 Found 169 hits with Last Name = 'ismail' and Initial = 'a'
Found 169 hits with Last Name = 'ismail' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-10
(Rattus norvegicus) | BDBM50366779
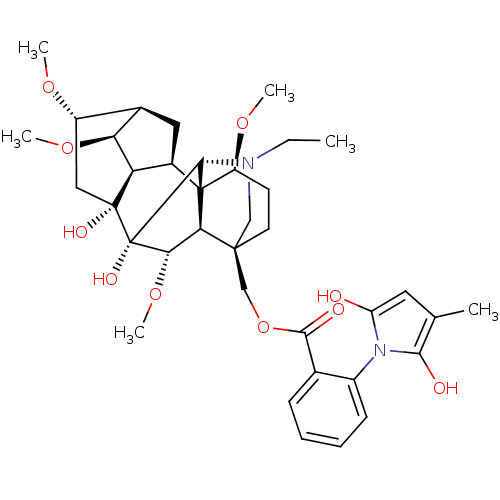
(METHYLLYCACONITINE)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)[C@H]23)[C@H]14 |r,wU:4.4,48.54,44.48,42.46,36.39,39.43,wD:28.52,35.36,31.32,29.31,47.50,25.27,32.34,TLB:23:4:28:44.42,4:47:36.29.35:48,1:2:28:44.42,45:44:36.29.35:48,44:47:24.23.25:3.48.2,42:36:32:29.30,THB:37:36:32:29.30,40:39:32:29.30,5:4:28:44.42,(5.29,-1.99,;6.48,-1.02,;7.91,-1.57,;7.91,-4.71,;8.6,-3.19,;9.67,-4.27,;9.27,-5.75,;10.35,-6.84,;11.83,-6.45,;9.94,-8.32,;8.46,-8.7,;8.06,-10.18,;9.14,-11.27,;10.63,-10.87,;11.02,-9.39,;12.51,-8.99,;13.7,-9.95,;13.62,-11.48,;14.98,-9.12,;14.58,-7.64,;15.55,-6.45,;13.05,-7.56,;11.52,-7.55,;7.27,-2.43,;7.27,-.88,;8.6,-.11,;8.59,1.42,;7.27,2.19,;9.93,-.88,;11.13,.09,;10.97,1.62,;12.38,2.24,;13.41,1.1,;17.14,1.1,;17.91,-.25,;12.64,-.24,;13.31,-1.62,;14.39,-2.7,;15.18,-1.64,;15.11,2.18,;16.19,3.26,;15.8,4.74,;12.66,-3.02,;13.74,-4.1,;11.15,-3.38,;10.82,-4.87,;12.29,-5.26,;9.94,-2.42,;9.88,1.15,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29-,30+,33-,34+,35-,36+,37+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha3-beta4 neuronal nicotinic acetylcholine receptor |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hyderabad Campus
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid as substrate incubated for 1 min prior to substrate addition measured for 25 secs by TMPD-based chrom... |
Bioorg Med Chem Lett 22: 6745-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.082
BindingDB Entry DOI: 10.7270/Q2C82BCM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hyderabad Campus
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cells using arachidonic acid as substrate incubated for 1 min prior to substrate addition me... |
Bioorg Med Chem Lett 22: 6745-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.082
BindingDB Entry DOI: 10.7270/Q2C82BCM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hyderabad Campus
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 expressed in insect cells using arachidonic acid as substrate incubated for 1 min prior to substrate addition me... |
Bioorg Med Chem Lett 22: 6745-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.082
BindingDB Entry DOI: 10.7270/Q2C82BCM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523300
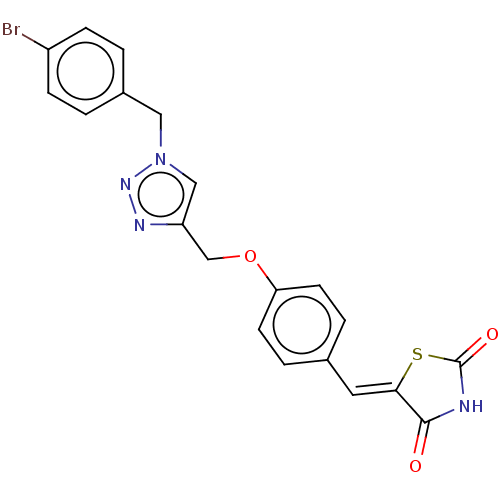
(CHEMBL4475190)Show SMILES Brc1ccc(Cn2cc(COc3ccc(\C=C4/SC(=O)NC4=O)cc3)nn2)cc1 Show InChI InChI=1S/C20H15BrN4O3S/c21-15-5-1-14(2-6-15)10-25-11-16(23-24-25)12-28-17-7-3-13(4-8-17)9-18-19(26)22-20(27)29-18/h1-9,11H,10,12H2,(H,22,26,27)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523290
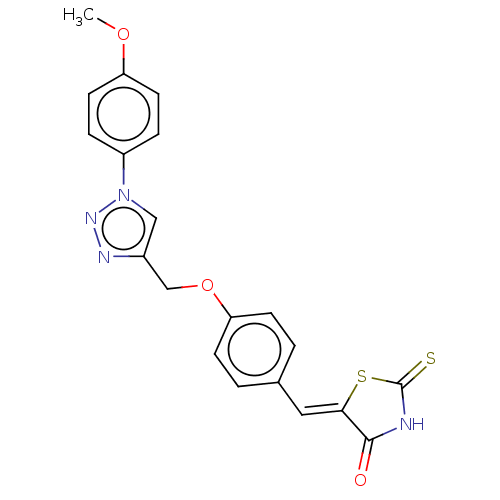
(CHEMBL4474290)Show SMILES COc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1 Show InChI InChI=1S/C20H16N4O3S2/c1-26-16-8-4-15(5-9-16)24-11-14(22-23-24)12-27-17-6-2-13(3-7-17)10-18-19(25)21-20(28)29-18/h2-11H,12H2,1H3,(H,21,25,28)/b18-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50369323
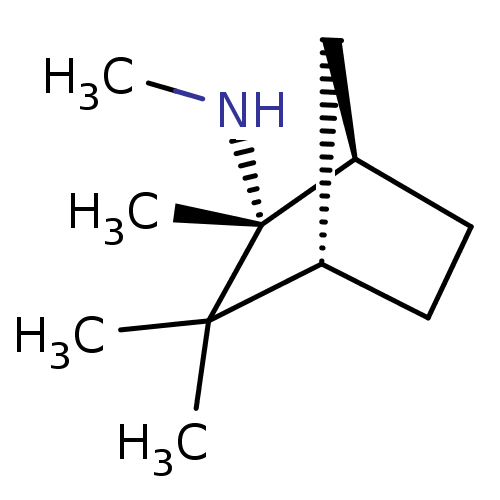
(MECAMYLAMINE)Show InChI InChI=1S/C11H21N/c1-10(2)8-5-6-9(7-8)11(10,3)12-4/h8-9,12H,5-7H2,1-4H3/t8-,9+,11+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against nicotinic acetylcholine receptor alpha3-beta4 |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523302
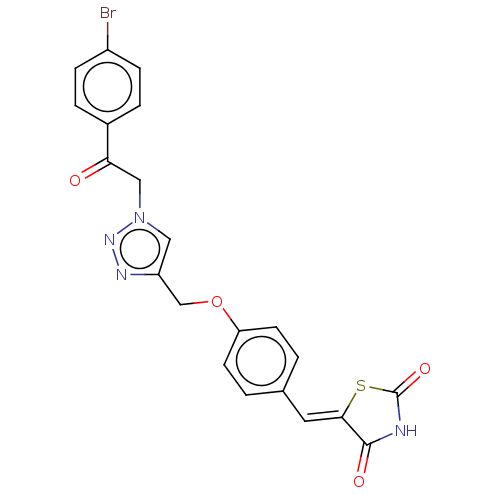
(CHEMBL4589007)Show SMILES Brc1ccc(cc1)C(=O)Cn1cc(COc2ccc(\C=C3/SC(=O)NC3=O)cc2)nn1 Show InChI InChI=1S/C21H15BrN4O4S/c22-15-5-3-14(4-6-15)18(27)11-26-10-16(24-25-26)12-30-17-7-1-13(2-8-17)9-19-20(28)23-21(29)31-19/h1-10H,11-12H2,(H,23,28,29)/b19-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464825

(CHEMBL4279764)Show SMILES OC(=O)c1ccc(cc1)-n1cc(CSc2nc3ccccc3c(=O)n2-c2ccc(Cl)cc2)nn1 Show InChI InChI=1S/C24H16ClN5O3S/c25-16-7-11-19(12-8-16)30-22(31)20-3-1-2-4-21(20)26-24(30)34-14-17-13-29(28-27-17)18-9-5-15(6-10-18)23(32)33/h1-13H,14H2,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523283
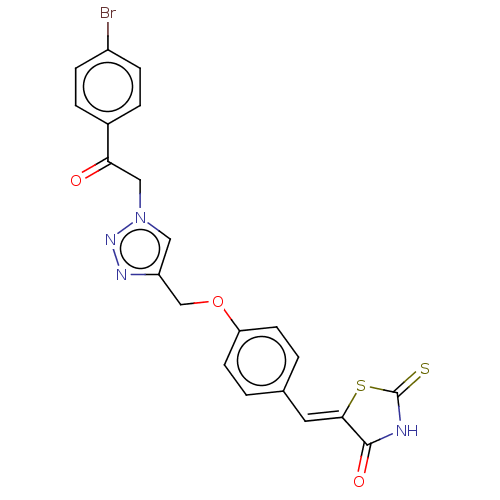
(CHEMBL4438502)Show SMILES Brc1ccc(cc1)C(=O)Cn1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1 Show InChI InChI=1S/C21H15BrN4O3S2/c22-15-5-3-14(4-6-15)18(27)11-26-10-16(24-25-26)12-29-17-7-1-13(2-8-17)9-19-20(28)23-21(30)31-19/h1-10H,11-12H2,(H,23,28,30)/b19-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523304
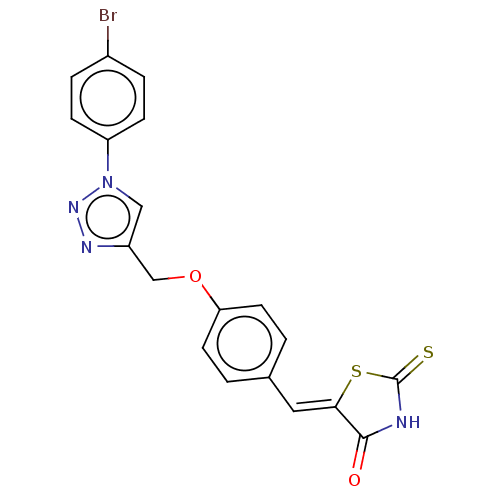
(CHEMBL4542774)Show SMILES Brc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1 Show InChI InChI=1S/C19H13BrN4O2S2/c20-13-3-5-15(6-4-13)24-10-14(22-23-24)11-26-16-7-1-12(2-8-16)9-17-18(25)21-19(27)28-17/h1-10H,11H2,(H,21,25,27)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523294
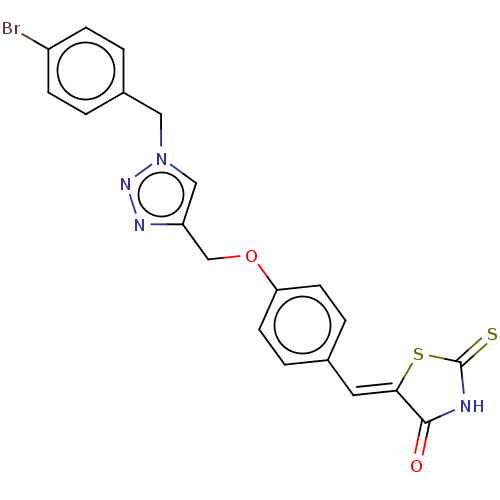
(CHEMBL4579799)Show SMILES Brc1ccc(Cn2cc(COc3ccc(\C=C4/SC(=S)NC4=O)cc3)nn2)cc1 Show InChI InChI=1S/C20H15BrN4O2S2/c21-15-5-1-14(2-6-15)10-25-11-16(23-24-25)12-27-17-7-3-13(4-8-17)9-18-19(26)22-20(28)29-18/h1-9,11H,10,12H2,(H,22,26,28)/b18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523284
(CHEMBL4461022)Show SMILES Brc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=O)NC3=O)cc2)nn1 Show InChI InChI=1S/C19H13BrN4O3S/c20-13-3-5-15(6-4-13)24-10-14(22-23-24)11-27-16-7-1-12(2-8-16)9-17-18(25)21-19(26)28-17/h1-10H,11H2,(H,21,25,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523287

(CHEMBL4447565)Show InChI InChI=1S/C13H9NO2S2/c1-2-7-16-10-5-3-9(4-6-10)8-11-12(15)14-13(17)18-11/h1,3-6,8H,7H2,(H,14,15,17)/b11-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523303
(CHEMBL4472170)Show SMILES COc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=O)NC3=O)cc2)nn1 Show InChI InChI=1S/C20H16N4O4S/c1-27-16-8-4-15(5-9-16)24-11-14(22-23-24)12-28-17-6-2-13(3-7-17)10-18-19(25)21-20(26)29-18/h2-11H,12H2,1H3,(H,21,25,26)/b18-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523289
(CHEMBL4436095)Show SMILES Clc1ccc(cc1)C(=O)Cn1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1 Show InChI InChI=1S/C21H15ClN4O3S2/c22-15-5-3-14(4-6-15)18(27)11-26-10-16(24-25-26)12-29-17-7-1-13(2-8-17)9-19-20(28)23-21(30)31-19/h1-10H,11-12H2,(H,23,28,30)/b19-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464822

(CHEMBL4290032)Show SMILES Clc1ccc(cc1)-n1cc(CSc2nc3ccccc3c(=O)n2-c2ccc(Br)cc2)nn1 Show InChI InChI=1S/C23H15BrClN5OS/c24-15-5-9-19(10-6-15)30-22(31)20-3-1-2-4-21(20)26-23(30)32-14-17-13-29(28-27-17)18-11-7-16(25)8-12-18/h1-13H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464817
(CHEMBL4279346)Show SMILES Brc1ccc(cc1)-n1cc(CSc2nc3ccccc3c(=O)n2-c2ccc(Br)cc2)nn1 Show InChI InChI=1S/C23H15Br2N5OS/c24-15-5-9-18(10-6-15)29-13-17(27-28-29)14-32-23-26-21-4-2-1-3-20(21)22(31)30(23)19-11-7-16(25)8-12-19/h1-13H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464845
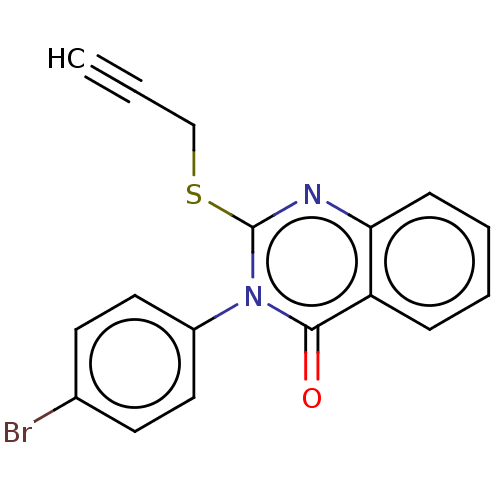
(CHEMBL4289748)Show InChI InChI=1S/C17H11BrN2OS/c1-2-11-22-17-19-15-6-4-3-5-14(15)16(21)20(17)13-9-7-12(18)8-10-13/h1,3-10H,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523292
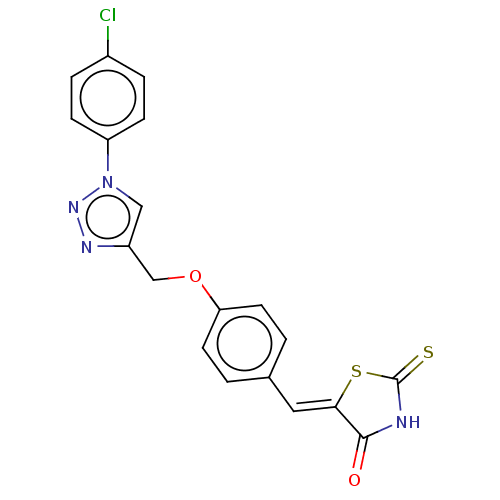
(CHEMBL4530072)Show SMILES Clc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1 Show InChI InChI=1S/C19H13ClN4O2S2/c20-13-3-5-15(6-4-13)24-10-14(22-23-24)11-26-16-7-1-12(2-8-16)9-17-18(25)21-19(27)28-17/h1-10H,11H2,(H,21,25,27)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464827

(CHEMBL4285920)Show SMILES Clc1ccc(cc1)-n1cc(CSc2nc3ccccc3c(=O)n2-c2ccccc2)nn1 Show InChI InChI=1S/C23H16ClN5OS/c24-16-10-12-18(13-11-16)28-14-17(26-27-28)15-31-23-25-21-9-5-4-8-20(21)22(30)29(23)19-6-2-1-3-7-19/h1-14H,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523288
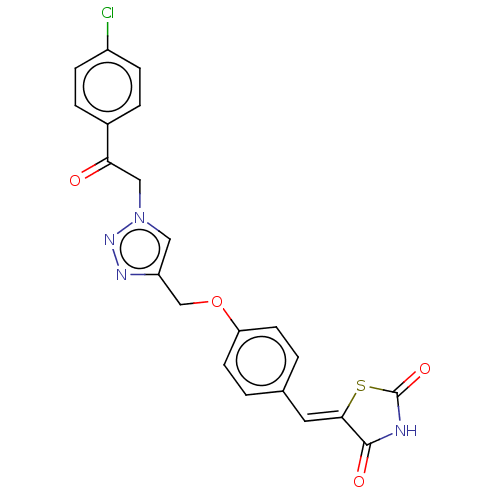
(CHEMBL4521668)Show SMILES Clc1ccc(cc1)C(=O)Cn1cc(COc2ccc(\C=C3/SC(=O)NC3=O)cc2)nn1 Show InChI InChI=1S/C21H15ClN4O4S/c22-15-5-3-14(4-6-15)18(27)11-26-10-16(24-25-26)12-30-17-7-1-13(2-8-17)9-19-20(28)23-21(29)31-19/h1-10H,11-12H2,(H,23,28,29)/b19-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464830

(CHEMBL4291092)Show SMILES Clc1ccc(cc1)-n1c(SCc2cn(nn2)-c2ccc(Br)cc2)nc2ccccc2c1=O Show InChI InChI=1S/C23H15BrClN5OS/c24-15-5-9-18(10-6-15)29-13-17(27-28-29)14-32-23-26-21-4-2-1-3-20(21)22(31)30(23)19-11-7-16(25)8-12-19/h1-13H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464831

(CHEMBL4287667)Show SMILES Brc1ccc(cc1)-n1c(SCc2cn(nn2)-c2ccccc2)nc2ccccc2c1=O Show InChI InChI=1S/C23H16BrN5OS/c24-16-10-12-19(13-11-16)29-22(30)20-8-4-5-9-21(20)25-23(29)31-15-17-14-28(27-26-17)18-6-2-1-3-7-18/h1-14H,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523299
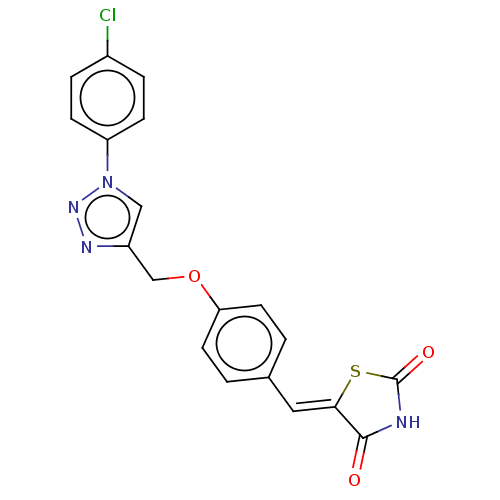
(CHEMBL4544031)Show SMILES Clc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=O)NC3=O)cc2)nn1 Show InChI InChI=1S/C19H13ClN4O3S/c20-13-3-5-15(6-4-13)24-10-14(22-23-24)11-27-16-7-1-12(2-8-16)9-17-18(25)21-19(26)28-17/h1-10H,11H2,(H,21,25,26)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523286
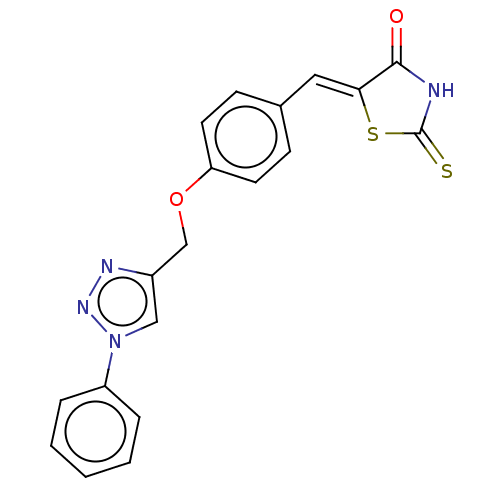
(CHEMBL4559991)Show SMILES O=C1NC(=S)S\C1=C/c1ccc(OCc2cn(nn2)-c2ccccc2)cc1 Show InChI InChI=1S/C19H14N4O2S2/c24-18-17(27-19(26)20-18)10-13-6-8-16(9-7-13)25-12-14-11-23(22-21-14)15-4-2-1-3-5-15/h1-11H,12H2,(H,20,24,26)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523301
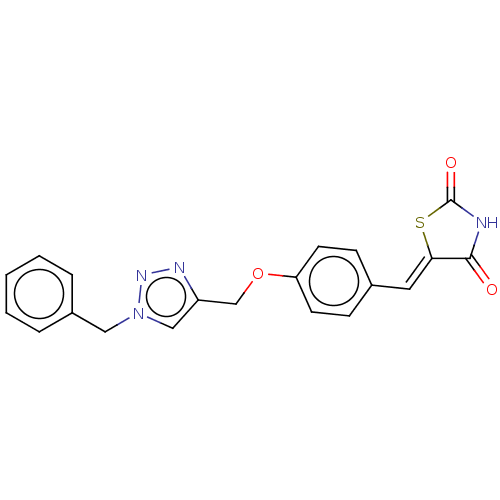
(CHEMBL4570970)Show SMILES O=C1NC(=O)\C(S1)=C\c1ccc(OCc2cn(Cc3ccccc3)nn2)cc1 Show InChI InChI=1S/C20H16N4O3S/c25-19-18(28-20(26)21-19)10-14-6-8-17(9-7-14)27-13-16-12-24(23-22-16)11-15-4-2-1-3-5-15/h1-10,12H,11,13H2,(H,21,25,26)/b18-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464824
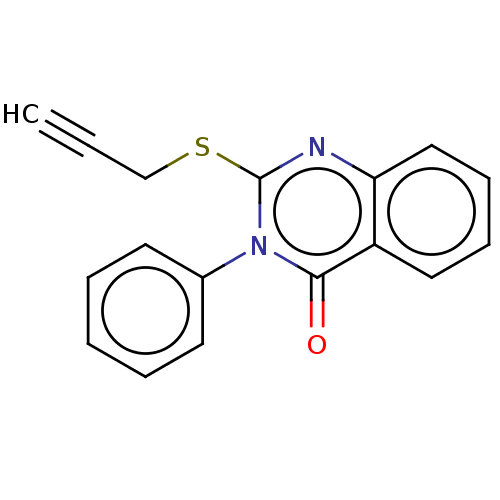
(CHEMBL4293133)Show InChI InChI=1S/C17H12N2OS/c1-2-12-21-17-18-15-11-7-6-10-14(15)16(20)19(17)13-8-4-3-5-9-13/h1,3-11H,12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523295
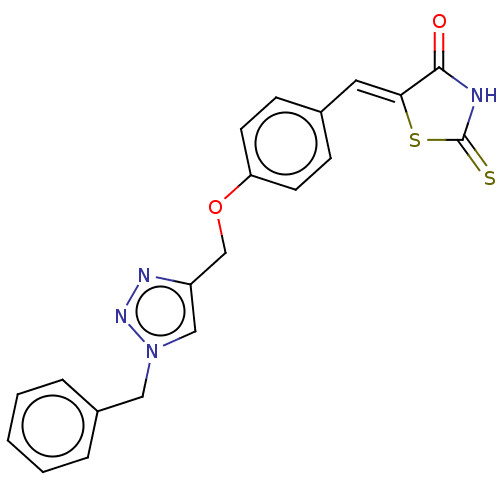
(CHEMBL4449234)Show SMILES O=C1NC(=S)S\C1=C/c1ccc(OCc2cn(Cc3ccccc3)nn2)cc1 Show InChI InChI=1S/C20H16N4O2S2/c25-19-18(28-20(27)21-19)10-14-6-8-17(9-7-14)26-13-16-12-24(23-22-16)11-15-4-2-1-3-5-15/h1-10,12H,11,13H2,(H,21,25,27)/b18-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523296
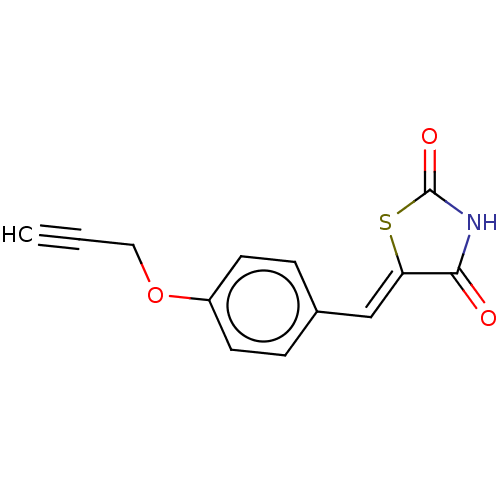
(CHEMBL4454251)Show InChI InChI=1S/C13H9NO3S/c1-2-7-17-10-5-3-9(4-6-10)8-11-12(15)14-13(16)18-11/h1,3-6,8H,7H2,(H,14,15,16)/b11-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464838
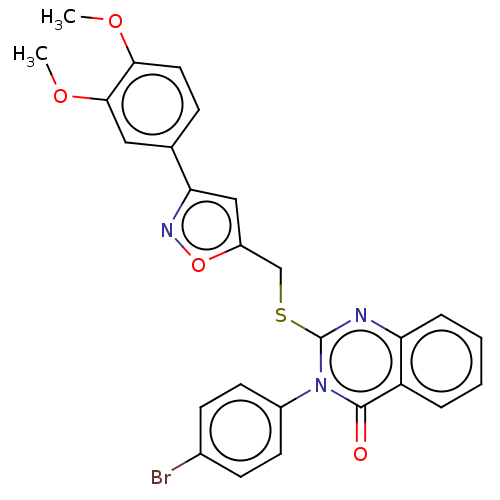
(CHEMBL4291293)Show SMILES COc1ccc(cc1OC)-c1cc(CSc2nc3ccccc3c(=O)n2-c2ccc(Br)cc2)on1 Show InChI InChI=1S/C26H20BrN3O4S/c1-32-23-12-7-16(13-24(23)33-2)22-14-19(34-29-22)15-35-26-28-21-6-4-3-5-20(21)25(31)30(26)18-10-8-17(27)9-11-18/h3-14H,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523285
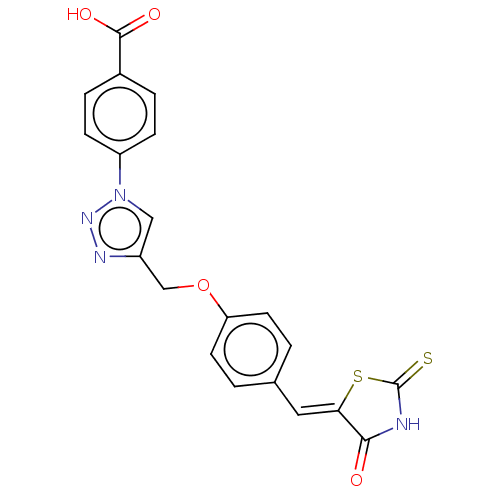
(CHEMBL4445953)Show SMILES OC(=O)c1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1 Show InChI InChI=1S/C20H14N4O4S2/c25-18-17(30-20(29)21-18)9-12-1-7-16(8-2-12)28-11-14-10-24(23-22-14)15-5-3-13(4-6-15)19(26)27/h1-10H,11H2,(H,26,27)(H,21,25,29)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464835

(CHEMBL4278472)Show SMILES COc1ccc(cc1)-c1cc(CSc2nc3ccccc3c(=O)n2-c2ccccc2)on1 Show InChI InChI=1S/C25H19N3O3S/c1-30-19-13-11-17(12-14-19)23-15-20(31-27-23)16-32-25-26-22-10-6-5-9-21(22)24(29)28(25)18-7-3-2-4-8-18/h2-15H,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523293
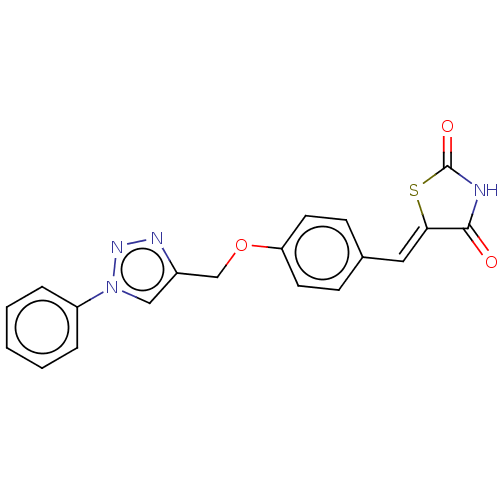
(CHEMBL4439919)Show SMILES O=C1NC(=O)\C(S1)=C\c1ccc(OCc2cn(nn2)-c2ccccc2)cc1 Show InChI InChI=1S/C19H14N4O3S/c24-18-17(27-19(25)20-18)10-13-6-8-16(9-7-13)26-12-14-11-23(22-21-14)15-4-2-1-3-5-15/h1-11H,12H2,(H,20,24,25)/b17-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464840

(CHEMBL4279063)Show InChI InChI=1S/C17H11ClN2OS/c1-2-11-22-17-19-15-6-4-3-5-14(15)16(21)20(17)13-9-7-12(18)8-10-13/h1,3-10H,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523298
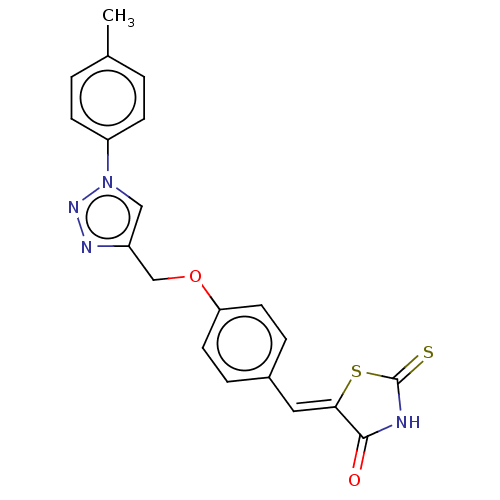
(CHEMBL4454204)Show SMILES Cc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1 Show InChI InChI=1S/C20H16N4O2S2/c1-13-2-6-16(7-3-13)24-11-15(22-23-24)12-26-17-8-4-14(5-9-17)10-18-19(25)21-20(27)28-18/h2-11H,12H2,1H3,(H,21,25,27)/b18-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464821

(CHEMBL4289328)Show InChI InChI=1S/C18H14N2O2S/c1-3-12-23-18-19-16-7-5-4-6-15(16)17(21)20(18)13-8-10-14(22-2)11-9-13/h1,4-11H,12H2,2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523297
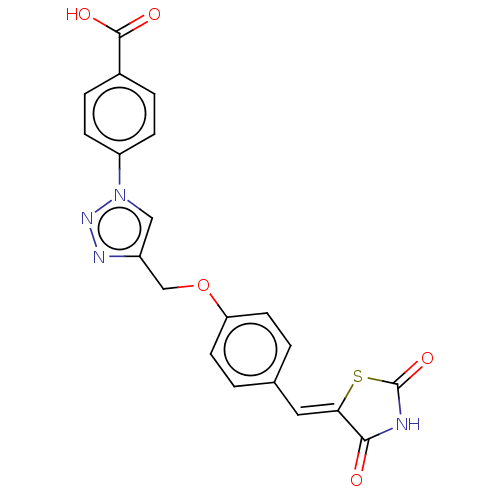
(CHEMBL4535128)Show SMILES OC(=O)c1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=O)NC3=O)cc2)nn1 Show InChI InChI=1S/C20H14N4O5S/c25-18-17(30-20(28)21-18)9-12-1-7-16(8-2-12)29-11-14-10-24(23-22-14)15-5-3-13(4-6-15)19(26)27/h1-10H,11H2,(H,26,27)(H,21,25,28)/b17-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50523291
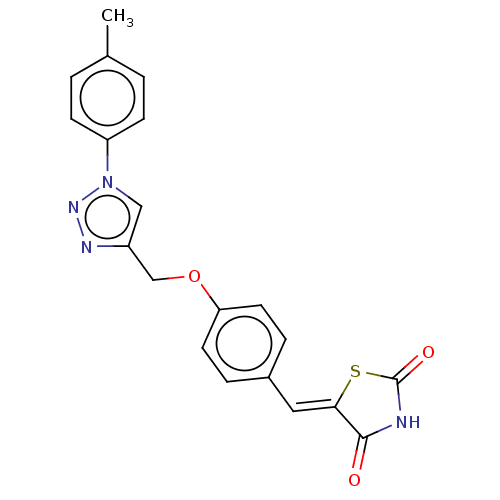
(CHEMBL4450483)Show SMILES Cc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=O)NC3=O)cc2)nn1 Show InChI InChI=1S/C20H16N4O3S/c1-13-2-6-16(7-3-13)24-11-15(22-23-24)12-27-17-8-4-14(5-9-17)10-18-19(25)21-20(26)28-18/h2-11H,12H2,1H3,(H,21,25,26)/b18-10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... |
Eur J Med Chem 167: 562-582 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.034
BindingDB Entry DOI: 10.7270/Q2DN48FJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464833

(CHEMBL4288111)Show SMILES COc1ccc(cc1)-n1c(SCc2cn(nn2)-c2ccc(Cl)cc2)nc2ccccc2c1=O Show InChI InChI=1S/C24H18ClN5O2S/c1-32-20-12-10-19(11-13-20)30-23(31)21-4-2-3-5-22(21)26-24(30)33-15-17-14-29(28-27-17)18-8-6-16(25)7-9-18/h2-14H,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464829

(CHEMBL4283242)Show SMILES Clc1ccc(cc1)-n1cc(CSc2nc3ccccc3c(=O)n2-c2ccc(Cl)cc2)nn1 Show InChI InChI=1S/C23H15Cl2N5OS/c24-15-5-9-18(10-6-15)29-13-17(27-28-29)14-32-23-26-21-4-2-1-3-20(21)22(31)30(23)19-11-7-16(25)8-12-19/h1-13H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464828
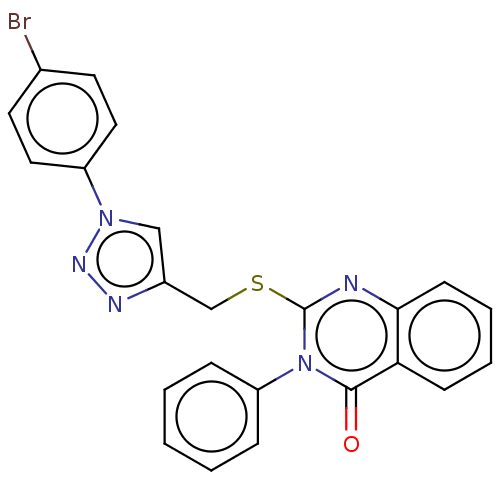
(CHEMBL4277993)Show SMILES Brc1ccc(cc1)-n1cc(CSc2nc3ccccc3c(=O)n2-c2ccccc2)nn1 Show InChI InChI=1S/C23H16BrN5OS/c24-16-10-12-18(13-11-16)28-14-17(26-27-28)15-31-23-25-21-9-5-4-8-20(21)22(30)29(23)19-6-2-1-3-7-19/h1-14H,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464820

(CHEMBL4288714)Show SMILES COc1ccc(cc1)-c1cc(CSc2nc3ccccc3c(=O)n2-c2ccc(Br)cc2)on1 Show InChI InChI=1S/C25H18BrN3O3S/c1-31-19-12-6-16(7-13-19)23-14-20(32-28-23)15-33-25-27-22-5-3-2-4-21(22)24(30)29(25)18-10-8-17(26)9-11-18/h2-14H,15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50464847

(CHEMBL4283447)Show SMILES Clc1ccc(cc1)-c1cc(CSc2nc3ccccc3c(=O)n2-c2ccc(Br)cc2)on1 Show InChI InChI=1S/C24H15BrClN3O2S/c25-16-7-11-18(12-8-16)29-23(30)20-3-1-2-4-21(20)27-24(29)32-14-19-13-22(28-31-19)15-5-9-17(26)10-6-15/h1-13H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha production using arachidonic acid as substrate by colorimetric method |
Eur J Med Chem 144: 635-650 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.065
BindingDB Entry DOI: 10.7270/Q2FX7D4C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data