 Found 151 hits with Last Name = 'desai' and Initial = 'aj'
Found 151 hits with Last Name = 'desai' and Initial = 'aj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-2] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728
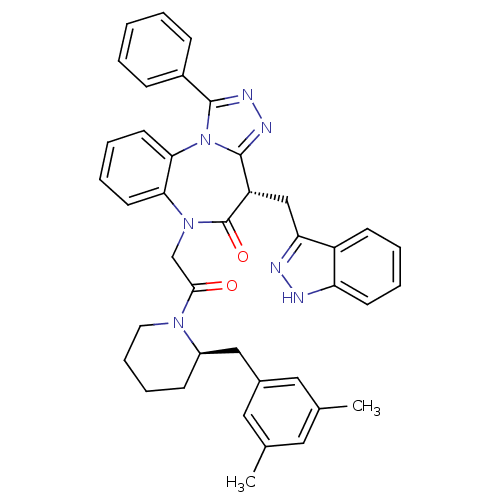
(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-2] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-2] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK2R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from wild-type human CCK1R at allosteric site expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-2] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK2R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.813 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK1R N2.61T mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK1R W6.48A mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK2R H7.39L mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK1R N2.61T mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-2] from human CCK2R H7.39L mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329178
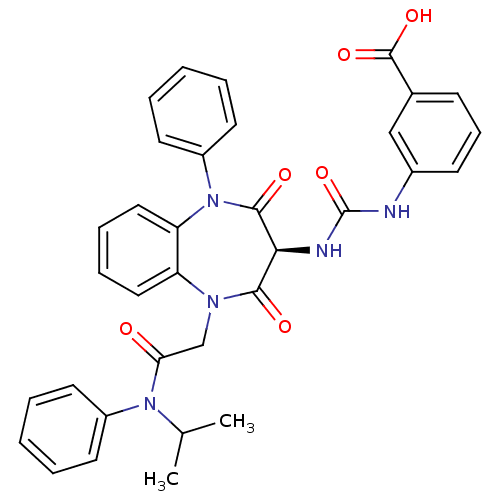
((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...)Show SMILES CC(C)N(C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)[C@@H](NC(=O)Nc2cccc(c2)C(O)=O)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C34H31N5O6/c1-22(2)38(25-14-5-3-6-15-25)29(40)21-37-27-18-9-10-19-28(27)39(26-16-7-4-8-17-26)32(42)30(31(37)41)36-34(45)35-24-13-11-12-23(20-24)33(43)44/h3-20,22,30H,21H2,1-2H3,(H,43,44)(H2,35,36,45)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ2 from CCK2R (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Agonist activity at human CCK1R V3.36A mutant expressed in CHO cells assessed as intracellular calcium response by fluorescence analysis |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from human CCK1R T3.28V, T3.29S mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50329178

((S)-3-(3-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl...)Show SMILES CC(C)N(C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)[C@@H](NC(=O)Nc2cccc(c2)C(O)=O)C1=O)c1ccccc1 |r| Show InChI InChI=1S/C34H31N5O6/c1-22(2)38(25-14-5-3-6-15-25)29(40)21-37-27-18-9-10-19-28(27)39(26-16-7-4-8-17-26)32(42)30(31(37)41)36-34(45)35-24-13-11-12-23(20-24)33(43)44/h3-20,22,30H,21H2,1-2H3,(H,43,44)(H2,35,36,45)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK from CCK2R (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50074832
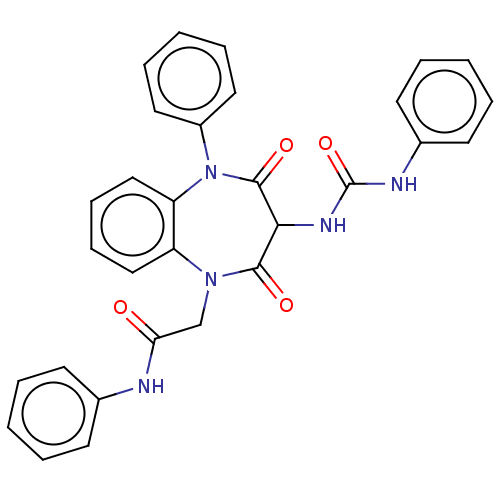
(CHEMBL156605)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C30H25N5O4/c36-26(31-21-12-4-1-5-13-21)20-34-24-18-10-11-19-25(24)35(23-16-8-3-9-17-23)29(38)27(28(34)37)33-30(39)32-22-14-6-2-7-15-22/h1-19,27H,20H2,(H,31,36)(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ2 from CCK2R TM7 (unknown origin) containing L7.39H chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from human CCK1R T3.28V, T3.29S mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK2R H7.39L mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK1R V3.36A mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419
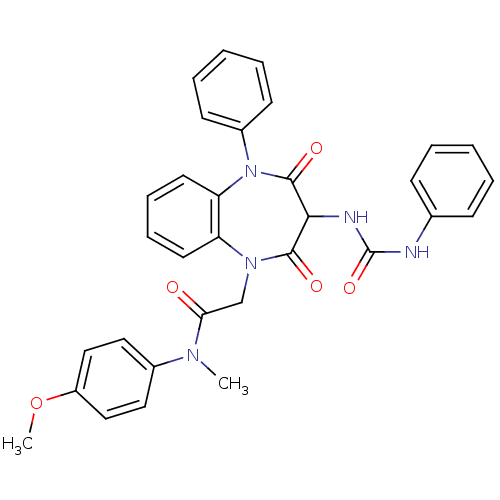
(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK from CCK2R TM3 (unknown origin) containing T3.28V, T3.29S chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-2] from human CCK2R H7.39L mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50074832

(CHEMBL156605)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C30H25N5O4/c36-26(31-21-12-4-1-5-13-21)20-34-24-18-10-11-19-25(24)35(23-16-8-3-9-17-23)29(38)27(28(34)37)33-30(39)32-22-14-6-2-7-15-22/h1-19,27H,20H2,(H,31,36)(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK from CCK2R TM7 (unknown origin) containing L7.39H chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ2 from CCK2R TM7 (unknown origin) containing L7.39H chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-BDZ-1] from human CCK1R M3.32A mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK1R V3.36A mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BDZ-1 from rat CCK1R Ala-317,321,325 non dimerizing mutant expressed in COS cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ1 from CCK2R (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK from CCK2R TM7 (unknown origin) containing L7.39H chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]-BDZ-1 from rat CCK1R Ala-317,321,325 non dimerizing mutant expressed in COS cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ2 from CCK2R TM3 (unknown origin) containing T3.28V, T3.29S chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50074832

(CHEMBL156605)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C30H25N5O4/c36-26(31-21-12-4-1-5-13-21)20-34-24-18-10-11-19-25(24)35(23-16-8-3-9-17-23)29(38)27(28(34)37)33-30(39)32-22-14-6-2-7-15-22/h1-19,27H,20H2,(H,31,36)(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ1 from CCK2R (unknown origin) expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50074832

(CHEMBL156605)Show SMILES O=C(CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)Nc1ccccc1 Show InChI InChI=1S/C30H25N5O4/c36-26(31-21-12-4-1-5-13-21)20-34-24-18-10-11-19-25(24)35(23-16-8-3-9-17-23)29(38)27(28(34)37)33-30(39)32-22-14-6-2-7-15-22/h1-19,27H,20H2,(H,31,36)(H2,32,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ2 from CCK2R TM6 (unknown origin) containing 16.51V, F6.52Y chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50380728

(CHEMBL2017835)Show SMILES Cc1cc(C)cc(C[C@H]2CCCCN2C(=O)CN2c3ccccc3-n3c(nnc3-c3ccccc3)[C@H](Cc3n[nH]c4ccccc34)C2=O)c1 |r| Show InChI InChI=1S/C40H39N7O2/c1-26-20-27(2)22-28(21-26)23-30-14-10-11-19-45(30)37(48)25-46-35-17-8-9-18-36(35)47-38(29-12-4-3-5-13-29)43-44-39(47)32(40(46)49)24-34-31-15-6-7-16-33(31)41-42-34/h3-9,12-13,15-18,20-22,30,32H,10-11,14,19,23-25H2,1-2H3,(H,41,42)/t30-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from wild-type human CCK1R expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]BDZ2 from CCK2R TM6 (unknown origin) containing 16.51V, F6.52Y chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK1R T3.28V, T3.29S mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50072419

(2-[2,4-Dioxo-5-phenyl-3-(3-phenyl-ureido)-2,3,4,5-...)Show SMILES COc1ccc(cc1)N(C)C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O Show InChI InChI=1S/C32H29N5O5/c1-35(23-17-19-25(42-2)20-18-23)28(38)21-36-26-15-9-10-16-27(26)37(24-13-7-4-8-14-24)31(40)29(30(36)39)34-32(41)33-22-11-5-3-6-12-22/h3-20,29H,21H2,1-2H3,(H2,33,34,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I]CCK from CCK1R TM2 (unknown origin) containing N2.61T chimeric mutant expressed in CHO cells |
Bioorg Med Chem Lett 25: 1849-55 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.051
BindingDB Entry DOI: 10.7270/Q2SJ1NB0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50329179

(2-((4S)-4-((1H-indol-3-yl)methyl)-5-oxo-1-phenyl-4...)Show SMILES CC(C)N(Cc1ccccc1)C(=O)CN1c2ccccc2-n2c(nnc2-c2ccccc2)[C@H](Cc2c[nH]c3ccccc23)C1=O |r| Show InChI InChI=1S/C37H34N6O2/c1-25(2)41(23-26-13-5-3-6-14-26)34(44)24-42-32-19-11-12-20-33(32)43-35(27-15-7-4-8-16-27)39-40-36(43)30(37(42)45)21-28-22-38-31-18-10-9-17-29(28)31/h3-20,22,25,30,38H,21,23-24H2,1-2H3/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Clinic
Curated by ChEMBL
| Assay Description
Displacement of [125I-CCK] from human CCK1R W6.48A mutant expressed in CHO cells after 60 mins by scintillation counter |
J Med Chem 58: 9562-77 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01110
BindingDB Entry DOI: 10.7270/Q29K4D33 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data