16(17)18)7-20(23)24/h1-2,6,8,13-15H,3-5,7,9-11H2,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50051568

((3S,6S,9S,12R,15S,18S)-9-(4-Amino-butyl)-3-benzyl-...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55)/t27-,34-,35-,36+,37-,38-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50378998
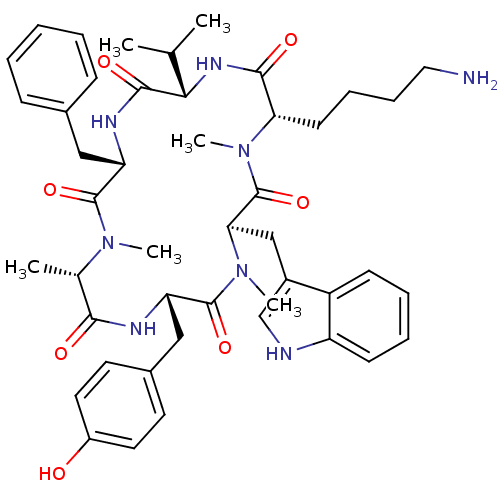
(CHEMBL2011465)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-43(58)50-36(24-30-14-8-7-9-15-30)44(59)52(4)29(3)41(56)49-37(25-31-19-21-33(55)22-20-31)45(60)54(6)39(26-32-27-48-35-17-11-10-16-34(32)35)46(61)53(5)38(42(57)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,56)(H,50,58)(H,51,57)/t29-,36-,37-,38-,39+,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50379004
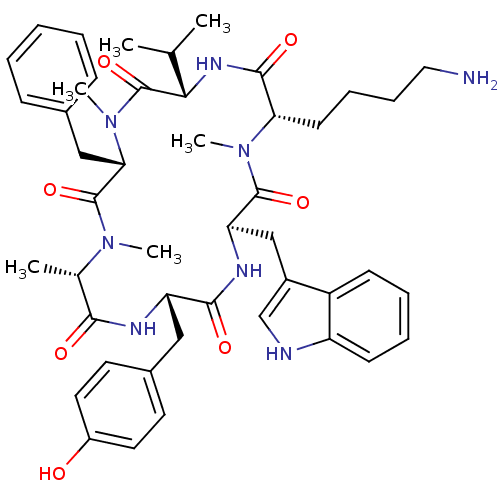
(CHEMBL2011466)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-46(61)54(6)39(25-30-14-8-7-9-15-30)45(60)52(4)29(3)41(56)49-36(24-31-19-21-33(55)22-20-31)42(57)50-37(26-32-27-48-35-17-11-10-16-34(32)35)44(59)53(5)38(43(58)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,56)(H,50,57)(H,51,58)/t29-,36-,37+,38-,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50378999

(CHEMBL2011464)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-43(58)50-36(24-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)48-35(23-30-18-20-32(54)21-19-30)41(56)49-37(25-31-26-47-34-16-10-9-15-33(31)34)45(60)53(5)38(42(57)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,55)(H,49,56)(H,50,58)(H,51,57)/t28-,35-,36-,37+,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50379000
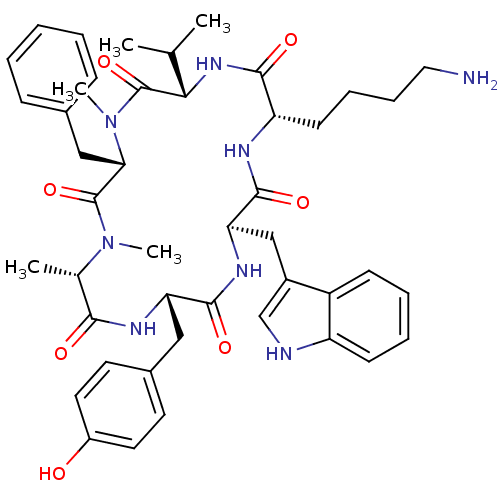
(CHEMBL2011463)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-45(60)53(5)38(24-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)49-36(23-30-18-20-32(54)21-19-30)42(57)50-37(25-31-26-47-34-16-10-9-15-33(31)34)43(58)48-35(41(56)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,58)(H,49,55)(H,50,57)(H,51,56)/t28-,35-,36-,37+,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50379001
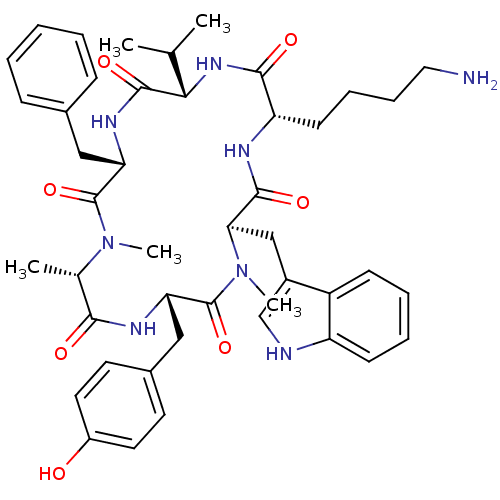
(CHEMBL2011462)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-43(58)50-36(23-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)49-37(24-30-18-20-32(54)21-19-30)45(60)53(5)38(25-31-26-47-34-16-10-9-15-33(31)34)42(57)48-35(41(56)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,57)(H,49,55)(H,50,58)(H,51,56)/t28-,35-,36-,37-,38+,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50118560

(CHEMBL262050 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2sc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:22:25:17.18.19,23:18:25:24.22.21,THB:21:22:17:25.20.19,21:20:17:24.22.23| Show InChI InChI=1S/C19H21NO4S2/c20-26(22,23)24-14-1-2-17-15(6-14)16(21)7-18(25-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase over-expressed in CHO cells |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase over-expressed in CHO cells |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50135162

(CHEMBL147705 | Sulfamic acid 2-adamantan-1-yl-4-ox...)Show SMILES NS(=O)(=O)Oc1ccc2oc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:23:18:25:24.22.21,23:22:25:17.18.19,THB:21:20:17:24.22.23,21:22:17:25.20.19| Show InChI InChI=1S/C19H21NO5S/c20-26(22,23)25-14-1-2-17-15(6-14)16(21)7-18(24-17)19-8-11-3-12(9-19)5-13(4-11)10-19/h1-2,6-7,11-13H,3-5,8-10H2,(H2,20,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase over-expressed in CHO cells |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against Steroid sulfatase expressed in CHO cells |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50379002
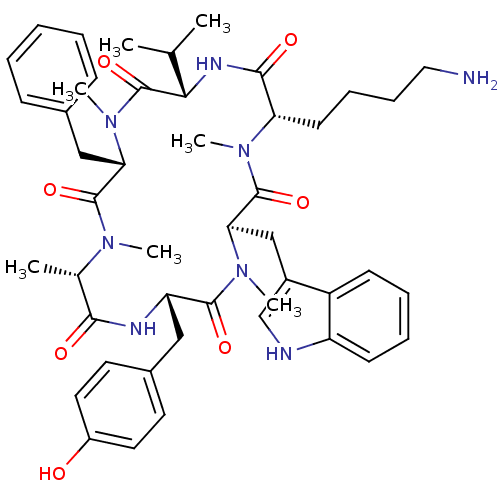
(CHEMBL2011467)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C47H62N8O7/c1-29(2)41-47(62)55(7)39(26-31-15-9-8-10-16-31)45(60)52(4)30(3)42(57)50-37(25-32-20-22-34(56)23-21-32)44(59)54(6)40(27-33-28-49-36-18-12-11-17-35(33)36)46(61)53(5)38(43(58)51-41)19-13-14-24-48/h8-12,15-18,20-23,28-30,37-41,49,56H,13-14,19,24-27,48H2,1-7H3,(H,50,57)(H,51,58)/t30-,37-,38-,39-,40+,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50379003
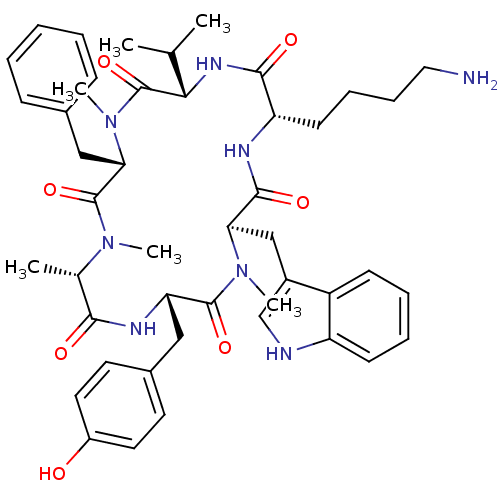
(CHEMBL2011461)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-46(61)54(6)39(25-30-14-8-7-9-15-30)45(60)52(4)29(3)41(56)50-37(24-31-19-21-33(55)22-20-31)44(59)53(5)38(26-32-27-48-35-17-11-10-16-34(32)35)43(58)49-36(42(57)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,58)(H,50,56)(H,51,57)/t29-,36-,37-,38+,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst2 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50379000

(CHEMBL2011463)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-45(60)53(5)38(24-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)49-36(23-30-18-20-32(54)21-19-30)42(57)50-37(25-31-26-47-34-16-10-9-15-33(31)34)43(58)48-35(41(56)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,58)(H,49,55)(H,50,57)(H,51,56)/t28-,35-,36-,37+,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134329

(CHEMBL122708 | Sulfamic acid (11R,12S,15S,16S)-13-...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)ccc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H23NO4S/c1-18-9-8-14-13-5-3-12(23-24(19,21)22)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,19,21,22)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human Steroid sulfatase |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50379003

(CHEMBL2011461)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-46(61)54(6)39(25-30-14-8-7-9-15-30)45(60)52(4)29(3)41(56)50-37(24-31-19-21-33(55)22-20-31)44(59)53(5)38(26-32-27-48-35-17-11-10-16-34(32)35)43(58)49-36(42(57)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,58)(H,50,56)(H,51,57)/t29-,36-,37-,38+,39-,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50051568

((3S,6S,9S,12R,15S,18S)-9-(4-Amino-butyl)-3-benzyl-...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55)/t27-,34-,35-,36+,37-,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50379003

(CHEMBL2011461)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-46(61)54(6)39(25-30-14-8-7-9-15-30)45(60)52(4)29(3)41(56)50-37(24-31-19-21-33(55)22-20-31)44(59)53(5)38(26-32-27-48-35-17-11-10-16-34(32)35)43(58)49-36(42(57)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,58)(H,50,56)(H,51,57)/t29-,36-,37-,38+,39-,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst3 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50134333

(CHEMBL331089 | Phenyl-acetic acid (1R,3R,5S)-8-(3,...)Show SMILES FC(F)(F)c1cc(cc(c1)S(=O)(=O)NC(=O)N1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)Cc1ccccc1)C(F)(F)F |THB:14:16:23.21.22:18.19| Show InChI InChI=1S/C24H22F6N2O5S/c25-23(26,27)15-9-16(24(28,29)30)11-20(10-15)38(35,36)31-22(34)32-17-6-7-18(32)13-19(12-17)37-21(33)8-14-4-2-1-3-5-14/h1-5,9-11,17-19H,6-8,12-13H2,(H,31,34)/t17-,18+,19+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Research Institute Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against purified human Steroid sulfatase |
Bioorg Med Chem Lett 13: 3673-7 (2003)
BindingDB Entry DOI: 10.7270/Q27S7N5G |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50379000

(CHEMBL2011463)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-45(60)53(5)38(24-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)49-36(23-30-18-20-32(54)21-19-30)42(57)50-37(25-31-26-47-34-16-10-9-15-33(31)34)43(58)48-35(41(56)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,58)(H,49,55)(H,50,57)(H,51,56)/t28-,35-,36-,37+,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst3 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50051568

((3S,6S,9S,12R,15S,18S)-9-(4-Amino-butyl)-3-benzyl-...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55)/t27-,34-,35-,36+,37-,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst3 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50379001

(CHEMBL2011462)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-43(58)50-36(23-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)49-37(24-30-18-20-32(54)21-19-30)45(60)53(5)38(25-31-26-47-34-16-10-9-15-33(31)34)42(57)48-35(41(56)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,57)(H,49,55)(H,50,58)(H,51,56)/t28-,35-,36-,37-,38+,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50379004

(CHEMBL2011466)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-46(61)54(6)39(25-30-14-8-7-9-15-30)45(60)52(4)29(3)41(56)49-36(24-31-19-21-33(55)22-20-31)42(57)50-37(26-32-27-48-35-17-11-10-16-34(32)35)44(59)53(5)38(43(58)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,56)(H,50,57)(H,51,58)/t29-,36-,37+,38-,39-,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50378998

(CHEMBL2011465)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-43(58)50-36(24-30-14-8-7-9-15-30)44(59)52(4)29(3)41(56)49-37(25-31-19-21-33(55)22-20-31)45(60)54(6)39(26-32-27-48-35-17-11-10-16-34(32)35)46(61)53(5)38(42(57)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,56)(H,50,58)(H,51,57)/t29-,36-,37-,38-,39+,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst3 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50379004

(CHEMBL2011466)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-46(61)54(6)39(25-30-14-8-7-9-15-30)45(60)52(4)29(3)41(56)49-36(24-31-19-21-33(55)22-20-31)42(57)50-37(26-32-27-48-35-17-11-10-16-34(32)35)44(59)53(5)38(43(58)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,56)(H,50,57)(H,51,58)/t29-,36-,37+,38-,39-,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst3 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50151137

(2-Adamantan-1-yl-4-oxo-4H-thiochromene-6-carboxyli...)Show SMILES OC(=O)c1ccc2sc(cc(=O)c2c1)C12CC3CC(CC(C3)C1)C2 |TLB:21:20:23:15.16.17,21:16:23:22.20.19,17:18:22:15.16.21,THB:17:16:22:23.18.19| Show InChI InChI=1S/C20H20O3S/c21-16-7-18(24-17-2-1-14(19(22)23)6-15(16)17)20-8-11-3-12(9-20)5-13(4-11)10-20/h1-2,6-7,11-13H,3-5,8-10H2,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Vienna
Curated by ChEMBL
| Assay Description
Inhibitory activity against human steroid sulfatase over-expressed in CHO cells |
J Med Chem 47: 4268-76 (2004)
Article DOI: 10.1021/jm0407916
BindingDB Entry DOI: 10.7270/Q2XW4KK4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50378998

(CHEMBL2011465)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C46H60N8O7/c1-28(2)40-43(58)50-36(24-30-14-8-7-9-15-30)44(59)52(4)29(3)41(56)49-37(25-31-19-21-33(55)22-20-31)45(60)54(6)39(26-32-27-48-35-17-11-10-16-34(32)35)46(61)53(5)38(42(57)51-40)18-12-13-23-47/h7-11,14-17,19-22,27-29,36-40,48,55H,12-13,18,23-26,47H2,1-6H3,(H,49,56)(H,50,58)(H,51,57)/t29-,36-,37-,38-,39+,40-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50379002

(CHEMBL2011467)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C47H62N8O7/c1-29(2)41-47(62)55(7)39(26-31-15-9-8-10-16-31)45(60)52(4)30(3)42(57)50-37(25-32-20-22-34(56)23-21-32)44(59)54(6)40(27-33-28-49-36-18-12-11-17-35(33)36)46(61)53(5)38(43(58)51-41)19-13-14-24-48/h8-12,15-18,20-23,28-30,37-41,49,56H,13-14,19,24-27,48H2,1-7H3,(H,50,57)(H,51,58)/t30-,37-,38-,39-,40+,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst3 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50379001

(CHEMBL2011462)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-43(58)50-36(23-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)49-37(24-30-18-20-32(54)21-19-30)45(60)53(5)38(25-31-26-47-34-16-10-9-15-33(31)34)42(57)48-35(41(56)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,57)(H,49,55)(H,50,58)(H,51,56)/t28-,35-,36-,37-,38+,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst3 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50378999

(CHEMBL2011464)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O |r| Show InChI InChI=1S/C45H58N8O7/c1-27(2)39-43(58)50-36(24-29-13-7-6-8-14-29)44(59)52(4)28(3)40(55)48-35(23-30-18-20-32(54)21-19-30)41(56)49-37(25-31-26-47-34-16-10-9-15-33(31)34)45(60)53(5)38(42(57)51-39)17-11-12-22-46/h6-10,13-16,18-21,26-28,35-39,47,54H,11-12,17,22-25,46H2,1-5H3,(H,48,55)(H,49,56)(H,50,58)(H,51,57)/t28-,35-,36-,37+,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50379002

(CHEMBL2011467)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)N(C)C(=O)[C@@H](Cc2c[nH]c3ccccc23)N(C)C(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](C)N(C)C(=O)[C@H](Cc2ccccc2)N(C)C1=O |r| Show InChI InChI=1S/C47H62N8O7/c1-29(2)41-47(62)55(7)39(26-31-15-9-8-10-16-31)45(60)52(4)30(3)42(57)50-37(25-32-20-22-34(56)23-21-32)44(59)54(6)40(27-33-28-49-36-18-12-11-17-35(33)36)46(61)53(5)38(43(58)51-41)19-13-14-24-48/h8-12,15-18,20-23,28-30,37-41,49,56H,13-14,19,24-27,48H2,1-7H3,(H,50,57)(H,51,58)/t30-,37-,38-,39-,40+,41-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human sst5 by in vitro receptor autoradiography assay |
ACS Med Chem Lett 2: 509-514 (2011)
Article DOI: 10.1021/ml200032v
BindingDB Entry DOI: 10.7270/Q2HQ40WP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data