 Found 292 hits with Last Name = 'nitta' and Initial = 'a'
Found 292 hits with Last Name = 'nitta' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276045
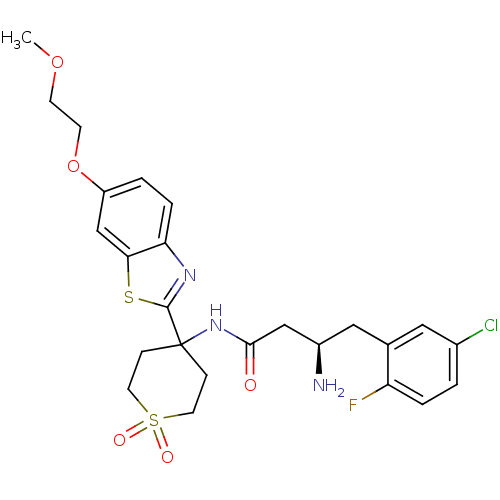
((R)-3-Amino-4-(5-chloro-2-fluoro-phenyl)-N-{4-[6-(...)Show SMILES COCCOc1ccc2nc(sc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1cc(Cl)ccc1F |r| Show InChI InChI=1S/C25H29ClFN3O5S2/c1-34-8-9-35-19-3-5-21-22(15-19)36-24(29-21)25(6-10-37(32,33)11-7-25)30-23(31)14-18(28)13-16-12-17(26)2-4-20(16)27/h2-5,12,15,18H,6-11,13-14,28H2,1H3,(H,30,31)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276047

((R)-3-Amino-N-{4-[6-(2-morpholin-4-yl-ethoxy)-benz...)Show SMILES N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1nc2ccc(OCCN3CCOCC3)cc2s1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C28H33F3N4O5S2/c29-21-17-23(31)22(30)14-18(21)13-19(32)15-26(36)34-28(3-11-42(37,38)12-4-28)27-33-24-2-1-20(16-25(24)41-27)40-10-7-35-5-8-39-9-6-35/h1-2,14,16-17,19H,3-13,15,32H2,(H,34,36)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394470

(CHEMBL2160099)Show SMILES OCCOc1ccccc1NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C24H26FN3O3/c25-20-8-7-18-13-17(5-6-19(18)14-20)15-28-10-9-21(16-28)26-24(30)27-22-3-1-2-4-23(22)31-12-11-29/h1-8,13-14,21,29H,9-12,15-16H2,(H2,26,27,30)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor in human eosinophils assessed as inhibition of CCL11-induced degranulation after 4 hrs by ELISA |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297172
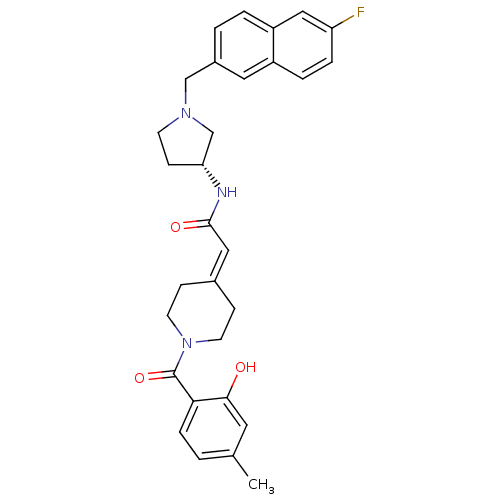
(CHEMBL560275 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-c1ccc(-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]/[#6](=O)-[#7]-[#6@@H]-2-[#6]-[#6]-[#7](-[#6]-c3ccc4cc(F)ccc4c3)-[#6]-2)c(-[#8])c1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-20-2-7-27(28(35)14-20)30(37)34-12-8-21(9-13-34)16-29(36)32-26-10-11-33(19-26)18-22-3-4-24-17-25(31)6-5-23(24)15-22/h2-7,14-17,26,35H,8-13,18-19H2,1H3,(H,32,36)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276001

((R)-3-Amino-N-{4-[6-(2-methoxy-ethoxy)-benzothiazo...)Show SMILES COCCOc1ccc2nc(sc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C25H28F3N3O5S2/c1-35-6-7-36-17-2-3-21-22(13-17)37-24(30-21)25(4-8-38(33,34)9-5-25)31-23(32)12-16(29)10-15-11-19(27)20(28)14-18(15)26/h2-3,11,13-14,16H,4-10,12,29H2,1H3,(H,31,32)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297171
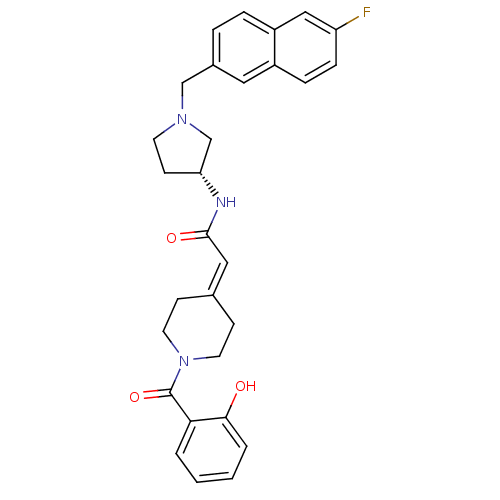
(CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1ccccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-24-8-7-22-15-21(5-6-23(22)17-24)18-32-12-11-25(19-32)31-28(35)16-20-9-13-33(14-10-20)29(36)26-3-1-2-4-27(26)34/h1-8,15-17,25,34H,9-14,18-19H2,(H,31,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells by functional inhibition curve analysis |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297181

(2-[1-(1,3-Benzodioxol-5-ylcarbonyl)piperidin-4-yli...)Show SMILES Fc1ccc2cc(-[#6]-[#7]-3-[#6]-[#6]-[#6@H](-[#6]-3)-[#7]-[#6](=O)\[#6]=[#6]-3/[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-c3ccc4-[#8]-[#6]-[#8]-c4c3)ccc2c1 |r| Show InChI InChI=1S/C30H30FN3O4/c31-25-5-3-22-13-21(1-2-23(22)15-25)17-33-10-9-26(18-33)32-29(35)14-20-7-11-34(12-8-20)30(36)24-4-6-27-28(16-24)38-19-37-27/h1-6,13-16,26H,7-12,17-19H2,(H,32,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394470

(CHEMBL2160099)Show SMILES OCCOc1ccccc1NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C24H26FN3O3/c25-20-8-7-18-13-17(5-6-19(18)14-20)15-28-10-9-21(16-28)26-24(30)27-22-3-1-2-4-23(22)31-12-11-29/h1-8,13-14,21,29H,9-12,15-16H2,(H2,26,27,30)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297183

(CHEMBL551738 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1ccc(cc1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-37-28-8-5-23(6-9-28)30(36)34-14-10-21(11-15-34)17-29(35)32-27-12-13-33(20-27)19-22-2-3-25-18-26(31)7-4-24(25)16-22/h2-9,16-18,27H,10-15,19-20H2,1H3,(H,32,35)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276046

((R)-3-amino-4-(5-chloro-2-fluorophenyl)-N-(4-(6-(2...)Show SMILES COCCOc1ccc2nc(sc2c1)C1(CCOCC1)NC(=O)C[C@H](N)Cc1cc(Cl)ccc1F |r| Show InChI InChI=1S/C25H29ClFN3O4S/c1-32-10-11-34-19-3-5-21-22(15-19)35-24(29-21)25(6-8-33-9-7-25)30-23(31)14-18(28)13-16-12-17(26)2-4-20(16)27/h2-5,12,15,18H,6-11,13-14,28H2,1H3,(H,30,31)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394487

(CHEMBL2160111)Show SMILES Oc1ccccc1S(=O)(=O)N1CCC(CC1)NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C27H31FN4O4S/c28-22-8-7-20-15-19(5-6-21(20)16-22)17-31-12-9-24(18-31)30-27(34)29-23-10-13-32(14-11-23)37(35,36)26-4-2-1-3-25(26)33/h1-8,15-16,23-24,33H,9-14,17-18H2,(H2,29,30,34)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50275954
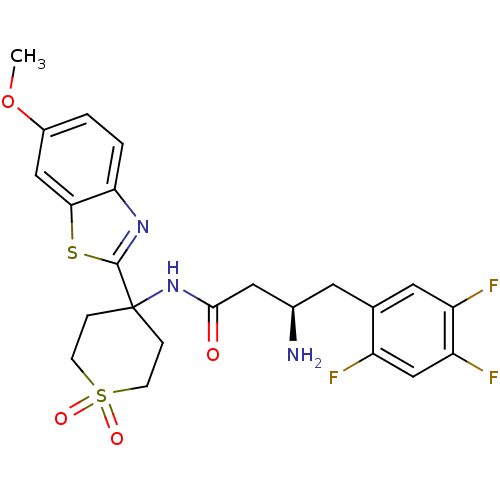
((R)-3-Amino-N-[4-(6-methoxy-benzothiazol-2-yl)-1,1...)Show SMILES COc1ccc2nc(sc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H24F3N3O4S2/c1-33-15-2-3-19-20(11-15)34-22(28-19)23(4-6-35(31,32)7-5-23)29-21(30)10-14(27)8-13-9-17(25)18(26)12-16(13)24/h2-3,9,11-12,14H,4-8,10,27H2,1H3,(H,29,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50275955
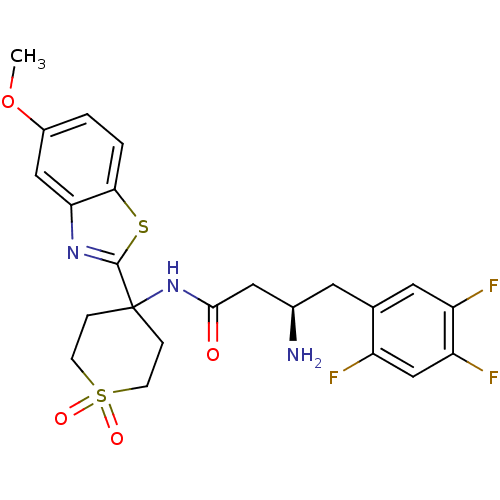
((R)-3-Amino-N-[4-(5-methoxy-benzothiazol-2-yl)-1,1...)Show SMILES COc1ccc2sc(nc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H24F3N3O4S2/c1-33-15-2-3-20-19(11-15)28-22(34-20)23(4-6-35(31,32)7-5-23)29-21(30)10-14(27)8-13-9-17(25)18(26)12-16(13)24/h2-3,9,11-12,14H,4-8,10,27H2,1H3,(H,29,30)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297177

(CHEMBL561535 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1ccc(-[#6](=O)-[#7]-2-[#6]-[#6]\[#6](-[#6]-[#6]-2)=[#6]/[#6](=O)-[#7]-[#6@@H]-2-[#6]-[#6]-[#7](-[#6]-c3ccc4cc(F)ccc4c3)-[#6]-2)c(-[#8])c1 |r| Show InChI InChI=1S/C30H32FN3O4/c1-38-26-6-7-27(28(35)17-26)30(37)34-12-8-20(9-13-34)15-29(36)32-25-10-11-33(19-25)18-21-2-3-23-16-24(31)5-4-22(23)14-21/h2-7,14-17,25,35H,8-13,18-19H2,1H3,(H,32,36)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430145
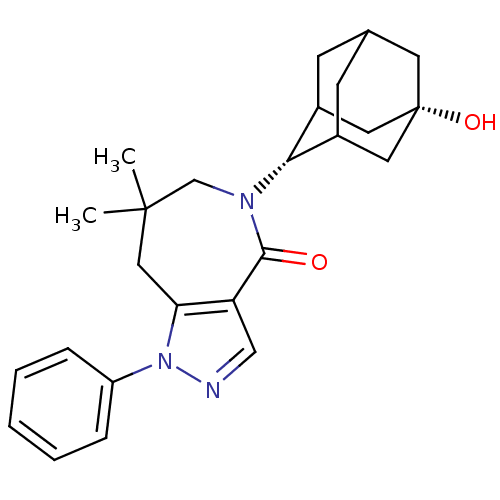
(CHEMBL2338266)Show SMILES CC1(C)CN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c2C1)-c1ccccc1 |r,wU:5.4,12.13,TLB:9:8:15:11.10.5,9:10:14.8.7:15,5:6:14:11.9.10,THB:5:10:14:7.6.15,4:5:14.8.7:15,(30.58,-17.72,;30.19,-19.21,;31.67,-18.81,;31.12,-20.44,;30.75,-21.94,;31.93,-22.93,;33.32,-22.33,;34.37,-23.56,;34.38,-25.15,;32.98,-25.72,;31.95,-24.46,;33.35,-24.79,;34.68,-24.29,;36.21,-24.27,;35.89,-25.55,;34.67,-22.8,;29.35,-22.57,;29.31,-24.11,;27.97,-21.87,;26.63,-22.62,;25.49,-21.59,;26.13,-20.18,;27.67,-20.35,;28.66,-19.17,;25.37,-18.85,;26.16,-17.52,;25.4,-16.18,;23.86,-16.16,;23.08,-17.5,;23.84,-18.84,)| Show InChI InChI=1S/C25H31N3O2/c1-24(2)13-21-20(14-26-28(21)19-6-4-3-5-7-19)23(29)27(15-24)22-17-8-16-9-18(22)12-25(30,10-16)11-17/h3-7,14,16-18,22,30H,8-13,15H2,1-2H3/t16?,17?,18?,22-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297182

(2-[1-(3,4-Dimethoxybenzoyl)piperidin-4-ylidene]-N-...)Show SMILES [#6]-[#8]-c1ccc(cc1-[#8]-[#6])-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C31H34FN3O4/c1-38-28-8-6-25(18-29(28)39-2)31(37)35-13-9-21(10-14-35)16-30(36)33-27-11-12-34(20-27)19-22-3-4-24-17-26(32)7-5-23(24)15-22/h3-8,15-18,27H,9-14,19-20H2,1-2H3,(H,33,36)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50276044

((3R)-3-amino-4-(2,4,5-trifluorophenyl)-N-{4-[6-(2-...)Show SMILES COCCOc1ccc2nc(sc2c1)C1(CCOCC1)NC(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C25H28F3N3O4S/c1-33-8-9-35-17-2-3-21-22(13-17)36-24(30-21)25(4-6-34-7-5-25)31-23(32)12-16(29)10-15-11-19(27)20(28)14-18(15)26/h2-3,11,13-14,16H,4-10,12,29H2,1H3,(H,31,32)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430135
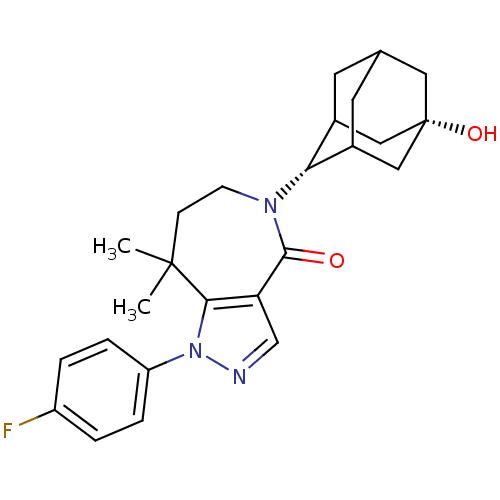
(CHEMBL2338251)Show SMILES CC1(C)CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1ccc(F)cc1 |r,wU:6.5,13.14,TLB:10:9:16:12.11.6,10:11:15.9.8:16,6:7:15:12.10.11,THB:6:11:15:8.7.16,5:6:15.9.8:16,(10.24,-45.05,;10.87,-46.46,;11.26,-44.96,;12.4,-46.5,;13.34,-47.73,;12.96,-49.22,;14.14,-50.21,;15.53,-49.62,;16.58,-50.84,;16.59,-52.43,;15.19,-53,;14.17,-51.74,;15.57,-52.07,;16.89,-51.57,;18.42,-51.55,;18.1,-52.83,;16.88,-50.08,;11.56,-49.85,;11.52,-51.39,;10.19,-49.15,;8.84,-49.91,;7.71,-48.87,;8.35,-47.46,;9.88,-47.64,;7.59,-46.13,;8.37,-44.8,;7.61,-43.46,;6.07,-43.45,;5.31,-42.11,;5.29,-44.79,;6.05,-46.12,)| Show InChI InChI=1S/C25H30FN3O2/c1-24(2)7-8-28(21-16-9-15-10-17(21)13-25(31,11-15)12-16)23(30)20-14-27-29(22(20)24)19-5-3-18(26)4-6-19/h3-6,14-17,21,31H,7-13H2,1-2H3/t15?,16?,17?,21-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297190
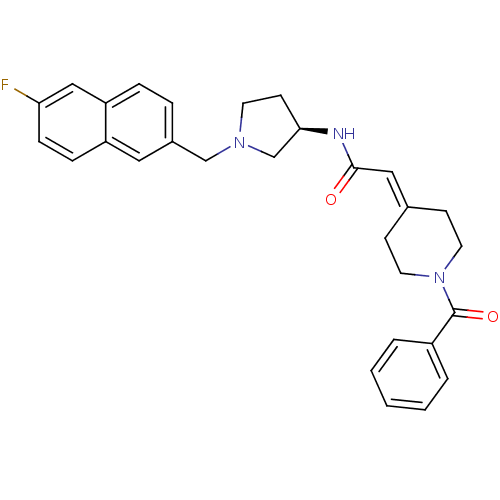
(2-(1-Benzoylpiperidin-4-ylidene)-N-{(3R)-1-[(6-flu...)Show SMILES Fc1ccc2cc(-[#6]-[#7]-3-[#6]-[#6]-[#6@H](-[#6]-3)-[#7]-[#6](=O)\[#6]=[#6]-3/[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-c3ccccc3)ccc2c1 |r| Show InChI InChI=1S/C29H30FN3O2/c30-26-9-8-24-16-22(6-7-25(24)18-26)19-32-13-12-27(20-32)31-28(34)17-21-10-14-33(15-11-21)29(35)23-4-2-1-3-5-23/h1-9,16-18,27H,10-15,19-20H2,(H,31,34)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297188
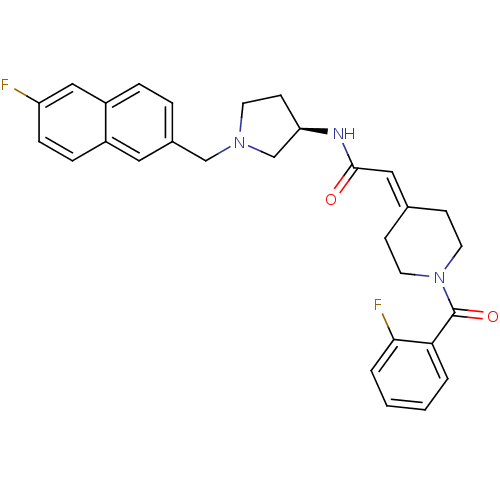
(2-[1-(2-Fluorobenzoyl)piperidin-4-ylidene]-N-{(3R)...)Show SMILES Fc1ccc2cc(-[#6]-[#7]-3-[#6]-[#6]-[#6@H](-[#6]-3)-[#7]-[#6](=O)\[#6]=[#6]-3/[#6]-[#6]-[#7](-[#6]-[#6]-3)-[#6](=O)-c3ccccc3F)ccc2c1 |r| Show InChI InChI=1S/C29H29F2N3O2/c30-24-8-7-22-15-21(5-6-23(22)17-24)18-33-12-11-25(19-33)32-28(35)16-20-9-13-34(14-10-20)29(36)26-3-1-2-4-27(26)31/h1-8,15-17,25H,9-14,18-19H2,(H,32,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50387669
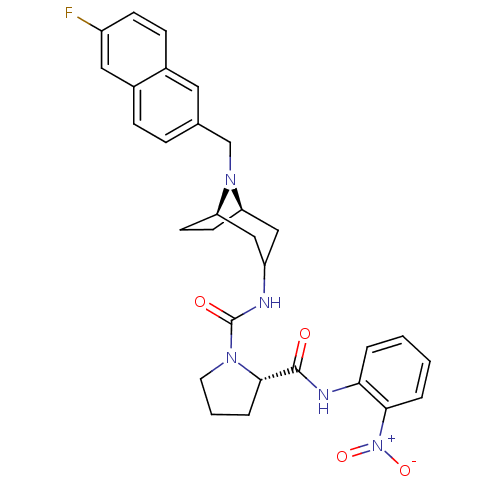
(CHEMBL2058071)Show SMILES [O-][N+](=O)c1ccccc1NC(=O)[C@@H]1CCCN1C(=O)NC1C[C@H]2CC[C@H](C1)N2Cc1ccc2cc(F)ccc2c1 |r,TLB:28:27:20.21.26:23.24,THB:19:20:27:23.24| Show InChI InChI=1S/C30H32FN5O4/c31-22-10-9-20-14-19(7-8-21(20)15-22)18-35-24-11-12-25(35)17-23(16-24)32-30(38)34-13-3-6-28(34)29(37)33-26-4-1-2-5-27(26)36(39)40/h1-2,4-5,7-10,14-15,23-25,28H,3,6,11-13,16-18H2,(H,32,38)(H,33,37)/t24-,25-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 |
Bioorg Med Chem Lett 22: 4951-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.042
BindingDB Entry DOI: 10.7270/Q2057H06 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297180
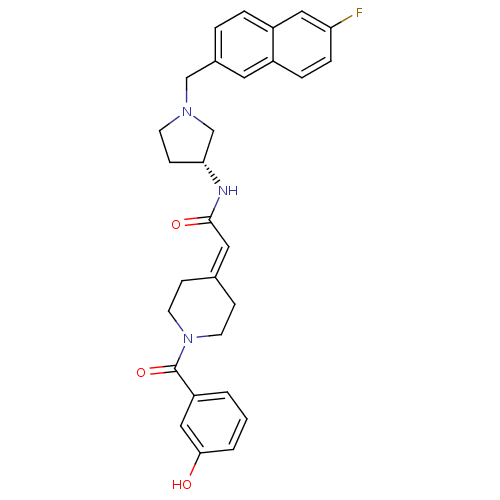
(CHEMBL556916 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1cccc(c1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-25-7-6-22-14-21(4-5-23(22)16-25)18-32-11-10-26(19-32)31-28(35)15-20-8-12-33(13-9-20)29(36)24-2-1-3-27(34)17-24/h1-7,14-17,26,34H,8-13,18-19H2,(H,31,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297179

(CHEMBL562923 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1ccc(cc1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-25-6-3-23-15-21(1-2-24(23)17-25)18-32-12-11-26(19-32)31-28(35)16-20-9-13-33(14-10-20)29(36)22-4-7-27(34)8-5-22/h1-8,15-17,26,34H,9-14,18-19H2,(H,31,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297185

(CHEMBL556227 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1ccccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-37-28-5-3-2-4-27(28)30(36)34-14-10-21(11-15-34)17-29(35)32-26-12-13-33(20-26)19-22-6-7-24-18-25(31)9-8-23(24)16-22/h2-9,16-18,26H,10-15,19-20H2,1H3,(H,32,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430139
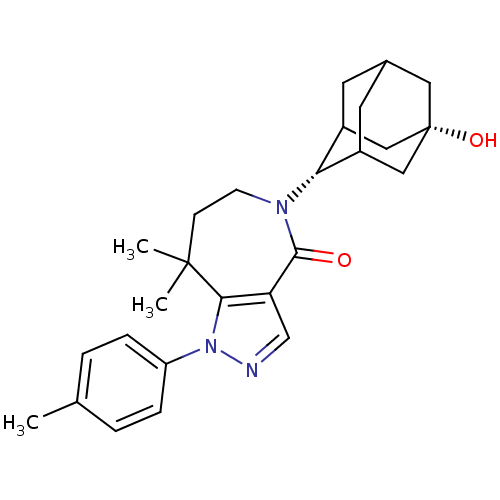
(CHEMBL2338247)Show SMILES Cc1ccc(cc1)-n1ncc2c1C(C)(C)CCN([C@H]1C3CC4CC1C[C@@](O)(C4)C3)C2=O |r,wU:18.19,25.28,TLB:22:21:28:24.23.18,22:23:27.21.20:28,18:19:27:24.22.23,THB:18:23:27:20.19.28,17:18:27.21.20:28,(7.11,-16.96,;7.87,-18.3,;9.41,-18.31,;10.17,-19.65,;9.38,-20.98,;7.85,-20.97,;7.09,-19.63,;10.14,-22.31,;9.5,-23.71,;10.64,-24.75,;11.98,-24,;11.68,-22.48,;12.67,-21.3,;12.03,-19.9,;13.06,-19.8,;14.2,-21.34,;15.13,-22.57,;14.76,-24.06,;15.94,-25.05,;17.33,-24.46,;18.38,-25.69,;18.39,-27.27,;16.99,-27.85,;15.96,-26.58,;17.36,-26.92,;18.68,-26.41,;20.22,-26.39,;19.89,-27.68,;18.68,-24.93,;13.36,-24.69,;13.32,-26.23,)| Show InChI InChI=1S/C26H33N3O2/c1-16-4-6-20(7-5-16)29-23-21(15-27-29)24(30)28(9-8-25(23,2)3)22-18-10-17-11-19(22)14-26(31,12-17)13-18/h4-7,15,17-19,22,31H,8-14H2,1-3H3/t17?,18?,19?,22-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297184
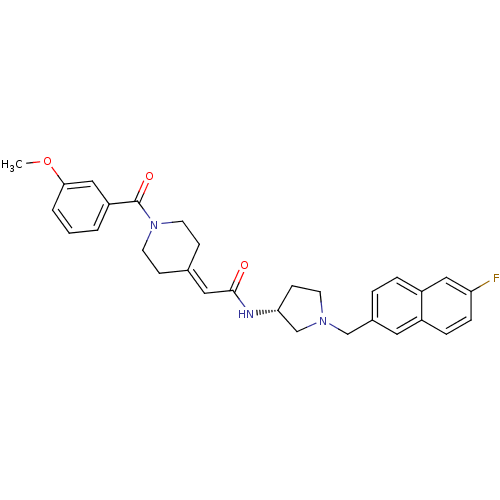
(CHEMBL557118 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C30H32FN3O3/c1-37-28-4-2-3-25(18-28)30(36)34-13-9-21(10-14-34)16-29(35)32-27-11-12-33(20-27)19-22-5-6-24-17-26(31)8-7-23(24)15-22/h2-8,15-18,27H,9-14,19-20H2,1H3,(H,32,35)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297174

(2-[1-(4-Fluoro-2-hydroxybenzoyl)piperidin-4-yliden...)Show SMILES [#8]-c1cc(F)ccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H29F2N3O3/c30-23-4-3-21-13-20(1-2-22(21)15-23)17-33-10-9-25(18-33)32-28(36)14-19-7-11-34(12-8-19)29(37)26-6-5-24(31)16-27(26)35/h1-6,13-16,25,35H,7-12,17-18H2,(H,32,36)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430136

(CHEMBL2338250)Show SMILES CC1(C)CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1cccc(F)c1 |r,wU:6.5,13.14,TLB:10:9:16:12.11.6,10:11:15.9.8:16,6:7:15:12.10.11,THB:6:11:15:8.7.16,5:6:15.9.8:16,(30.53,-32.58,;31.16,-33.98,;31.55,-32.48,;32.69,-34.02,;33.63,-35.25,;33.26,-36.74,;34.43,-37.73,;35.83,-37.14,;36.88,-38.37,;36.88,-39.95,;35.48,-40.53,;34.46,-39.26,;35.86,-39.59,;37.18,-39.09,;38.71,-39.07,;38.39,-40.36,;37.18,-37.61,;31.85,-37.37,;31.82,-38.91,;30.48,-36.68,;29.13,-37.43,;28,-36.39,;28.64,-34.99,;30.17,-35.16,;27.88,-33.65,;28.66,-32.33,;27.91,-30.99,;26.37,-30.97,;25.58,-32.31,;24.04,-32.3,;26.35,-33.64,)| Show InChI InChI=1S/C25H30FN3O2/c1-24(2)6-7-28(21-16-8-15-9-17(21)13-25(31,11-15)12-16)23(30)20-14-27-29(22(20)24)19-5-3-4-18(26)10-19/h3-5,10,14-17,21,31H,6-9,11-13H2,1-2H3/t15?,16?,17?,21-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297173
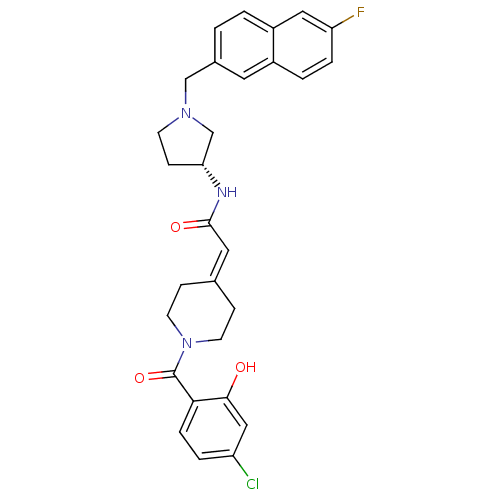
(2-[1-(4-Chloro-2-hydroxybenzoyl)piperidin-4-yliden...)Show SMILES [#8]-c1cc(Cl)ccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H29ClFN3O3/c30-23-4-6-26(27(35)16-23)29(37)34-11-7-19(8-12-34)14-28(36)32-25-9-10-33(18-25)17-20-1-2-22-15-24(31)5-3-21(22)13-20/h1-6,13-16,25,35H,7-12,17-18H2,(H,32,36)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50401518
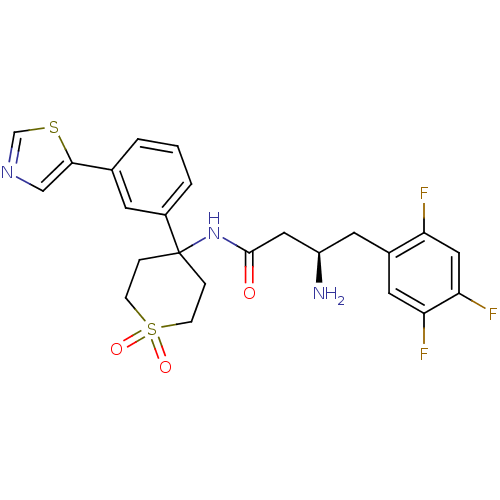
(CHEMBL2206469)Show SMILES N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1cccc(c1)-c1cncs1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C24H24F3N3O3S2/c25-19-12-21(27)20(26)10-16(19)9-18(28)11-23(31)30-24(4-6-35(32,33)7-5-24)17-3-1-2-15(8-17)22-13-29-14-34-22/h1-3,8,10,12-14,18H,4-7,9,11,28H2,(H,30,31)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco2 cell extracts using Ala-Pro-AFC as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 22: 7036-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.099
BindingDB Entry DOI: 10.7270/Q2DV1M17 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50430140

(CHEMBL2338246)Show SMILES Cc1cccc(c1)-n1ncc2c1C(C)(C)CCN([C@H]1C3CC4CC1C[C@@](O)(C4)C3)C2=O |r,wU:18.19,25.28,TLB:22:21:28:24.23.18,22:23:27.21.20:28,18:19:27:24.22.23,THB:18:23:27:20.19.28,17:18:27.21.20:28,(23.2,-5.1,;24.74,-5.11,;25.52,-3.77,;27.06,-3.79,;27.82,-5.13,;27.03,-6.45,;25.5,-6.45,;27.79,-7.79,;27.15,-9.19,;28.28,-10.23,;29.63,-9.48,;29.32,-7.96,;30.31,-6.78,;29.68,-5.38,;30.71,-5.28,;31.85,-6.82,;32.78,-8.05,;32.41,-9.54,;33.59,-10.53,;34.98,-9.94,;36.03,-11.17,;36.04,-12.75,;34.64,-13.33,;33.61,-12.06,;35.01,-12.4,;36.33,-11.89,;37.87,-11.87,;37.54,-13.16,;36.33,-10.41,;31.01,-10.17,;30.97,-11.71,)| Show InChI InChI=1S/C26H33N3O2/c1-16-5-4-6-20(9-16)29-23-21(15-27-29)24(30)28(8-7-25(23,2)3)22-18-10-17-11-19(22)14-26(31,12-17)13-18/h4-6,9,15,17-19,22,31H,7-8,10-14H2,1-3H3/t17?,18?,19?,22-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430132
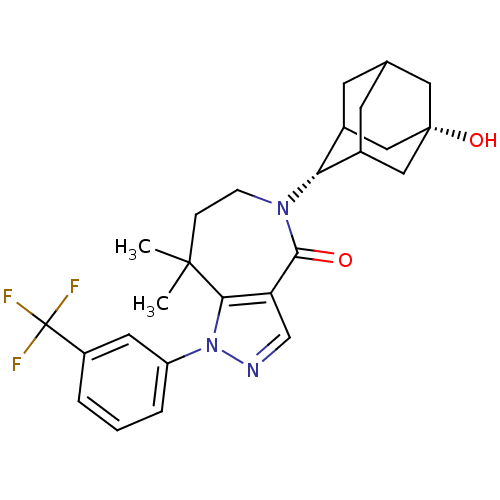
(CHEMBL2338254)Show SMILES CC1(C)CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1cccc(c1)C(F)(F)F |r,wU:6.5,13.14,TLB:10:9:16:12.11.6,10:11:15.9.8:16,6:7:15:12.10.11,THB:6:11:15:8.7.16,5:6:15.9.8:16,(12.82,-7.27,;13.45,-8.67,;13.84,-7.17,;14.98,-8.71,;15.92,-9.94,;15.55,-11.43,;16.72,-12.42,;18.11,-11.83,;19.16,-13.06,;19.17,-14.64,;17.77,-15.22,;16.75,-13.95,;18.15,-14.29,;19.47,-13.78,;21,-13.76,;20.68,-15.05,;19.47,-12.3,;14.14,-12.06,;14.11,-13.6,;12.77,-11.37,;11.42,-12.12,;10.29,-11.08,;10.93,-9.68,;12.46,-9.85,;10.17,-8.34,;10.95,-7.02,;10.2,-5.68,;8.65,-5.66,;7.87,-7,;8.64,-8.34,;6.33,-6.99,;5.57,-5.66,;5.56,-8.32,;4.79,-6.98,)| Show InChI InChI=1S/C26H30F3N3O2/c1-24(2)6-7-31(21-16-8-15-9-17(21)13-25(34,11-15)12-16)23(33)20-14-30-32(22(20)24)19-5-3-4-18(10-19)26(27,28)29/h3-5,10,14-17,21,34H,6-9,11-13H2,1-2H3/t15?,16?,17?,21-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50387649

(CHEMBL2057751)Show SMILES Nc1ccc2n(C3CCN(CC3)C(=O)NC3C[C@H]4CC[C@H](C3)N4Cc3ccc4cc(F)ccc4c3)c(=O)[nH]c2c1 |r,TLB:23:22:15.16.21:18.19,THB:14:15:22:18.19| Show InChI InChI=1S/C31H35FN6O2/c32-22-4-3-20-13-19(1-2-21(20)14-22)18-37-26-6-7-27(37)17-24(16-26)34-30(39)36-11-9-25(10-12-36)38-29-8-5-23(33)15-28(29)35-31(38)40/h1-5,8,13-15,24-27H,6-7,9-12,16-18,33H2,(H,34,39)(H,35,40)/t26-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 |
Bioorg Med Chem Lett 22: 4951-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.042
BindingDB Entry DOI: 10.7270/Q2057H06 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50430141
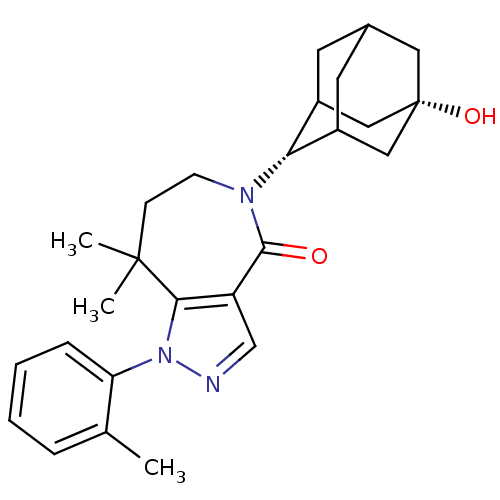
(CHEMBL2338245)Show SMILES Cc1ccccc1-n1ncc2c1C(C)(C)CCN([C@H]1C3CC4CC1C[C@@](O)(C4)C3)C2=O |r,wU:18.19,25.28,TLB:22:21:28:24.23.18,22:23:27.21.20:28,18:19:27:24.22.23,THB:18:23:27:20.19.28,17:18:27.21.20:28,(6.79,-8.68,;7.57,-7.35,;6.81,-6.01,;7.59,-4.68,;9.13,-4.69,;9.89,-6.03,;9.11,-7.36,;9.86,-8.69,;9.22,-10.1,;10.36,-11.13,;11.7,-10.38,;11.4,-8.86,;12.39,-7.68,;11.75,-6.28,;12.78,-6.18,;13.92,-7.72,;14.85,-8.95,;14.48,-10.45,;15.66,-11.44,;17.05,-10.84,;18.1,-12.07,;18.11,-13.65,;16.71,-14.23,;15.68,-12.96,;17.08,-13.3,;18.41,-12.8,;19.94,-12.78,;19.61,-14.06,;18.4,-11.31,;13.08,-11.08,;13.04,-12.62,)| Show InChI InChI=1S/C26H33N3O2/c1-16-6-4-5-7-21(16)29-23-20(15-27-29)24(30)28(9-8-25(23,2)3)22-18-10-17-11-19(22)14-26(31,12-17)13-18/h4-7,15,17-19,22,31H,8-14H2,1-3H3/t17?,18?,19?,22-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297171

(CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...)Show SMILES [#8]-c1ccccc1-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H30FN3O3/c30-24-8-7-22-15-21(5-6-23(22)17-24)18-32-12-11-25(19-32)31-28(35)16-20-9-13-33(14-10-20)29(36)26-3-1-2-4-27(26)34/h1-8,15-17,25,34H,9-14,18-19H2,(H,31,35)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430147

(CHEMBL2338264)Show SMILES CC1(C)CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1ccccc1 |r,wU:6.5,13.14,TLB:10:9:16:12.11.6,10:11:15.9.8:16,6:7:15:12.10.11,THB:6:11:15:8.7.16,5:6:15.9.8:16,(29.24,-4.12,;29.87,-5.53,;30.63,-4.18,;31.41,-5.57,;32.34,-6.8,;31.97,-8.29,;33.15,-9.28,;34.54,-8.69,;35.59,-9.91,;35.6,-11.5,;34.2,-12.08,;33.17,-10.81,;34.57,-11.14,;35.89,-10.64,;37.43,-10.62,;37.1,-11.9,;35.89,-9.15,;30.57,-8.92,;30.53,-10.46,;29.19,-8.22,;27.85,-8.98,;26.71,-7.94,;27.35,-6.53,;28.89,-6.71,;26.59,-5.2,;27.38,-3.87,;26.62,-2.54,;25.08,-2.52,;24.3,-3.86,;25.06,-5.19,)| Show InChI InChI=1S/C25H31N3O2/c1-24(2)8-9-27(21-17-10-16-11-18(21)14-25(30,12-16)13-17)23(29)20-15-26-28(22(20)24)19-6-4-3-5-7-19/h3-7,15-18,21,30H,8-14H2,1-2H3/t16?,17?,18?,21-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50430147

(CHEMBL2338264)Show SMILES CC1(C)CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1ccccc1 |r,wU:6.5,13.14,TLB:10:9:16:12.11.6,10:11:15.9.8:16,6:7:15:12.10.11,THB:6:11:15:8.7.16,5:6:15.9.8:16,(29.24,-4.12,;29.87,-5.53,;30.63,-4.18,;31.41,-5.57,;32.34,-6.8,;31.97,-8.29,;33.15,-9.28,;34.54,-8.69,;35.59,-9.91,;35.6,-11.5,;34.2,-12.08,;33.17,-10.81,;34.57,-11.14,;35.89,-10.64,;37.43,-10.62,;37.1,-11.9,;35.89,-9.15,;30.57,-8.92,;30.53,-10.46,;29.19,-8.22,;27.85,-8.98,;26.71,-7.94,;27.35,-6.53,;28.89,-6.71,;26.59,-5.2,;27.38,-3.87,;26.62,-2.54,;25.08,-2.52,;24.3,-3.86,;25.06,-5.19,)| Show InChI InChI=1S/C25H31N3O2/c1-24(2)8-9-27(21-17-10-16-11-18(21)14-25(30,12-16)13-17)23(29)20-15-26-28(22(20)24)19-6-4-3-5-7-19/h3-7,15-18,21,30H,8-14H2,1-2H3/t16?,17?,18?,21-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430134
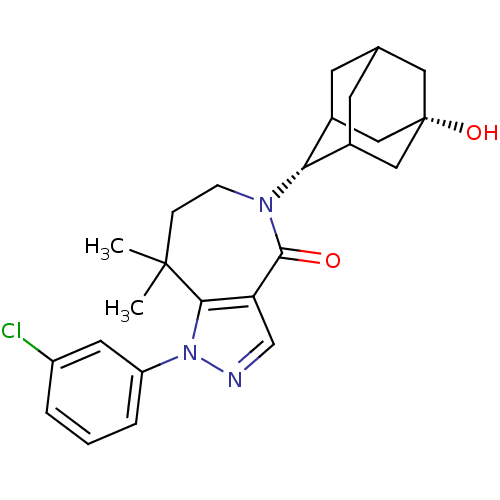
(CHEMBL2338252)Show SMILES CC1(C)CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1cccc(Cl)c1 |r,wU:6.5,13.14,TLB:10:9:16:12.11.6,10:11:15.9.8:16,6:7:15:12.10.11,THB:6:11:15:8.7.16,5:6:15.9.8:16,(25.72,-45.66,;26.36,-47.06,;26.75,-45.56,;27.89,-47.1,;28.82,-48.33,;28.45,-49.82,;29.63,-50.81,;31.02,-50.22,;32.07,-51.45,;32.08,-53.03,;30.68,-53.61,;29.65,-52.34,;31.05,-52.68,;32.37,-52.18,;33.91,-52.15,;33.58,-53.44,;32.37,-50.69,;27.05,-50.45,;27.01,-51.99,;25.67,-49.76,;24.33,-50.51,;23.19,-49.47,;23.83,-48.07,;25.37,-48.24,;23.07,-46.74,;23.86,-45.41,;23.1,-44.07,;21.56,-44.06,;20.78,-45.39,;19.24,-45.39,;21.54,-46.73,)| Show InChI InChI=1S/C25H30ClN3O2/c1-24(2)6-7-28(21-16-8-15-9-17(21)13-25(31,11-15)12-16)23(30)20-14-27-29(22(20)24)19-5-3-4-18(26)10-19/h3-5,10,14-17,21,31H,6-9,11-13H2,1-2H3/t15?,16?,17?,21-,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394482

(CHEMBL2159867)Show SMILES OCCCCOc1ccccc1NC(=O)N[C@@H]1CCN(Cc2ccc3cc(F)ccc3c2)C1 |r| Show InChI InChI=1S/C26H30FN3O3/c27-22-10-9-20-15-19(7-8-21(20)16-22)17-30-12-11-23(18-30)28-26(32)29-24-5-1-2-6-25(24)33-14-4-3-13-31/h1-2,5-10,15-16,23,31H,3-4,11-14,17-18H2,(H2,28,29,32)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430142

(CHEMBL2338244)Show SMILES CC1(C)CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1ccccn1 |r,wU:6.5,13.14,TLB:10:9:16:12.11.6,10:11:15.9.8:16,6:7:15:12.10.11,THB:6:11:15:8.7.16,5:6:15.9.8:16,(8.51,-43.7,;8.91,-45.2,;9.67,-43.86,;10.44,-45.24,;11.38,-46.47,;11.01,-47.96,;12.18,-48.95,;13.57,-48.36,;14.62,-49.58,;14.63,-51.16,;13.23,-51.74,;12.21,-50.47,;13.6,-50.81,;14.93,-50.31,;16.46,-50.29,;16.13,-51.57,;14.92,-48.82,;9.6,-48.59,;9.57,-50.13,;8.23,-47.89,;6.89,-48.65,;5.76,-47.61,;6.39,-46.2,;7.93,-46.38,;5.64,-44.88,;6.42,-43.55,;5.66,-42.21,;4.12,-42.2,;3.34,-43.53,;4.11,-44.87,)| Show InChI InChI=1S/C24H30N4O2/c1-23(2)6-8-27(20-16-9-15-10-17(20)13-24(30,11-15)12-16)22(29)18-14-26-28(21(18)23)19-5-3-4-7-25-19/h3-5,7,14-17,20,30H,6,8-13H2,1-2H3/t15?,16?,17?,20-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430138
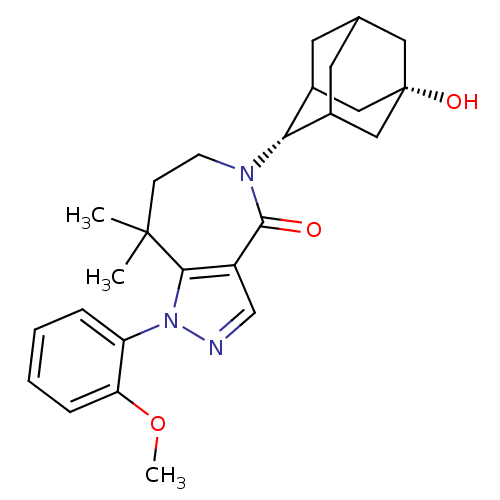
(CHEMBL2338248)Show SMILES COc1ccccc1-n1ncc2c1C(C)(C)CCN([C@H]1C3CC4CC1C[C@@](O)(C4)C3)C2=O |r,wU:19.20,26.29,TLB:23:22:29:25.24.19,23:24:28.22.21:29,19:20:28:25.23.24,THB:19:24:28:21.20.29,18:19:28.22.21:29,(23.46,-21.38,;25,-21.39,;25.78,-20.06,;25.01,-18.73,;25.79,-17.39,;27.34,-17.4,;28.09,-18.74,;27.31,-20.07,;28.07,-21.4,;27.43,-22.81,;28.56,-23.85,;29.91,-23.09,;29.6,-21.58,;30.59,-20.4,;29.96,-18.99,;30.98,-18.9,;32.12,-20.44,;33.06,-21.67,;32.68,-23.16,;33.86,-24.15,;35.25,-23.56,;36.3,-24.78,;36.31,-26.37,;34.91,-26.94,;33.89,-25.68,;35.29,-26.01,;36.61,-25.51,;38.14,-25.49,;37.82,-26.77,;36.6,-24.02,;31.28,-23.79,;31.25,-25.33,)| Show InChI InChI=1S/C26H33N3O3/c1-25(2)8-9-28(22-17-10-16-11-18(22)14-26(31,12-16)13-17)24(30)19-15-27-29(23(19)25)20-6-4-5-7-21(20)32-3/h4-7,15-18,22,31H,8-14H2,1-3H3/t16?,17?,18?,22-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430137
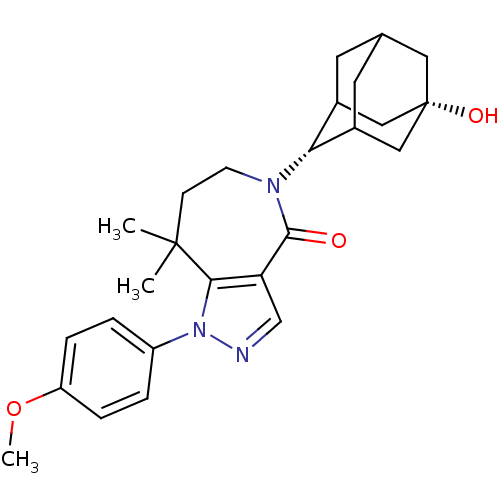
(CHEMBL2338249)Show SMILES COc1ccc(cc1)-n1ncc2c1C(C)(C)CCN([C@H]1C3CC4CC1C[C@@](O)(C4)C3)C2=O |r,wU:19.20,26.29,TLB:23:22:29:25.24.19,23:24:28.22.21:29,19:20:28:25.23.24,THB:19:24:28:21.20.29,18:19:28.22.21:29,(8.21,-30.35,;7.44,-31.68,;8.2,-33.02,;9.74,-33.03,;10.5,-34.37,;9.71,-35.7,;8.18,-35.69,;7.42,-34.36,;10.47,-37.03,;9.83,-38.44,;10.96,-39.48,;12.31,-38.72,;12,-37.21,;12.99,-36.03,;12.36,-34.62,;13.39,-34.53,;14.53,-36.07,;15.46,-37.3,;15.09,-38.79,;16.27,-39.78,;17.66,-39.19,;18.71,-40.41,;18.72,-42,;17.32,-42.57,;16.29,-41.31,;17.69,-41.64,;19.01,-41.14,;20.55,-41.12,;20.22,-42.4,;19.01,-39.65,;13.68,-39.42,;13.65,-40.96,)| Show InChI InChI=1S/C26H33N3O3/c1-25(2)8-9-28(22-17-10-16-11-18(22)14-26(31,12-16)13-17)24(30)21-15-27-29(23(21)25)19-4-6-20(32-3)7-5-19/h4-7,15-18,22,31H,8-14H2,1-3H3/t16?,17?,18?,22-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50394481

(CHEMBL2159868)Show SMILES OCCOc1ccc(NC(=O)N[C@@H]2CCN(Cc3ccc4cc(F)ccc4c3)C2)cc1 |r| Show InChI InChI=1S/C24H26FN3O3/c25-20-4-3-18-13-17(1-2-19(18)14-20)15-28-10-9-22(16-28)27-24(30)26-21-5-7-23(8-6-21)31-12-11-29/h1-8,13-14,22,29H,9-12,15-16H2,(H2,26,27,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor |
Bioorg Med Chem Lett 22: 6876-81 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.035
BindingDB Entry DOI: 10.7270/Q2QC04MN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50401519
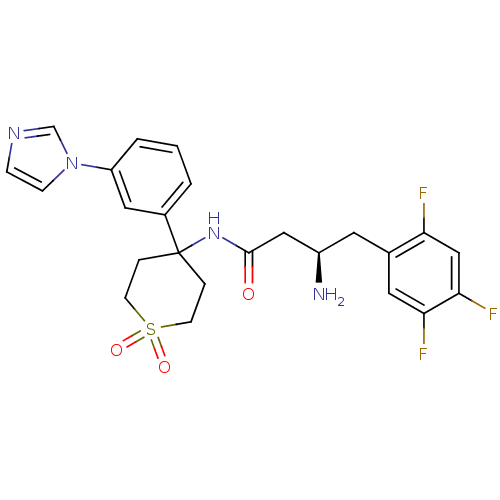
(CHEMBL2203319)Show SMILES N[C@@H](CC(=O)NC1(CCS(=O)(=O)CC1)c1cccc(c1)-n1ccnc1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C24H25F3N4O3S/c25-20-14-22(27)21(26)11-16(20)10-18(28)13-23(32)30-24(4-8-35(33,34)9-5-24)17-2-1-3-19(12-17)31-7-6-29-15-31/h1-3,6-7,11-12,14-15,18H,4-5,8-10,13,28H2,(H,30,32)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 in human Caco2 cell extracts using Ala-Pro-AFC as substrate after 20 mins by fluorescence assay |
Bioorg Med Chem Lett 22: 7036-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.099
BindingDB Entry DOI: 10.7270/Q2DV1M17 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430148
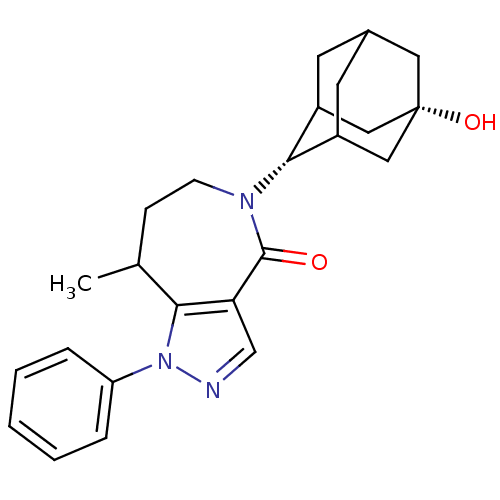
(CHEMBL2338263)Show SMILES CC1CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1ccccc1 |r,wU:5.4,12.13,TLB:9:8:15:11.10.5,9:10:14.8.7:15,5:6:14:11.9.10,THB:5:10:14:7.6.15,4:5:14.8.7:15,(9.34,-4.09,;9.97,-5.5,;11.51,-5.54,;12.44,-6.77,;12.07,-8.26,;13.25,-9.25,;14.64,-8.66,;15.69,-9.88,;15.7,-11.47,;14.3,-12.05,;13.27,-10.78,;14.67,-11.11,;15.99,-10.61,;17.53,-10.59,;17.2,-11.87,;15.99,-9.12,;10.67,-8.89,;10.63,-10.43,;9.29,-8.19,;7.95,-8.95,;6.81,-7.91,;7.45,-6.5,;8.99,-6.68,;6.69,-5.17,;7.48,-3.84,;6.72,-2.5,;5.18,-2.49,;4.4,-3.83,;5.16,-5.16,)| Show InChI InChI=1S/C24H29N3O2/c1-15-7-8-26(22-17-9-16-10-18(22)13-24(29,11-16)12-17)23(28)20-14-25-27(21(15)20)19-5-3-2-4-6-19/h2-6,14-18,22,29H,7-13H2,1H3/t15?,16?,17?,18?,22-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50264210
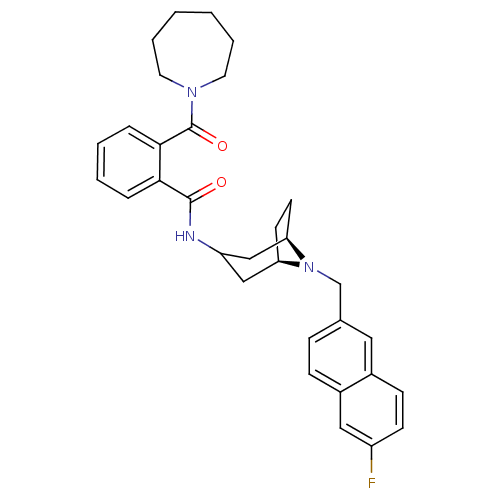
(2-(Azepan-1-ylcarbonyl)-N-{(3-exo)-8-[(6-fluoro-2-...)Show SMILES Fc1ccc2cc(CN3[C@@H]4CC[C@@H]3CC(C4)NC(=O)c3ccccc3C(=O)N3CCCCCC3)ccc2c1 |r,TLB:7:8:14.15.13:10.11,THB:16:14:8:10.11| Show InChI InChI=1S/C32H36FN3O2/c33-25-12-11-23-17-22(9-10-24(23)18-25)21-36-27-13-14-28(36)20-26(19-27)34-31(37)29-7-3-4-8-30(29)32(38)35-15-5-1-2-6-16-35/h3-4,7-12,17-18,26-28H,1-2,5-6,13-16,19-21H2,(H,34,37)/t27-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR3 receptor expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium influx by spectrophotometry |
Bioorg Med Chem 16: 8607-18 (2008)
Article DOI: 10.1016/j.bmc.2008.08.006
BindingDB Entry DOI: 10.7270/Q21J9BPZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50275905
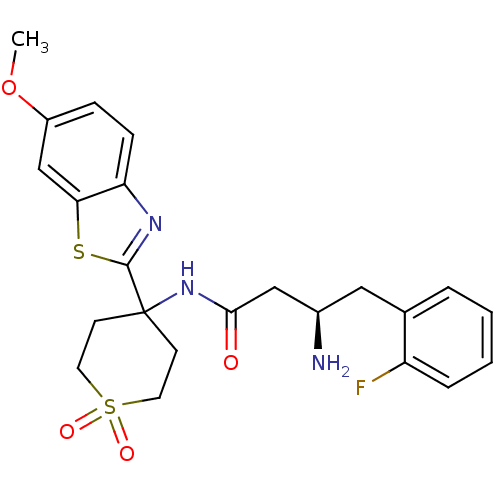
((R)-3-Amino-4-(2-fluoro-phenyl)-N-[4-(6-methoxy-be...)Show SMILES COc1ccc2nc(sc2c1)C1(CCS(=O)(=O)CC1)NC(=O)C[C@H](N)Cc1ccccc1F |r| Show InChI InChI=1S/C23H26FN3O4S2/c1-31-17-6-7-19-20(14-17)32-22(26-19)23(8-10-33(29,30)11-9-23)27-21(28)13-16(25)12-15-4-2-3-5-18(15)24/h2-7,14,16H,8-13,25H2,1H3,(H,27,28)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Caco2 cells-derived DPP4 |
Bioorg Med Chem Lett 18: 5435-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.042
BindingDB Entry DOI: 10.7270/Q2QV3MC8 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50297187
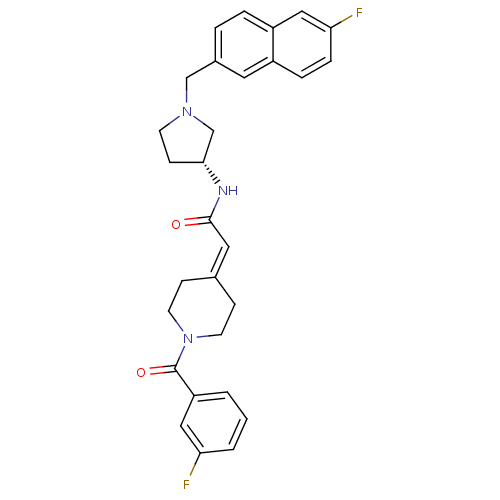
((R)-2-(1-(3-fluorobenzoyl)piperidin-4-ylidene)-N-(...)Show SMILES Fc1cccc(c1)-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/[#6](=O)-[#7]-[#6@@H]-1-[#6]-[#6]-[#7](-[#6]-c2ccc3cc(F)ccc3c2)-[#6]-1 |r| Show InChI InChI=1S/C29H29F2N3O2/c30-25-3-1-2-24(17-25)29(36)34-12-8-20(9-13-34)15-28(35)32-27-10-11-33(19-27)18-21-4-5-23-16-26(31)7-6-22(23)14-21/h1-7,14-17,27H,8-13,18-19H2,(H,32,35)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux |
Bioorg Med Chem 17: 5989-6002 (2009)
Article DOI: 10.1016/j.bmc.2009.06.066
BindingDB Entry DOI: 10.7270/Q2WD40NV |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50430148

(CHEMBL2338263)Show SMILES CC1CCN([C@H]2C3CC4CC2C[C@@](O)(C4)C3)C(=O)c2cnn(c12)-c1ccccc1 |r,wU:5.4,12.13,TLB:9:8:15:11.10.5,9:10:14.8.7:15,5:6:14:11.9.10,THB:5:10:14:7.6.15,4:5:14.8.7:15,(9.34,-4.09,;9.97,-5.5,;11.51,-5.54,;12.44,-6.77,;12.07,-8.26,;13.25,-9.25,;14.64,-8.66,;15.69,-9.88,;15.7,-11.47,;14.3,-12.05,;13.27,-10.78,;14.67,-11.11,;15.99,-10.61,;17.53,-10.59,;17.2,-11.87,;15.99,-9.12,;10.67,-8.89,;10.63,-10.43,;9.29,-8.19,;7.95,-8.95,;6.81,-7.91,;7.45,-6.5,;8.99,-6.68,;6.69,-5.17,;7.48,-3.84,;6.72,-2.5,;5.18,-2.49,;4.4,-3.83,;5.16,-5.16,)| Show InChI InChI=1S/C24H29N3O2/c1-15-7-8-26(22-17-9-16-10-18(22)13-24(29,11-16)12-17)23(28)20-14-25-27(21(15)20)19-5-3-2-4-6-19/h2-6,14-18,22,29H,7-13H2,1H3/t15?,16?,17?,18?,22-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in HEK293 cells using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50430140

(CHEMBL2338246)Show SMILES Cc1cccc(c1)-n1ncc2c1C(C)(C)CCN([C@H]1C3CC4CC1C[C@@](O)(C4)C3)C2=O |r,wU:18.19,25.28,TLB:22:21:28:24.23.18,22:23:27.21.20:28,18:19:27:24.22.23,THB:18:23:27:20.19.28,17:18:27.21.20:28,(23.2,-5.1,;24.74,-5.11,;25.52,-3.77,;27.06,-3.79,;27.82,-5.13,;27.03,-6.45,;25.5,-6.45,;27.79,-7.79,;27.15,-9.19,;28.28,-10.23,;29.63,-9.48,;29.32,-7.96,;30.31,-6.78,;29.68,-5.38,;30.71,-5.28,;31.85,-6.82,;32.78,-8.05,;32.41,-9.54,;33.59,-10.53,;34.98,-9.94,;36.03,-11.17,;36.04,-12.75,;34.64,-13.33,;33.61,-12.06,;35.01,-12.4,;36.33,-11.89,;37.87,-11.87,;37.54,-13.16,;36.33,-10.41,;31.01,-10.17,;30.97,-11.71,)| Show InChI InChI=1S/C26H33N3O2/c1-16-5-4-6-20(9-16)29-23-21(15-27-29)24(30)28(8-7-25(23,2)3)22-18-10-17-11-19(22)14-26(31,12-17)13-18/h4-6,9,15,17-19,22,31H,7-8,10-14H2,1-3H3/t17?,18?,19?,22-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human liver microsomes using cortisone and NADPH as substrate by HTRF assay |
Bioorg Med Chem Lett 23: 1617-21 (2013)
Article DOI: 10.1016/j.bmcl.2013.01.090
BindingDB Entry DOI: 10.7270/Q27P90RZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data