 Found 218 hits with Last Name = 'marquart' and Initial = 'a'
Found 218 hits with Last Name = 'marquart' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50073850
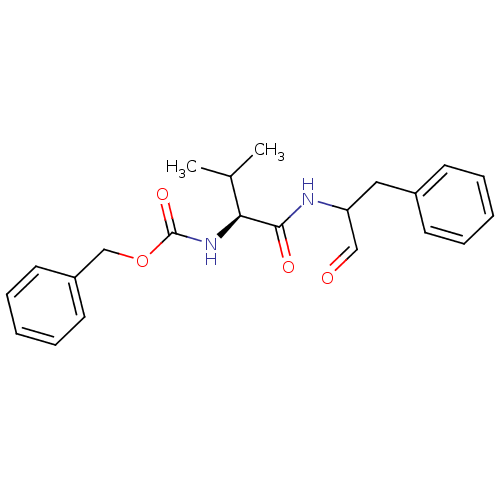
((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Gallus gallus) | BDBM50073850

((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O |r| Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27)/t19?,20-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against chicken gizzard smooth muscle calpain |
Bioorg Med Chem Lett 9: 139-40 (1999)
BindingDB Entry DOI: 10.7270/Q2DN4476 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080206
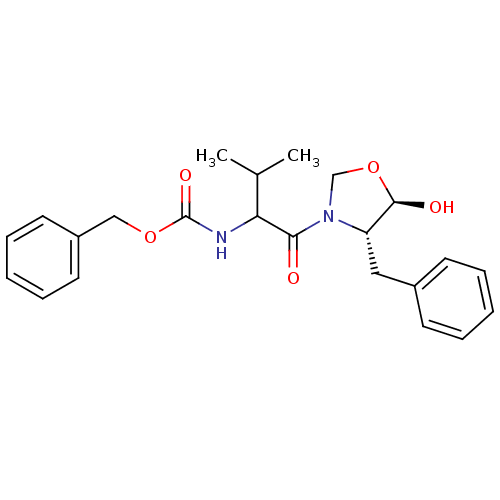
(CHEMBL311735 | [1-((4S,5R)-4-Benzyl-5-hydroxy-oxaz...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N1CO[C@@H](O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H28N2O5/c1-16(2)20(24-23(28)29-14-18-11-7-4-8-12-18)21(26)25-15-30-22(27)19(25)13-17-9-5-3-6-10-17/h3-12,16,19-20,22,27H,13-15H2,1-2H3,(H,24,28)/t19-,20?,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282635
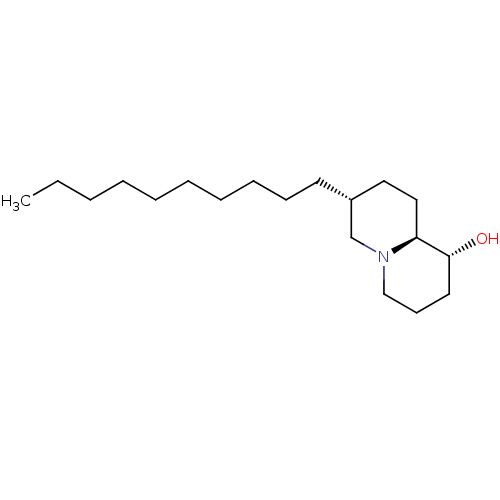
((1R,7R,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Bos taurus (bovine)) | BDBM50080206

(CHEMBL311735 | [1-((4S,5R)-4-Benzyl-5-hydroxy-oxaz...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N1CO[C@@H](O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H28N2O5/c1-16(2)20(24-23(28)29-14-18-11-7-4-8-12-18)21(26)25-15-30-22(27)19(25)13-17-9-5-3-6-10-17/h3-12,16,19-20,22,27H,13-15H2,1-2H3,(H,24,28)/t19-,20?,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against bovine cathepsin B. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080207

(CHEMBL80142 | {1-[((S)-1-Formyl-2-phenyl-ethyl)-hy...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N(CO)[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H28N2O5/c1-17(2)21(24-23(29)30-15-19-11-7-4-8-12-19)22(28)25(16-27)20(14-26)13-18-9-5-3-6-10-18/h3-12,14,17,20-21,27H,13,15-16H2,1-2H3,(H,24,29)/t20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080209
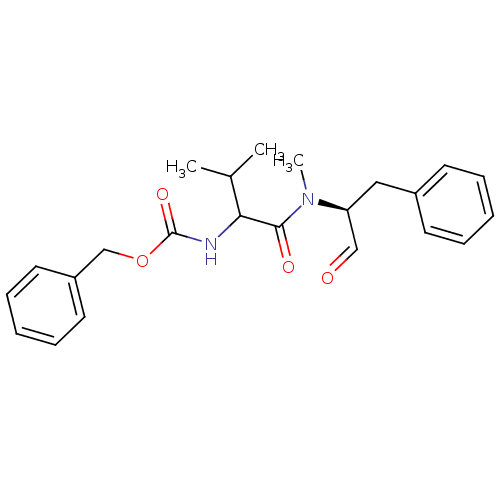
(CHEMBL78324 | {1-[((S)-1-Formyl-2-phenyl-ethyl)-me...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N(C)[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H28N2O4/c1-17(2)21(24-23(28)29-16-19-12-8-5-9-13-19)22(27)25(3)20(15-26)14-18-10-6-4-7-11-18/h4-13,15,17,20-21H,14,16H2,1-3H3,(H,24,28)/t20-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50282634
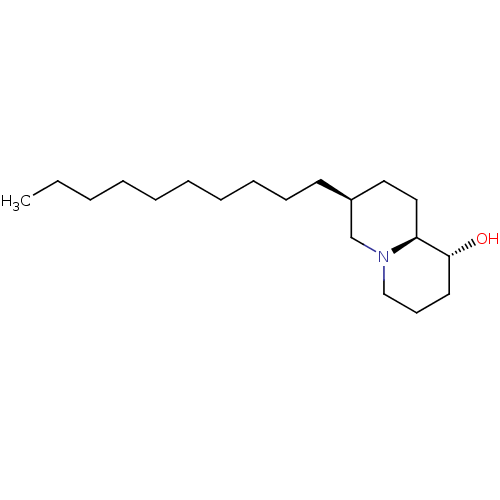
((1R,7S,9aS)-7-Decyl-octahydro-quinolizin-1-ol | CH...)Show InChI InChI=1S/C19H37NO/c1-2-3-4-5-6-7-8-9-11-17-13-14-18-19(21)12-10-15-20(18)16-17/h17-19,21H,2-16H2,1H3/t17-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its activity to inhibit rat liver 2,3-oxidosqualene-lanosterol cyclase, activity expressed as Ki |
Bioorg Med Chem Lett 4: 1317-1318 (1994)
Article DOI: 10.1016/S0960-894X(01)80352-8
BindingDB Entry DOI: 10.7270/Q23T9H5P |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50366611
(CHEMBL1794816)Show SMILES O[C@H]1O[C@@H]2CCC[C@H](NC(=O)OCc3ccccc3)C(=O)N2[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H26N2O5/c26-21-18(24-23(28)29-15-17-10-5-2-6-11-17)12-7-13-20-25(21)19(22(27)30-20)14-16-8-3-1-4-9-16/h1-6,8-11,18-20,22,27H,7,12-15H2,(H,24,28)/t18-,19+,20+,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
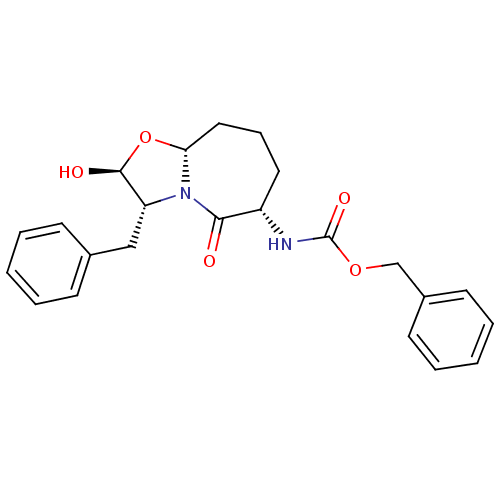
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080210
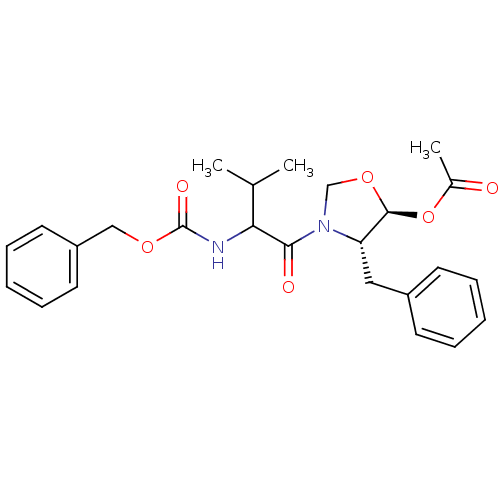
(Acetic acid (4S,5S)-4-benzyl-3-(2-benzyloxycarbony...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)N1CO[C@@H](OC(C)=O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C25H30N2O6/c1-17(2)22(26-25(30)31-15-20-12-8-5-9-13-20)23(29)27-16-32-24(33-18(3)28)21(27)14-19-10-6-4-7-11-19/h4-13,17,21-22,24H,14-16H2,1-3H3,(H,26,30)/t21-,22?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Gallus gallus) | BDBM50073851
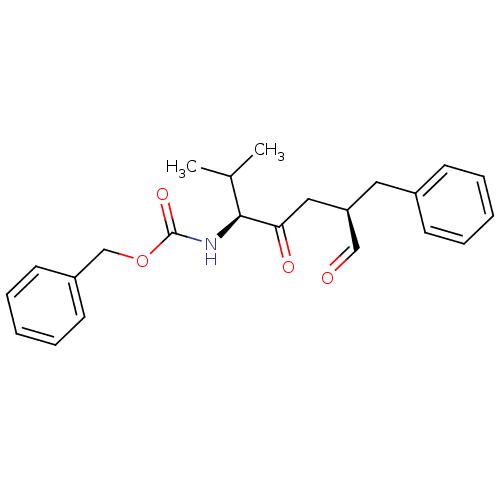
(((1S,4R)-4-Benzyl-1-isopropyl-2,5-dioxo-pentyl)-ca...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)C[C@@H](Cc1ccccc1)C=O Show InChI InChI=1S/C23H27NO4/c1-17(2)22(24-23(27)28-16-19-11-7-4-8-12-19)21(26)14-20(15-25)13-18-9-5-3-6-10-18/h3-12,15,17,20,22H,13-14,16H2,1-2H3,(H,24,27)/t20-,22+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against chicken gizzard smooth muscle calpain |
Bioorg Med Chem Lett 9: 139-40 (1999)
BindingDB Entry DOI: 10.7270/Q2DN4476 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50366612
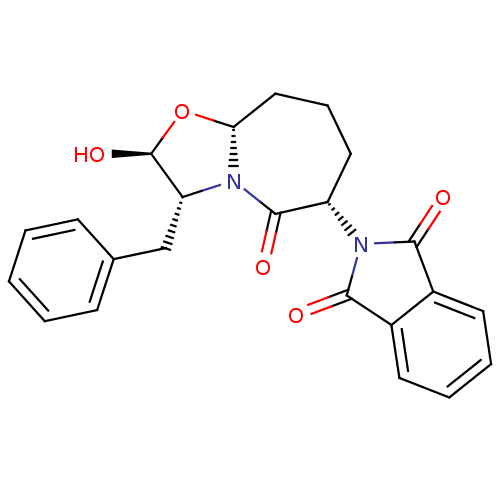
(CHEMBL1794817)Show SMILES O[C@H]1O[C@@H]2CCC[C@H](N3C(=O)c4ccccc4C3=O)C(=O)N2[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C23H22N2O5/c26-20-15-9-4-5-10-16(15)21(27)25(20)17-11-6-12-19-24(22(17)28)18(23(29)30-19)13-14-7-2-1-3-8-14/h1-5,7-10,17-19,23,29H,6,11-13H2/t17-,18+,19+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Gallus gallus) | BDBM50073849

(((S)-1-Isopropyl-4-methyl-2,5-dioxo-pentyl)-carbam...)Show InChI InChI=1S/C17H23NO4/c1-12(2)16(15(20)9-13(3)10-19)18-17(21)22-11-14-7-5-4-6-8-14/h4-8,10,12-13,16H,9,11H2,1-3H3,(H,18,21)/t13?,16-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against chicken gizzard smooth muscle calpain |
Bioorg Med Chem Lett 9: 139-40 (1999)
BindingDB Entry DOI: 10.7270/Q2DN4476 |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50080208
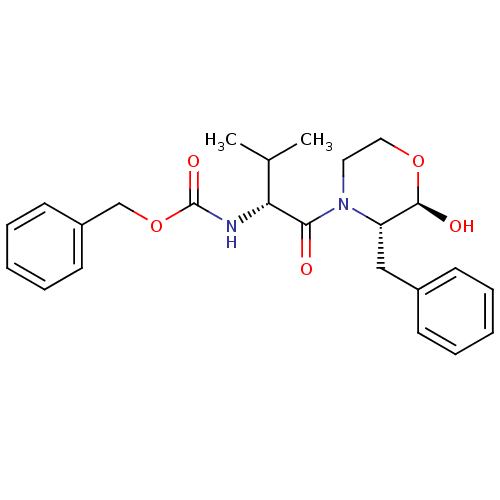
(CHEMBL73869 | [(R)-1-((2R,3S)-3-Benzyl-2-hydroxy-m...)Show SMILES CC(C)[C@@H](NC(=O)OCc1ccccc1)C(=O)N1CCO[C@@H](O)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C24H30N2O5/c1-17(2)21(25-24(29)31-16-19-11-7-4-8-12-19)22(27)26-13-14-30-23(28)20(26)15-18-9-5-3-6-10-18/h3-12,17,20-21,23,28H,13-16H2,1-2H3,(H,25,29)/t20-,21+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Tested for inhibitory activity against calpain. |
Bioorg Med Chem Lett 9: 2365-70 (1999)
BindingDB Entry DOI: 10.7270/Q2F1907J |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50474994
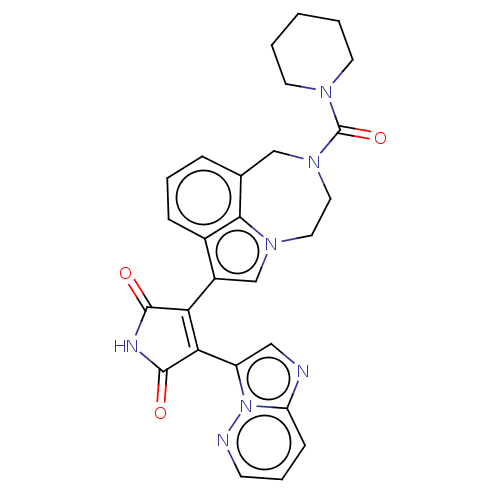
(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475007

(Bisarylmaleimide 2)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H23N5O5/c33-25-21(19-13-28-12-16-4-9-37-24(16)19)22(26(34)29-25)20-15-31-5-6-32(27(35)30-7-10-36-11-8-30)14-17-2-1-3-18(20)23(17)31/h1-4,9,12-13,15H,5-8,10-11,14H2,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475025

(CHEMBL181339)Show SMILES O=C(C1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-23(24(27(35)30-26)21-14-29-22-6-1-2-9-33(21)22)20-16-31-10-11-32(28(36)17-7-12-37-13-8-17)15-18-4-3-5-19(20)25(18)31/h1-6,9,14,16-17H,7-8,10-13,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475024

(CHEMBL181371)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H18N4O4/c1-13(29)27-6-7-28-12-18(16-4-2-3-15(11-27)21(16)28)20-19(23(30)26-24(20)31)17-10-25-9-14-5-8-32-22(14)17/h2-5,8-10,12H,6-7,11H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475018
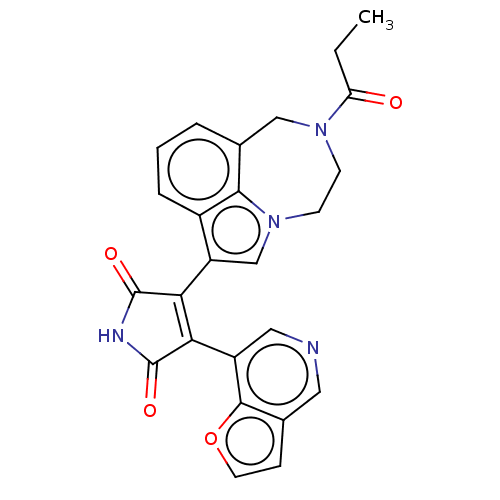
(CHEMBL181518)Show SMILES CCC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:10| Show InChI InChI=1S/C25H20N4O4/c1-2-19(30)28-7-8-29-13-18(16-5-3-4-15(12-28)22(16)29)21-20(24(31)27-25(21)32)17-11-26-10-14-6-9-33-23(14)17/h3-6,9-11,13H,2,7-8,12H2,1H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475031

(CHEMBL359871)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4CCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H21N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-7,13H,8-12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475029

(CHEMBL180779)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H21N5O4/c1-28(2)25(33)30-8-7-29-13-18(16-5-3-4-15(12-30)21(16)29)20-19(23(31)27-24(20)32)17-11-26-10-14-6-9-34-22(14)17/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150700
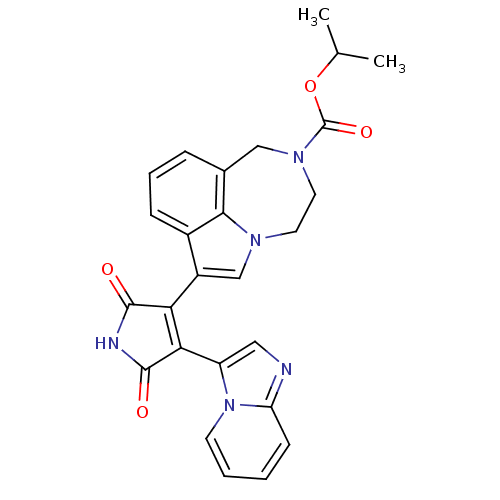
(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150698
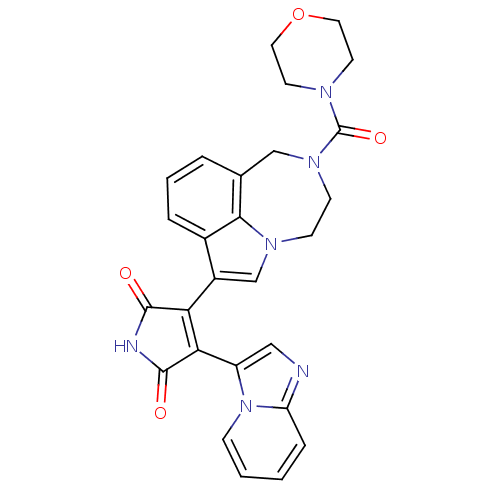
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475008

(CHEMBL369090)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3ccn4ncccc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(19-6-8-33-21(19)5-2-7-28-33)23(26(35)29-25)20-16-31-9-10-32(27(36)30-11-13-37-14-12-30)15-17-3-1-4-18(20)24(17)31/h1-8,16H,9-15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475022

(CHEMBL361765)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H19N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-8,11,13H,9-10,12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701
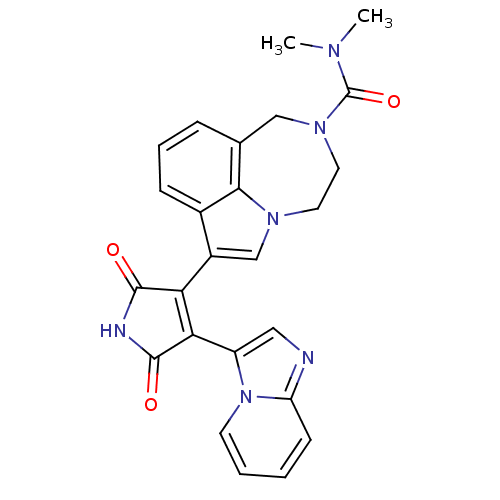
(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474994

(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475010
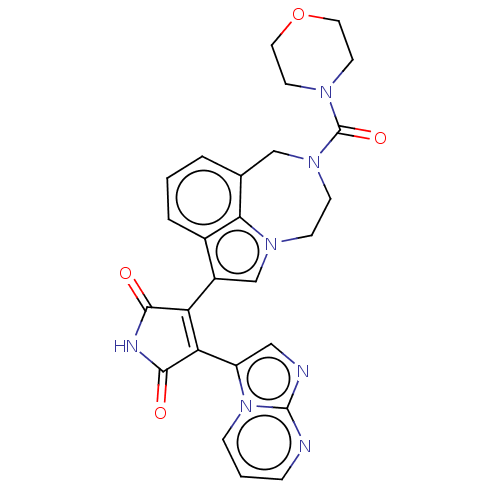
(CHEMBL369316)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C26H23N7O4/c34-23-20(21(24(35)29-23)19-13-28-25-27-5-2-6-33(19)25)18-15-31-7-8-32(26(36)30-9-11-37-12-10-30)14-16-3-1-4-17(18)22(16)31/h1-6,13,15H,7-12,14H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475001
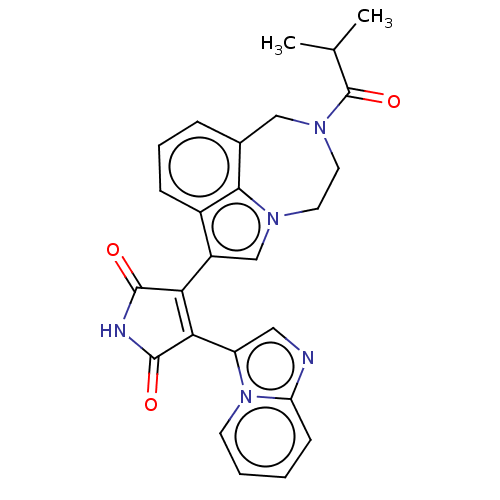
(CHEMBL368246)Show SMILES CC(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H23N5O3/c1-15(2)26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475004

(CHEMBL369572)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-24-21(22(25(36)30-24)20-14-29-26-28-8-5-11-34(20)26)19-16-32-12-13-33(27(37)31-9-2-1-3-10-31)15-17-6-4-7-18(19)23(17)32/h4-8,11,14,16H,1-3,9-10,12-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475014
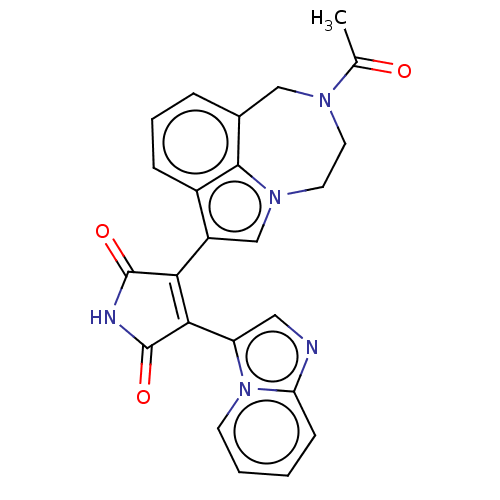
(CHEMBL361948)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N5O3/c1-14(30)27-9-10-28-13-17(16-6-4-5-15(12-27)22(16)28)20-21(24(32)26-23(20)31)18-11-25-19-7-2-3-8-29(18)19/h2-8,11,13H,9-10,12H2,1H3,(H,26,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475006

(CHEMBL178851)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cnccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-13-29-21-14-28-7-10-34(20)21)19-16-32-11-12-33(27(37)31-8-2-1-3-9-31)15-17-5-4-6-18(19)24(17)32/h4-7,10,13-14,16H,1-3,8-9,11-12,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474996

(CHEMBL178646)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-22(20-14-29-13-17-7-12-37-25(17)20)23(27(35)30-26)21-16-32-10-11-33(28(36)31-8-2-1-3-9-31)15-18-5-4-6-19(21)24(18)32/h4-7,12-14,16H,1-3,8-11,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475000

(CHEMBL181296)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4OCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N3O5/c1-13(28)26-8-9-27-11-17(15-5-2-4-14(10-26)21(15)27)20-19(23(29)25-24(20)30)16-6-3-7-18-22(16)32-12-31-18/h2-7,11H,8-10,12H2,1H3,(H,25,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475011

(CHEMBL178850)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H22N4O4/c1-28(2)26(33)30-11-10-29-14-19(17-7-4-6-16(13-30)22(17)29)21-20(24(31)27-25(21)32)18-8-3-5-15-9-12-34-23(15)18/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475016
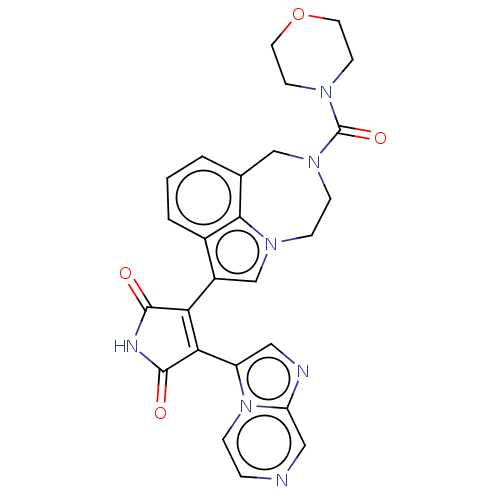
(CHEMBL361007)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cnccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C26H23N7O4/c34-24-21(22(25(35)29-24)19-12-28-20-13-27-4-5-33(19)20)18-15-31-6-7-32(26(36)30-8-10-37-11-9-30)14-16-2-1-3-17(18)23(16)31/h1-5,12-13,15H,6-11,14H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475021
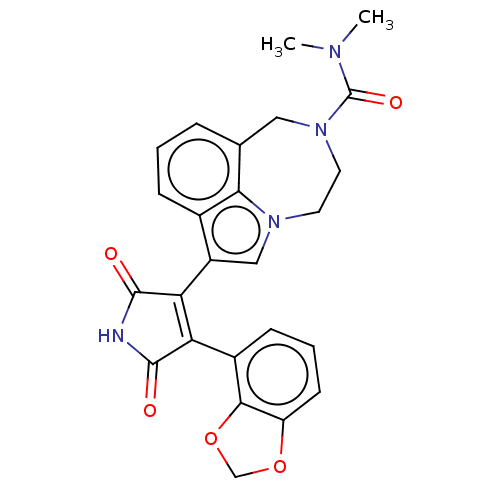
(CHEMBL445649)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4OCOc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N4O5/c1-27(2)25(32)29-10-9-28-12-17(15-6-3-5-14(11-29)21(15)28)20-19(23(30)26-24(20)31)16-7-4-8-18-22(16)34-13-33-18/h3-8,12H,9-11,13H2,1-2H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475009
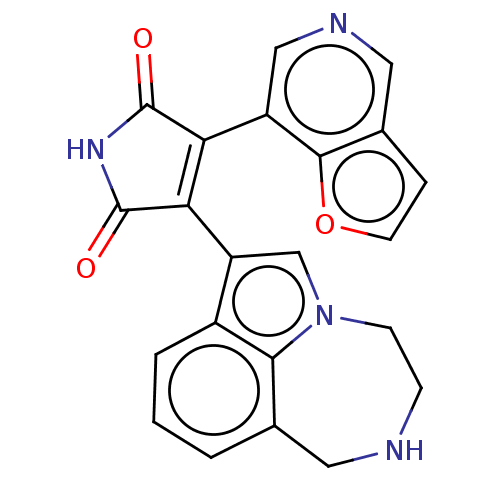
(CHEMBL361635)Show SMILES O=C1NC(=O)C(=C1c1cn2CCNCc3cccc1c23)c1cncc2ccoc12 |c:5| Show InChI InChI=1S/C22H16N4O3/c27-21-17(15-10-24-9-13-4-7-29-20(13)15)18(22(28)25-21)16-11-26-6-5-23-8-12-2-1-3-14(16)19(12)26/h1-4,7,9-11,23H,5-6,8H2,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475005
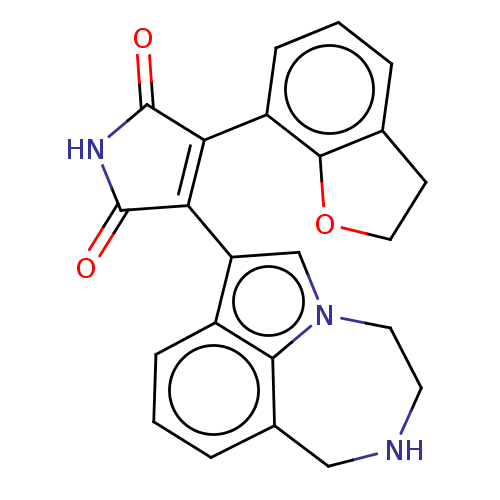
(CHEMBL178820)Show SMILES O=C1NC(=O)C(=C1c1cn2CCNCc3cccc1c23)c1cccc2CCOc12 |c:5| Show InChI InChI=1S/C23H19N3O3/c27-22-18(16-6-1-3-13-7-10-29-21(13)16)19(23(28)25-22)17-12-26-9-8-24-11-14-4-2-5-15(17)20(14)26/h1-6,12,24H,7-11H2,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474993
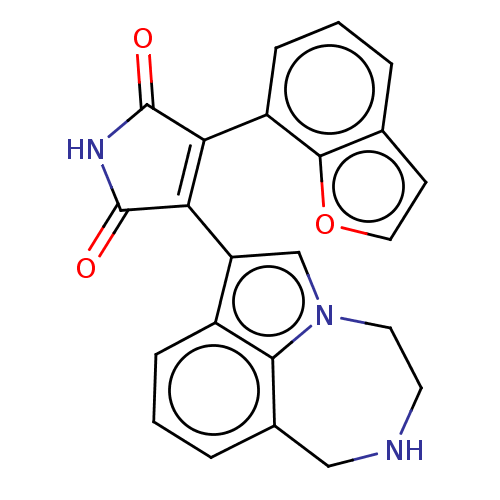
(CHEMBL361996)Show SMILES O=C1NC(=O)C(=C1c1cn2CCNCc3cccc1c23)c1cccc2ccoc12 |c:5| Show InChI InChI=1S/C23H17N3O3/c27-22-18(16-6-1-3-13-7-10-29-21(13)16)19(23(28)25-22)17-12-26-9-8-24-11-14-4-2-5-15(17)20(14)26/h1-7,10,12,24H,8-9,11H2,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data