 Found 913 hits with Last Name = 'mollica' and Initial = 'a'
Found 913 hits with Last Name = 'mollica' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107
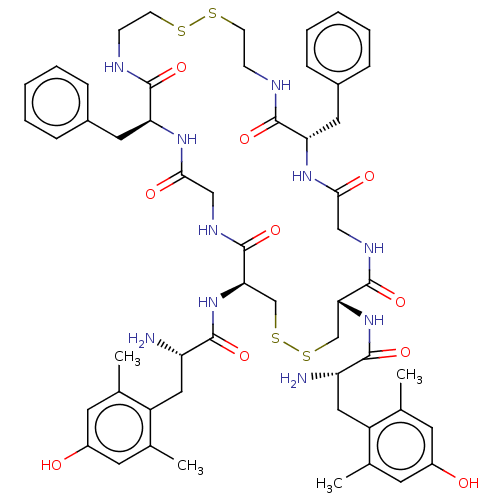
(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21125
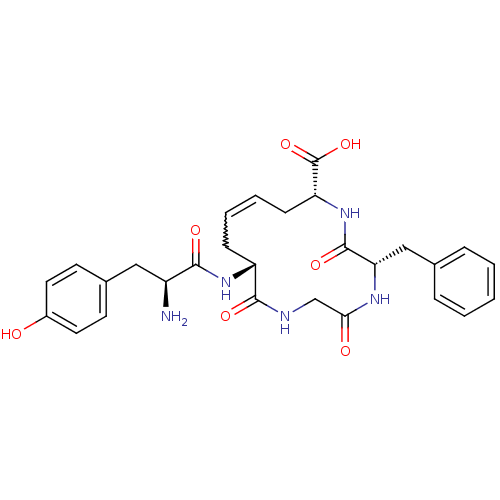
((5S,8R,10Z,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CC=CC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O |r,w:15.15| Show InChI InChI=1S/C28H33N5O7/c29-20(14-18-10-12-19(34)13-11-18)25(36)32-21-8-4-5-9-22(28(39)40)33-27(38)23(15-17-6-2-1-3-7-17)31-24(35)16-30-26(21)37/h1-7,10-13,20-23,34H,8-9,14-16,29H2,(H,30,37)(H,31,35)(H,32,36)(H,33,38)(H,39,40)/t20-,21+,22+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | -53.5 | n/a | n/a | 0.830 | n/a | n/a | 7.4 | 25 |
Universita degli Studi
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 50: 3138-3142 (2007)
Article DOI: 10.1021/jm061048b
BindingDB Entry DOI: 10.7270/Q28G8J0K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21129
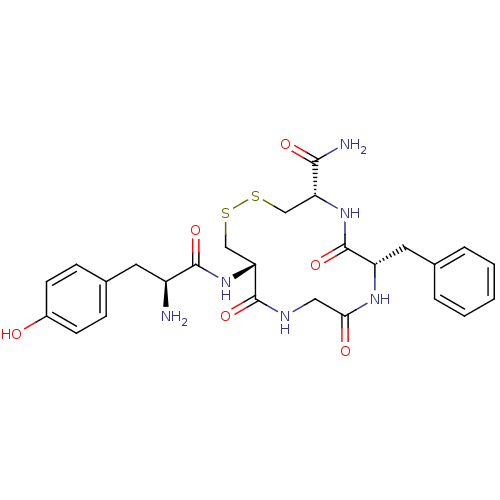
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(N)=O Show InChI InChI=1S/C26H32N6O6S2/c27-18(10-16-6-8-17(33)9-7-16)24(36)32-21-14-40-39-13-20(23(28)35)31-26(38)19(11-15-4-2-1-3-5-15)30-22(34)12-29-25(21)37/h1-9,18-21,33H,10-14,27H2,(H2,28,35)(H,29,37)(H,30,34)(H,31,38)(H,32,36)/t18-,19-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 50: 3138-3142 (2007)
Article DOI: 10.1021/jm061048b
BindingDB Entry DOI: 10.7270/Q28G8J0K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21126
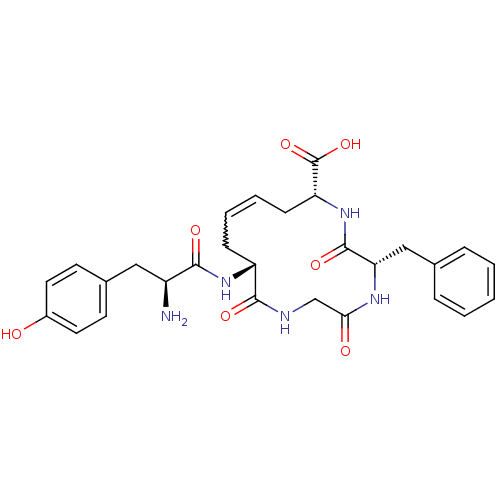
((5S,8R,10E,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CC=CC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O |w:15.15| Show InChI InChI=1S/C28H33N5O7/c29-20(14-18-10-12-19(34)13-11-18)25(36)32-21-8-4-5-9-22(28(39)40)33-27(38)23(15-17-6-2-1-3-7-17)31-24(35)16-30-26(21)37/h1-7,10-13,20-23,34H,8-9,14-16,29H2,(H,30,37)(H,31,35)(H,32,36)(H,33,38)(H,39,40)/t20-,21+,22+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | -52.8 | n/a | n/a | 0.880 | n/a | n/a | 7.4 | 25 |
Universita degli Studi
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 50: 3138-3142 (2007)
Article DOI: 10.1021/jm061048b
BindingDB Entry DOI: 10.7270/Q28G8J0K |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50428089
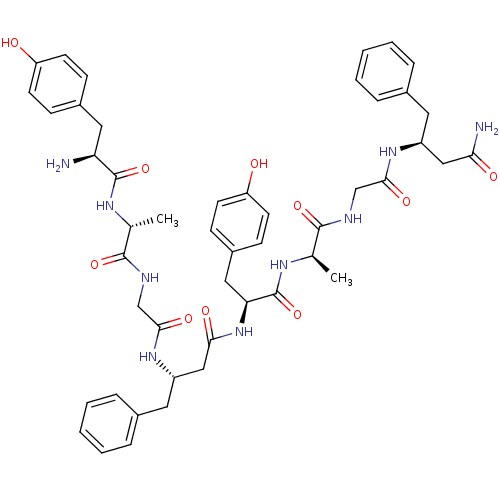
(CHEMBL2323577)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@H](CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](C)C(=O)NCC(=O)N[C@H](CC(N)=O)Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C48H59N9O10/c1-29(53-47(66)39(49)23-33-13-17-37(58)18-14-33)45(64)51-28-44(63)56-36(22-32-11-7-4-8-12-32)26-42(61)57-40(24-34-15-19-38(59)20-16-34)48(67)54-30(2)46(65)52-27-43(62)55-35(25-41(50)60)21-31-9-5-3-6-10-31/h3-20,29-30,35-36,39-40,58-59H,21-28,49H2,1-2H3,(H2,50,60)(H,51,64)(H,52,65)(H,53,66)(H,54,67)(H,55,62)(H,56,63)(H,57,61)/t29-,30-,35+,36+,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]Deltorphin from DOR in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
J Med Chem 56: 3419-23 (2013)
Article DOI: 10.1021/jm301456c
BindingDB Entry DOI: 10.7270/Q2X63P9H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21014
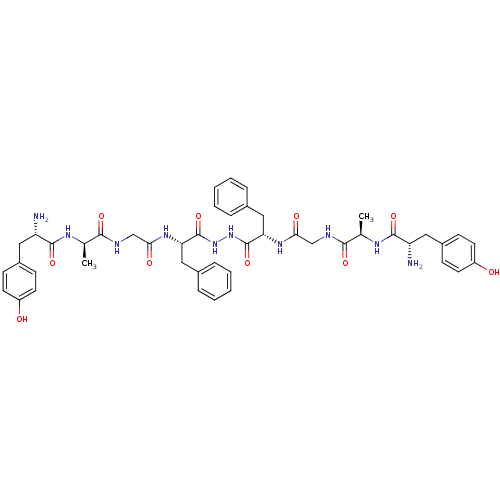
((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C46H56N10O10/c1-27(51-43(63)35(47)21-31-13-17-33(57)18-14-31)41(61)49-25-39(59)53-37(23-29-9-5-3-6-10-29)45(65)55-56-46(66)38(24-30-11-7-4-8-12-30)54-40(60)26-50-42(62)28(2)52-44(64)36(48)22-32-15-19-34(58)20-16-32/h3-20,27-28,35-38,57-58H,21-26,47-48H2,1-2H3,(H,49,61)(H,50,62)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,65)(H,56,66)/t27-,28-,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21123
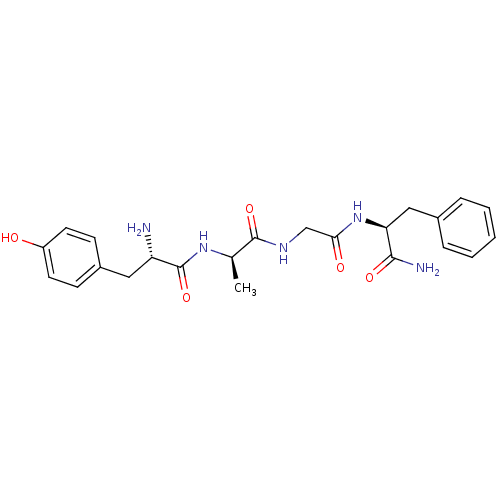
((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C23H29N5O5/c1-14(27-23(33)18(24)11-16-7-9-17(29)10-8-16)22(32)26-13-20(30)28-19(21(25)31)12-15-5-3-2-4-6-15/h2-10,14,18-19,29H,11-13,24H2,1H3,(H2,25,31)(H,26,32)(H,27,33)(H,28,30)/t14-,18+,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biological Research Centre of the Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes after 60 mins by liquid scintillation analysis |
Eur J Med Chem 178: 571-588 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.037
BindingDB Entry DOI: 10.7270/Q2Z60SD8 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21129

((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(N)=O Show InChI InChI=1S/C26H32N6O6S2/c27-18(10-16-6-8-17(33)9-7-16)24(36)32-21-14-40-39-13-20(23(28)35)31-26(38)19(11-15-4-2-1-3-5-15)30-22(34)12-29-25(21)37/h1-9,18-21,33H,10-14,27H2,(H2,28,35)(H,29,37)(H,30,34)(H,31,38)(H,32,36)/t18-,19-,20+,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.822 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 50: 3138-3142 (2007)
Article DOI: 10.1021/jm061048b
BindingDB Entry DOI: 10.7270/Q28G8J0K |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50275107

(CHEMBL4130303)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H70N10O10S4/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)63-45-29-77-78-30-46(64-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)54(74)60-28-48(68)62-44(24-36-13-9-6-10-14-36)52(72)58-16-18-76-75-17-15-57-51(71)43(23-35-11-7-5-8-12-35)61-47(67)27-59-53(45)73/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,73)(H,60,74)(H,61,67)(H,62,68)(H,63,69)(H,64,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from KOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50595851

(CHEMBL5192735)Show SMILES CCCC[C@@H](NC(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00237
BindingDB Entry DOI: 10.7270/Q21Z48F7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50595850

(CHEMBL5188021)Show SMILES CCCC[C@@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00237
BindingDB Entry DOI: 10.7270/Q21Z48F7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50428089

(CHEMBL2323577)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@H](CC(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](C)C(=O)NCC(=O)N[C@H](CC(N)=O)Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C48H59N9O10/c1-29(53-47(66)39(49)23-33-13-17-37(58)18-14-33)45(64)51-28-44(63)56-36(22-32-11-7-4-8-12-32)26-42(61)57-40(24-34-15-19-38(59)20-16-34)48(67)54-30(2)46(65)52-27-43(62)55-35(25-41(50)60)21-31-9-5-3-6-10-31/h3-20,29-30,35-36,39-40,58-59H,21-28,49H2,1-2H3,(H2,50,60)(H,51,64)(H,52,65)(H,53,66)(H,54,67)(H,55,62)(H,56,63)(H,57,61)/t29-,30-,35+,36+,39+,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
J Med Chem 56: 3419-23 (2013)
Article DOI: 10.1021/jm301456c
BindingDB Entry DOI: 10.7270/Q2X63P9H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50595847

(CHEMBL5199097)Show SMILES CCCC[C@@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1cccc(F)c1)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00237
BindingDB Entry DOI: 10.7270/Q21Z48F7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21126

((5S,8R,10E,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CC=CC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O |w:15.15| Show InChI InChI=1S/C28H33N5O7/c29-20(14-18-10-12-19(34)13-11-18)25(36)32-21-8-4-5-9-22(28(39)40)33-27(38)23(15-17-6-2-1-3-7-17)31-24(35)16-30-26(21)37/h1-7,10-13,20-23,34H,8-9,14-16,29H2,(H,30,37)(H,31,35)(H,32,36)(H,33,38)(H,39,40)/t20-,21+,22+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | -50.7 | n/a | n/a | 2.65 | n/a | n/a | 7.4 | 25 |
Universita degli Studi
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 50: 3138-3142 (2007)
Article DOI: 10.1021/jm061048b
BindingDB Entry DOI: 10.7270/Q28G8J0K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50457149
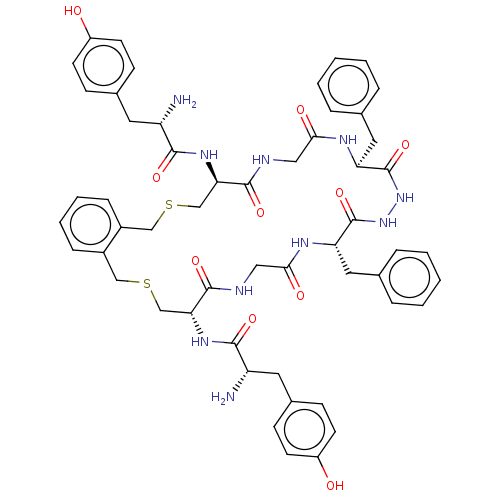
(CHEMBL4213910)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H62N10O10S2.2C2HF3O2/c55-41(23-35-15-19-39(65)20-16-35)49(69)61-45-31-75-29-37-13-7-8-14-38(37)30-76-32-46(62-50(70)42(56)24-36-17-21-40(66)22-18-36)52(72)58-28-48(68)60-44(26-34-11-5-2-6-12-34)54(74)64-63-53(73)43(25-33-9-3-1-4-10-33)59-47(67)27-57-51(45)71;2*3-2(4,5)1(6)7/h1-22,41-46,65-66H,23-32,55-56H2,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)(H,63,73)(H,64,74);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21125

((5S,8R,10Z,13R)-13-[(2S)-2-amino-3-(4-hydroxypheny...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CC=CC[C@@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O)C(O)=O |r,w:15.15| Show InChI InChI=1S/C28H33N5O7/c29-20(14-18-10-12-19(34)13-11-18)25(36)32-21-8-4-5-9-22(28(39)40)33-27(38)23(15-17-6-2-1-3-7-17)31-24(35)16-30-26(21)37/h1-7,10-13,20-23,34H,8-9,14-16,29H2,(H,30,37)(H,31,35)(H,32,36)(H,33,38)(H,39,40)/t20-,21+,22+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.35 | -50.6 | n/a | n/a | 2.35 | n/a | n/a | 7.4 | 25 |
Universita degli Studi
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 50: 3138-3142 (2007)
Article DOI: 10.1021/jm061048b
BindingDB Entry DOI: 10.7270/Q28G8J0K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21014

((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C46H56N10O10/c1-27(51-43(63)35(47)21-31-13-17-33(57)18-14-31)41(61)49-25-39(59)53-37(23-29-9-5-3-6-10-29)45(65)55-56-46(66)38(24-30-11-7-4-8-12-30)54-40(60)26-50-42(62)28(2)52-44(64)36(48)22-32-15-19-34(58)20-16-32/h3-20,27-28,35-38,57-58H,21-26,47-48H2,1-2H3,(H,49,61)(H,50,62)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,65)(H,56,66)/t27-,28-,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
J Med Chem 56: 3419-23 (2013)
Article DOI: 10.1021/jm301456c
BindingDB Entry DOI: 10.7270/Q2X63P9H |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21008
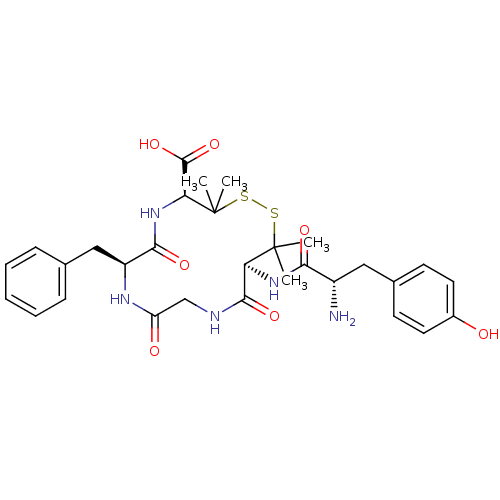
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi
| Assay Description
Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... |
J Med Chem 50: 3138-3142 (2007)
Article DOI: 10.1021/jm061048b
BindingDB Entry DOI: 10.7270/Q28G8J0K |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50457147
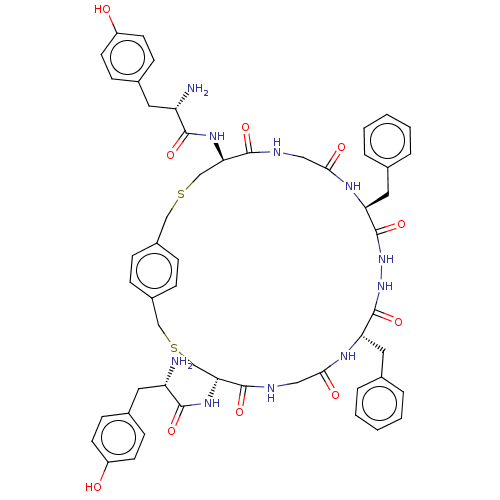
(CHEMBL4209510)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccc(CSC[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)NNC(=O)[C@H](Cc3ccccc3)NC(=O)CNC1=O)cc2 |r| Show InChI InChI=1S/C54H62N10O10S2.2C2HF3O2/c55-41(23-35-15-19-39(65)20-16-35)49(69)61-45-31-75-29-37-11-13-38(14-12-37)30-76-32-46(62-50(70)42(56)24-36-17-21-40(66)22-18-36)52(72)58-28-48(68)60-44(26-34-9-5-2-6-10-34)54(74)64-63-53(73)43(25-33-7-3-1-4-8-33)59-47(67)27-57-51(45)71;2*3-2(4,5)1(6)7/h1-22,41-46,65-66H,23-32,55-56H2,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)(H,63,73)(H,64,74);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50087065
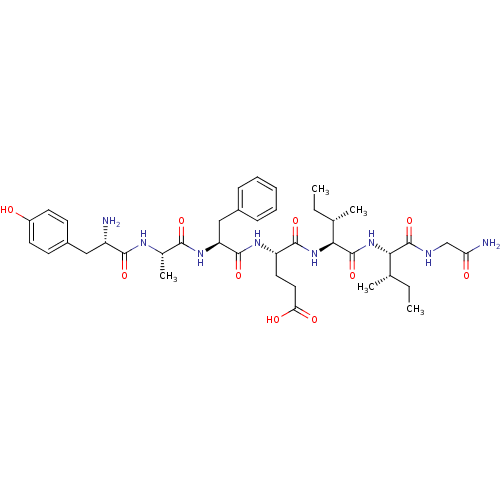
(4-(2-{2-[2-Amino-3-(4-hydroxy-phenyl)-propionylami...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)CC)C(=O)NCC(N)=O Show InChI InChI=1S/C40H58N8O10/c1-6-22(3)33(39(57)43-21-31(42)50)48-40(58)34(23(4)7-2)47-37(55)29(17-18-32(51)52)45-38(56)30(20-25-11-9-8-10-12-25)46-35(53)24(5)44-36(54)28(41)19-26-13-15-27(49)16-14-26/h8-16,22-24,28-30,33-34,49H,6-7,17-21,41H2,1-5H3,(H2,42,50)(H,43,57)(H,44,54)(H,45,56)(H,46,53)(H,47,55)(H,48,58)(H,51,52)/t22-,23-,24-,28-,29-,30-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi "G. d'Annunzio" di Chieti-Pescara
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ile5,6deltorphin-2 from delta opioid receptor in Wistar rat brain membranes by liquid scintillation counting |
Eur J Med Chem 68: 167-77 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.044
BindingDB Entry DOI: 10.7270/Q2D79FCG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50457148

(CHEMBL4204649)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2cccc(CSC[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)NNC(=O)[C@H](Cc3ccccc3)NC(=O)CNC1=O)c2 |r| Show InChI InChI=1S/C54H62N10O10S2.2C2HF3O2/c55-41(23-35-14-18-39(65)19-15-35)49(69)61-45-31-75-29-37-12-7-13-38(22-37)30-76-32-46(62-50(70)42(56)24-36-16-20-40(66)21-17-36)52(72)58-28-48(68)60-44(26-34-10-5-2-6-11-34)54(74)64-63-53(73)43(25-33-8-3-1-4-9-33)59-47(67)27-57-51(45)71;2*3-2(4,5)1(6)7/h1-22,41-46,65-66H,23-32,55-56H2,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)(H,63,73)(H,64,74);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi "G. d'Annunzio" di Chieti-Pescara
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting |
Eur J Med Chem 68: 167-77 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.044
BindingDB Entry DOI: 10.7270/Q2D79FCG |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50026764
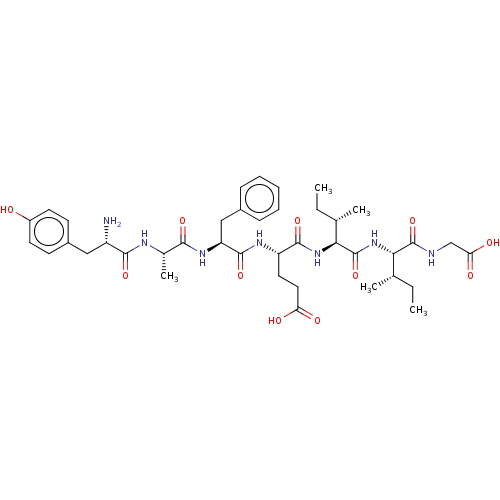
(CHEMBL3331509)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)[C@@H](C)CC)C(=O)NCC(O)=O |r| Show InChI InChI=1S/C40H57N7O11/c1-6-22(3)33(39(57)42-21-32(51)52)47-40(58)34(23(4)7-2)46-37(55)29(17-18-31(49)50)44-38(56)30(20-25-11-9-8-10-12-25)45-35(53)24(5)43-36(54)28(41)19-26-13-15-27(48)16-14-26/h8-16,22-24,28-30,33-34,48H,6-7,17-21,41H2,1-5H3,(H,42,57)(H,43,54)(H,44,56)(H,45,53)(H,46,55)(H,47,58)(H,49,50)(H,51,52)/t22-,23-,24-,28-,29-,30-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ile5,6deltorphin II from delta opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition b... |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50026762
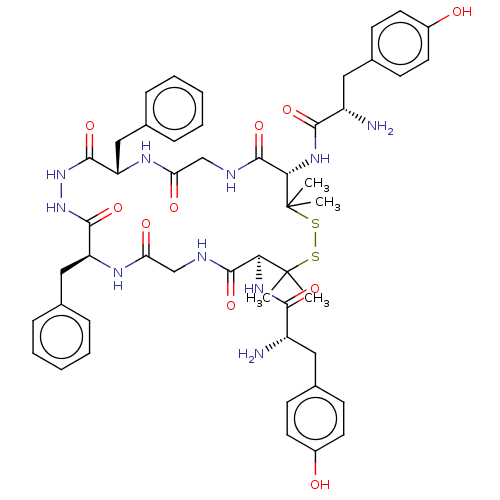
(CHEMBL3331510)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r| Show InChI InChI=1S/C50H62N10O10S2/c1-49(2)41(57-43(65)35(51)23-31-15-19-33(61)20-16-31)47(69)53-27-39(63)55-37(25-29-11-7-5-8-12-29)45(67)59-60-46(68)38(26-30-13-9-6-10-14-30)56-40(64)28-54-48(70)42(50(3,4)72-71-49)58-44(66)36(52)24-32-17-21-34(62)22-18-32/h5-22,35-38,41-42,61-62H,23-28,51-52H2,1-4H3,(H,53,69)(H,54,70)(H,55,63)(H,56,64)(H,57,65)(H,58,66)(H,59,67)(H,60,68)/t35-,36-,37-,38-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50399881
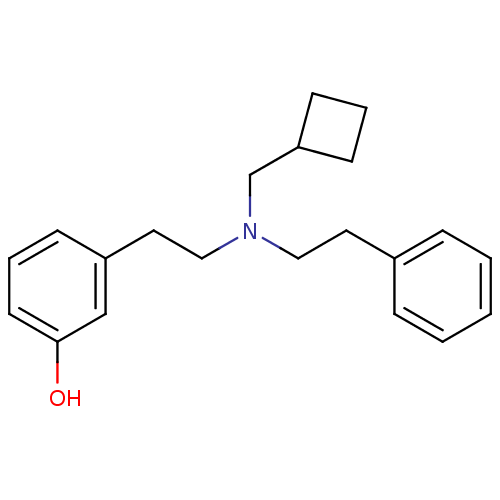
(CHEMBL2180639)Show InChI InChI=1S/C21H27NO/c23-21-11-5-8-19(16-21)13-15-22(17-20-9-4-10-20)14-12-18-6-2-1-3-7-18/h1-3,5-8,11,16,20,23H,4,9-10,12-15,17H2 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00237
BindingDB Entry DOI: 10.7270/Q21Z48F7 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50116436
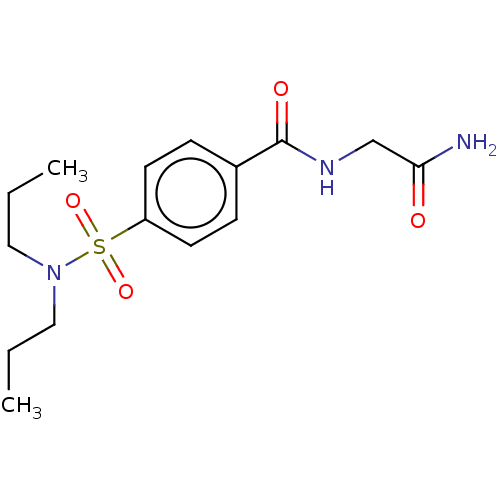
(CHEMBL3604416)Show InChI InChI=1S/C15H23N3O4S/c1-3-9-18(10-4-2)23(21,22)13-7-5-12(6-8-13)15(20)17-11-14(16)19/h5-8H,3-4,9-11H2,1-2H3,(H2,16,19)(H,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
'G. D'Annunzio' University of Chieti-Pescara
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-9 incubated for 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem 23: 5311-8 (2015)
Article DOI: 10.1016/j.bmc.2015.07.066
BindingDB Entry DOI: 10.7270/Q2D50PQR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111
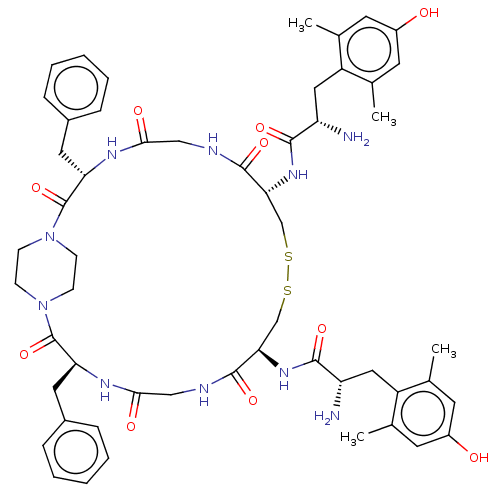
(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from DOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50515052

(CHEMBL4575678)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)NCCCCCn1cc(C(=O)c2cccc3ccccc23)c2ccccc12 |r| Show InChI InChI=1S/C49H53N7O7/c1-32(54-48(62)41(50)27-34-21-23-36(57)24-22-34)47(61)52-30-45(59)55-42(28-33-13-4-2-5-14-33)49(63)53-29-44(58)51-25-10-3-11-26-56-31-40(38-18-8-9-20-43(38)56)46(60)39-19-12-16-35-15-6-7-17-37(35)39/h2,4-9,12-24,31-32,41-42,57H,3,10-11,25-30,50H2,1H3,(H,51,58)(H,52,61)(H,53,63)(H,54,62)(H,55,59)/t32-,41+,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biological Research Centre of the Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in Wistar rat brain membranes after 60 mins by liquid scintillation analysis |
Eur J Med Chem 178: 571-588 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.037
BindingDB Entry DOI: 10.7270/Q2Z60SD8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50295263
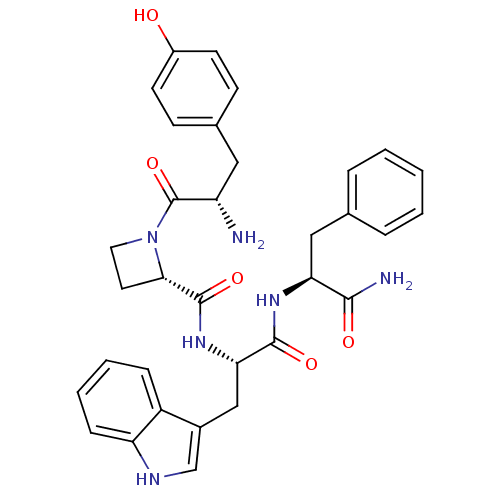
((S)-N-((S)-1-((S)-1-amino-1-oxo-3-phenylpropan-2-y...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1CC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C33H36N6O5/c34-25(16-21-10-12-23(40)13-11-21)33(44)39-15-14-29(39)32(43)38-28(18-22-19-36-26-9-5-4-8-24(22)26)31(42)37-27(30(35)41)17-20-6-2-1-3-7-20/h1-13,19,25,27-29,36,40H,14-18,34H2,(H2,35,41)(H,37,42)(H,38,43)/t25-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Roma
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in rat brain membrane by liquid scintillation counting |
Bioorg Med Chem Lett 19: 4115-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.008
BindingDB Entry DOI: 10.7270/Q2ZS2WJX |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50457149

(CHEMBL4213910)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccccc2CSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C54H62N10O10S2.2C2HF3O2/c55-41(23-35-15-19-39(65)20-16-35)49(69)61-45-31-75-29-37-13-7-8-14-38(37)30-76-32-46(62-50(70)42(56)24-36-17-21-40(66)22-18-36)52(72)58-28-48(68)60-44(26-34-11-5-2-6-12-34)54(74)64-63-53(73)43(25-33-9-3-1-4-10-33)59-47(67)27-57-51(45)71;2*3-2(4,5)1(6)7/h1-22,41-46,65-66H,23-32,55-56H2,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)(H,63,73)(H,64,74);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]lleDelt2 from DOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275108

(CHEMBL4129668)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C50H62N10O10S4/c51-37(23-33-11-15-35(61)16-12-33)45(65)59-41-29-73-74-30-42(60-46(66)38(52)24-34-13-17-36(62)18-14-34)50(70)56-28-44(64)58-40(26-32-9-5-2-6-10-32)48(68)54-20-22-72-71-21-19-53-47(67)39(25-31-7-3-1-4-8-31)57-43(63)27-55-49(41)69/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,69)(H,56,70)(H,57,63)(H,58,64)(H,59,65)(H,60,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50595846

(CHEMBL5183917)Show SMILES CCCC[C@@H](NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)C(=O)N[C@H](CCCNC(N)=N)C(N)=O |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00237
BindingDB Entry DOI: 10.7270/Q21Z48F7 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275108

(CHEMBL4129668)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)NCCSSCCNC(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r| Show InChI InChI=1S/C50H62N10O10S4/c51-37(23-33-11-15-35(61)16-12-33)45(65)59-41-29-73-74-30-42(60-46(66)38(52)24-34-13-17-36(62)18-14-34)50(70)56-28-44(64)58-40(26-32-9-5-2-6-10-32)48(68)54-20-22-72-71-21-19-53-47(67)39(25-31-7-3-1-4-8-31)57-43(63)27-55-49(41)69/h1-18,37-42,61-62H,19-30,51-52H2,(H,53,67)(H,54,68)(H,55,69)(H,56,70)(H,57,63)(H,58,64)(H,59,65)(H,60,66)/t37-,38-,39-,40-,41+,42+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50457147

(CHEMBL4209510)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CSCc2ccc(CSC[C@@H](NC(=O)[C@@H](N)Cc3ccc(O)cc3)C(=O)NCC(=O)N[C@@H](Cc3ccccc3)C(=O)NNC(=O)[C@H](Cc3ccccc3)NC(=O)CNC1=O)cc2 |r| Show InChI InChI=1S/C54H62N10O10S2.2C2HF3O2/c55-41(23-35-15-19-39(65)20-16-35)49(69)61-45-31-75-29-37-11-13-38(14-12-37)30-76-32-46(62-50(70)42(56)24-36-17-21-40(66)22-18-36)52(72)58-28-48(68)60-44(26-34-9-5-2-6-10-34)54(74)64-63-53(73)43(25-33-7-3-1-4-8-33)59-47(67)27-57-51(45)71;2*3-2(4,5)1(6)7/h1-22,41-46,65-66H,23-32,55-56H2,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)(H,63,73)(H,64,74);2*(H,6,7)/t41-,42-,43-,44-,45+,46+;;/m0../s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]lleDelt2 from DOR in Wistar rat whole brain membranes after 45 mins by liquid scintillation counting method |
ACS Med Chem Lett 8: 858-863 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00210
BindingDB Entry DOI: 10.7270/Q2S1853J |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275111

(CHEMBL4126803)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@@H](N)Cc2c(C)cc(O)cc2C)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC1=O |r,wU:10.10,15.15,61.65,wD:20.21,24.26,43.45,(24.8,-11.26,;26.16,-10.47,;26.16,-8.93,;27.49,-8.16,;27.49,-6.62,;28.83,-8.93,;28.83,-10.47,;30.19,-11.26,;27.49,-11.24,;27.49,-12.78,;26.16,-13.55,;24.82,-12.78,;26.16,-15.09,;24.83,-15.86,;27.49,-15.86,;27.49,-17.41,;26.16,-18.18,;26.16,-19.72,;28.83,-22.8,;28.83,-24.34,;30.16,-25.11,;30.16,-26.65,;28.82,-27.42,;27.49,-26.65,;28.82,-28.96,;27.49,-29.74,;30.16,-29.74,;30.16,-31.28,;28.82,-32.05,;27.46,-31.26,;28.82,-33.59,;30.16,-34.36,;30.16,-35.9,;31.5,-33.59,;31.5,-32.05,;32.86,-31.26,;31.49,-24.34,;31.49,-22.8,;32.83,-25.11,;34.17,-24.34,;35.5,-25.11,;35.5,-26.65,;36.83,-24.34,;36.83,-22.8,;38.17,-22.03,;39.5,-22.8,;40.83,-22.03,;42.17,-22.8,;42.17,-24.34,;40.83,-25.11,;39.5,-24.34,;35.5,-22.03,;34.17,-22.8,;35.5,-20.49,;34.17,-19.72,;34.17,-18.18,;35.5,-17.41,;36.83,-18.18,;36.83,-19.72,;35.5,-15.86,;36.83,-15.09,;34.17,-15.09,;34.17,-13.55,;35.5,-12.78,;36.83,-13.55,;38.17,-12.78,;38.17,-11.24,;36.83,-10.47,;35.5,-11.24,;32.83,-15.86,;31.49,-15.09,;31.49,-13.55,;30.16,-15.86,;30.16,-17.41,;28.83,-18.18,;28.83,-19.72,)| Show InChI InChI=1S/C54H68N10O10S2/c1-31-19-37(65)20-32(2)39(31)25-41(55)49(69)61-45-29-75-76-30-46(62-50(70)42(56)26-40-33(3)21-38(66)22-34(40)4)52(72)58-28-48(68)60-44(24-36-13-9-6-10-14-36)54(74)64-17-15-63(16-18-64)53(73)43(23-35-11-7-5-8-12-35)59-47(67)27-57-51(45)71/h5-14,19-22,41-46,65-66H,15-18,23-30,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t41-,42-,43-,44-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50116389
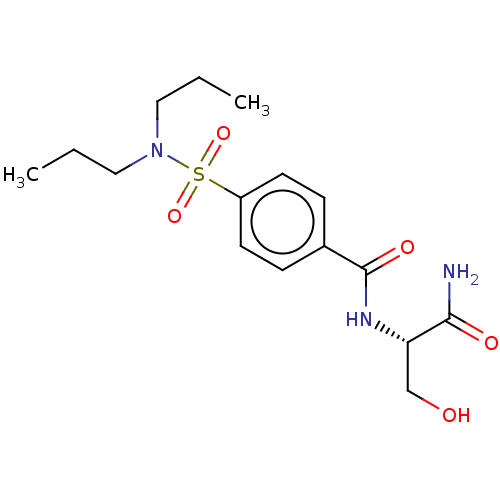
(CHEMBL3604509)Show SMILES CCCN(CCC)S(=O)(=O)c1ccc(cc1)C(=O)N[C@@H](CO)C(N)=O |r| Show InChI InChI=1S/C16H25N3O5S/c1-3-9-19(10-4-2)25(23,24)13-7-5-12(6-8-13)16(22)18-14(11-20)15(17)21/h5-8,14,20H,3-4,9-11H2,1-2H3,(H2,17,21)(H,18,22)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
'G. D'Annunzio' University of Chieti-Pescara
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase-9 incubated for 15 mins by stopped-flow CO2 hydration assay |
Bioorg Med Chem 23: 5311-8 (2015)
Article DOI: 10.1016/j.bmc.2015.07.066
BindingDB Entry DOI: 10.7270/Q2D50PQR |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21014

((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C46H56N10O10/c1-27(51-43(63)35(47)21-31-13-17-33(57)18-14-31)41(61)49-25-39(59)53-37(23-29-9-5-3-6-10-29)45(65)55-56-46(66)38(24-30-11-7-4-8-12-30)54-40(60)26-50-42(62)28(2)52-44(64)36(48)22-32-15-19-34(58)20-16-32/h3-20,27-28,35-38,57-58H,21-26,47-48H2,1-2H3,(H,49,61)(H,50,62)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,65)(H,56,66)/t27-,28-,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]Deltorphin from DOR in Sprague-Dawley rat brain membrane after 180 mins by scintillation counting analysis |
J Med Chem 56: 3419-23 (2013)
Article DOI: 10.1021/jm301456c
BindingDB Entry DOI: 10.7270/Q2X63P9H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21014

((2S)-2-amino-N-[(1R)-1-[({[(1S)-1-{N'-[(2S)-2-{2-[...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)NNC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C46H56N10O10/c1-27(51-43(63)35(47)21-31-13-17-33(57)18-14-31)41(61)49-25-39(59)53-37(23-29-9-5-3-6-10-29)45(65)55-56-46(66)38(24-30-11-7-4-8-12-30)54-40(60)26-50-42(62)28(2)52-44(64)36(48)22-32-15-19-34(58)20-16-32/h3-20,27-28,35-38,57-58H,21-26,47-48H2,1-2H3,(H,49,61)(H,50,62)(H,51,63)(H,52,64)(H,53,59)(H,54,60)(H,55,65)(H,56,66)/t27-,28-,35+,36+,37+,38+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara"G. d'Annunzio"
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid receptor in Wistar rat brain membranes by liquid scintillation counting based competition binding assay |
ACS Med Chem Lett 5: 1032-6 (2014)
Article DOI: 10.1021/ml500241n
BindingDB Entry DOI: 10.7270/Q23T9JS5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50275109

(CHEMBL4128853)Show SMILES CC1(C)SSC(C)(C)[C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N2CCN(CC2)C(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)[C@H]1NC(=O)[C@@H](N)Cc1ccc(O)cc1 |r,wU:66.72,47.50,8.8,wD:62.67,12.13,29.30,(46.1,-18.88,;47.68,-18.88,;46.89,-17.52,;47.68,-20.42,;50.35,-23.5,;50.34,-25.04,;49.55,-26.39,;48.77,-25.03,;51.68,-25.81,;51.68,-27.35,;50.34,-28.12,;49.01,-27.35,;50.34,-29.66,;49.01,-30.44,;51.68,-30.44,;51.68,-31.98,;53.02,-32.75,;53.02,-34.29,;51.68,-35.06,;51.68,-36.6,;50.34,-34.29,;50.34,-32.75,;53.01,-25.04,;53.01,-23.5,;54.35,-25.81,;55.69,-25.04,;57.02,-25.81,;57.02,-27.35,;58.35,-25.04,;58.35,-23.5,;59.69,-22.73,;61.02,-23.5,;62.35,-22.73,;63.69,-23.5,;63.69,-25.04,;62.35,-25.81,;61.02,-25.04,;57.02,-22.73,;55.69,-23.5,;57.02,-21.19,;55.69,-20.42,;55.69,-18.88,;57.02,-18.11,;58.35,-18.88,;58.35,-20.42,;57.02,-16.56,;58.35,-15.79,;55.69,-15.79,;55.69,-14.25,;57.02,-13.48,;58.35,-14.25,;59.69,-13.48,;59.69,-11.94,;58.35,-11.17,;57.02,-11.94,;54.35,-16.56,;53.01,-15.79,;53.01,-14.25,;51.68,-16.56,;51.68,-18.11,;50.35,-18.88,;50.35,-20.42,;49.01,-18.11,;49.01,-16.56,;47.68,-15.79,;46.35,-16.56,;47.68,-14.25,;46.34,-13.48,;49.01,-13.48,;49.01,-11.94,;47.68,-11.17,;47.68,-9.63,;49.01,-8.86,;49.01,-7.32,;50.35,-9.63,;50.35,-11.17,)| Show InChI InChI=1S/C54H68N10O10S2/c1-53(2)45(61-47(69)39(55)27-35-15-19-37(65)20-16-35)49(71)57-31-43(67)59-41(29-33-11-7-5-8-12-33)51(73)63-23-25-64(26-24-63)52(74)42(30-34-13-9-6-10-14-34)60-44(68)32-58-50(72)46(54(3,4)76-75-53)62-48(70)40(56)28-36-17-21-38(66)22-18-36/h5-22,39-42,45-46,65-66H,23-32,55-56H2,1-4H3,(H,57,71)(H,58,72)(H,59,67)(H,60,68)(H,61,69)(H,62,70)/t39-,40-,41-,42-,45+,46+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR (unknown origin) expressed in HEK cells |
Bioorg Med Chem 26: 3664-3667 (2018)
Article DOI: 10.1016/j.bmc.2018.05.045
BindingDB Entry DOI: 10.7270/Q2JW8HD5 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1/2
(Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50353747

(CHEMBL561013 | JWH-018)Show InChI InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biological Research Centre of the Hungarian Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]WIN-55,212-2 from CB1R/CB2R in Wistar rat brain membranes after 60 mins by liquid scintillation analysis |
Eur J Med Chem 178: 571-588 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.037
BindingDB Entry DOI: 10.7270/Q2Z60SD8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































