 Found 136 hits with Last Name = 'gouda' and Initial = 'am'
Found 136 hits with Last Name = 'gouda' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50614343
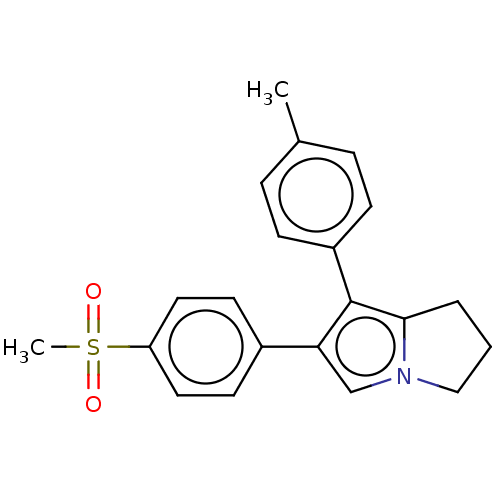
(CHEMBL597733)Show SMILES Cc1ccc(cc1)-c1c2CCCn2cc1-c1ccc(cc1)S(C)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50614341

(CHEMBL5269462)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cn2CCCc2c1-c1ccc(Cl)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614346
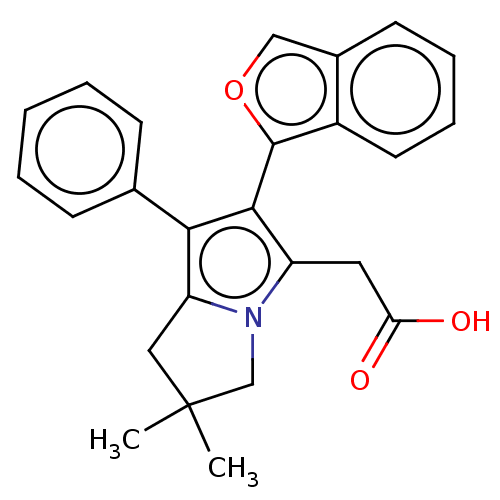
(CHEMBL5271431)Show SMILES CC1(C)Cc2c(c(c(CC(O)=O)n2C1)-c1occ2ccccc12)-c1ccccc1 |(12.77,-20.04,;13.55,-21.39,;12.06,-21.78,;14.79,-20.48,;16.04,-21.39,;17.58,-21.39,;18.06,-22.86,;16.81,-23.76,;16.8,-25.3,;15.47,-26.07,;14.14,-25.3,;15.47,-27.61,;15.56,-22.85,;14.02,-22.85,;19.52,-23.34,;19.99,-24.8,;21.53,-24.79,;22.01,-23.34,;23.42,-22.71,;23.59,-21.18,;22.34,-20.27,;20.93,-20.9,;20.77,-22.43,;18.49,-20.15,;20.02,-20.32,;20.93,-19.08,;20.31,-17.67,;18.77,-17.51,;17.86,-18.75,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50051317

((3-Chloro-phenyl)-(5,6-dimethyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C14H13ClN4/c1-8-9(2)18-13-12(8)14(17-7-16-13)19-11-5-3-4-10(15)6-11/h3-7H,1-2H3,(H2,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50051297
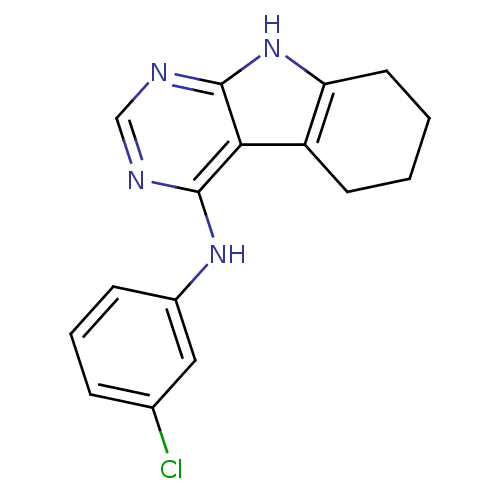
((3-Chloro-phenyl)-(6,7,8,9-tetrahydro-5H-pyrimido[...)Show InChI InChI=1S/C16H15ClN4/c17-10-4-3-5-11(8-10)20-15-14-12-6-1-2-7-13(12)21-16(14)19-9-18-15/h3-5,8-9H,1-2,6-7H2,(H2,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50614358

(CHEMBL5290904) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50614346

(CHEMBL5271431)Show SMILES CC1(C)Cc2c(c(c(CC(O)=O)n2C1)-c1occ2ccccc12)-c1ccccc1 |(12.77,-20.04,;13.55,-21.39,;12.06,-21.78,;14.79,-20.48,;16.04,-21.39,;17.58,-21.39,;18.06,-22.86,;16.81,-23.76,;16.8,-25.3,;15.47,-26.07,;14.14,-25.3,;15.47,-27.61,;15.56,-22.85,;14.02,-22.85,;19.52,-23.34,;19.99,-24.8,;21.53,-24.79,;22.01,-23.34,;23.42,-22.71,;23.59,-21.18,;22.34,-20.27,;20.93,-20.9,;20.77,-22.43,;18.49,-20.15,;20.02,-20.32,;20.93,-19.08,;20.31,-17.67,;18.77,-17.51,;17.86,-18.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614342
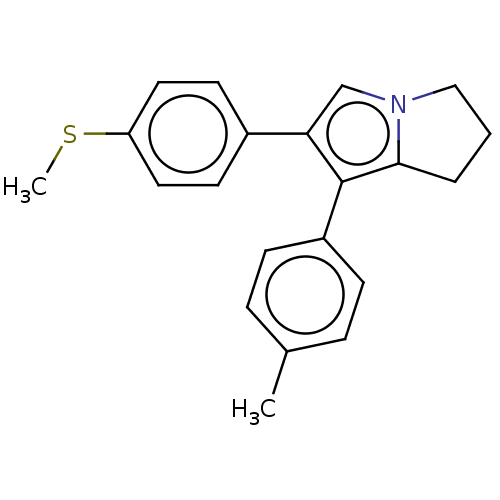
(CHEMBL5279593) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50614351
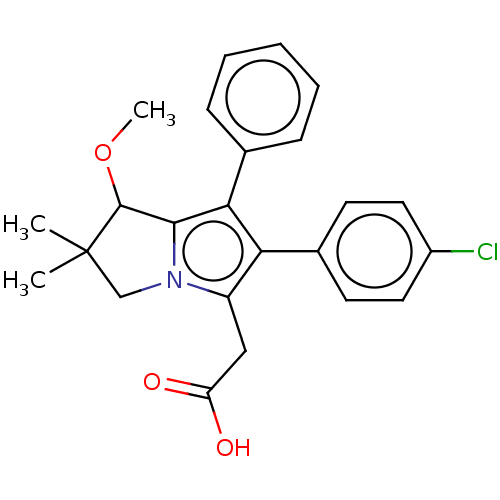
(CHEMBL5268642)Show SMILES COC1c2c(c(c(CC(O)=O)n2CC1(C)C)-c1ccc(Cl)cc1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614344

(CHEMBL5269592)Show SMILES CC1(C)Cn2c(CC(O)=O)c(c(c2C1=O)-c1ccccc1)-c1ccc(Cl)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614348

(CHEMBL5280353)Show SMILES CC1(C)Cc2c(c(-c3ccsc3)c(CC(O)=O)n2C1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50614352

(CHEMBL5277274)Show SMILES COC1c2c(c(c(C)n2CC1(C)C)-c1ccc(Cl)cc1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614351

(CHEMBL5268642)Show SMILES COC1c2c(c(c(CC(O)=O)n2CC1(C)C)-c1ccc(Cl)cc1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261639

(CHEMBL4076689)Show SMILES O=C(Nc1ccccc1)c1c(N=Cc2cccnc2)c(C#N)c2CCCn12 Show InChI InChI=1S/C21H17N5O/c22-12-17-18-9-5-11-26(18)20(21(27)25-16-7-2-1-3-8-16)19(17)24-14-15-6-4-10-23-13-15/h1-4,6-8,10,13-14H,5,9,11H2,(H,25,27)/b24-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50614356

(CHEMBL5270458) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261633

(CHEMBL4079032)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3ccc4ccccc4c3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C27H22N4O/c1-18-8-12-22(13-9-18)30-27(32)26-25(23(16-28)24-7-4-14-31(24)26)29-17-19-10-11-20-5-2-3-6-21(20)15-19/h2-3,5-6,8-13,15,17H,4,7,14H2,1H3,(H,30,32)/b29-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261638
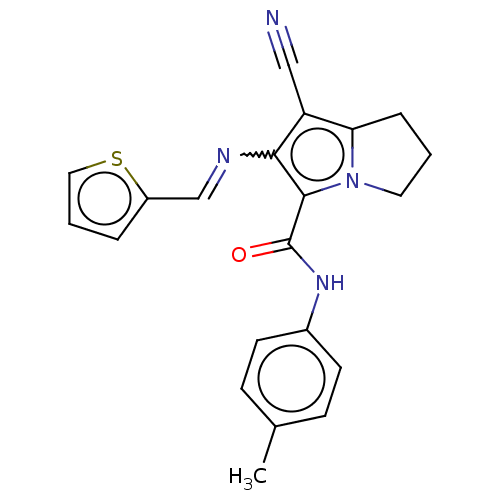
(CHEMBL4073779)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3cccs3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H18N4OS/c1-14-6-8-15(9-7-14)24-21(26)20-19(23-13-16-4-3-11-27-16)17(12-22)18-5-2-10-25(18)20/h3-4,6-9,11,13H,2,5,10H2,1H3,(H,24,26)/b23-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614347

(CHEMBL5284424)Show SMILES CC1(C)Cc2c(c(-c3ccco3)c(CC(O)=O)n2C1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261640

(CHEMBL4096865)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3cccnc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C22H19N5O/c1-15-6-8-17(9-7-15)26-22(28)21-20(25-14-16-4-2-10-24-13-16)18(12-23)19-5-3-11-27(19)21/h2,4,6-10,13-14H,3,5,11H2,1H3,(H,26,28)/b25-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50614340
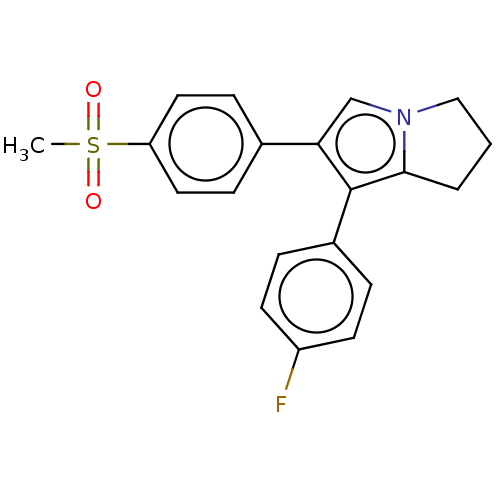
(CHEMBL5286930)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cn2CCCc2c1-c1ccc(F)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261634
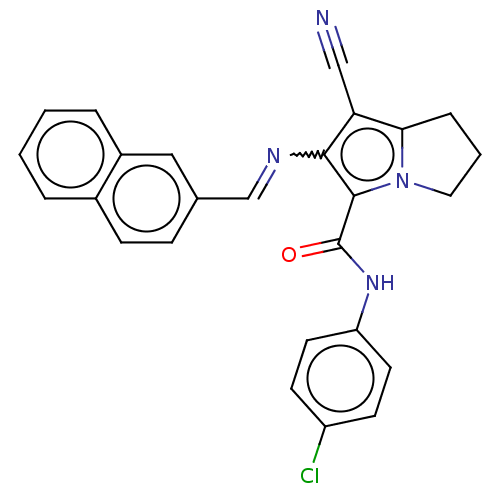
(CHEMBL4097045)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3ccc4ccccc4c3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C26H19ClN4O/c27-20-9-11-21(12-10-20)30-26(32)25-24(22(15-28)23-6-3-13-31(23)25)29-16-17-7-8-18-4-1-2-5-19(18)14-17/h1-2,4-5,7-12,14,16H,3,6,13H2,(H,30,32)/b29-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50038649
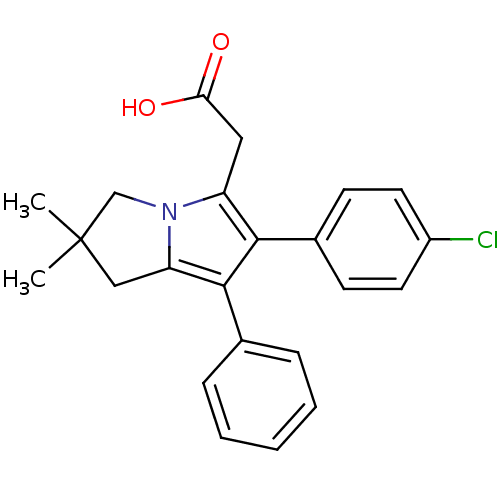
(2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...)Show SMILES CC1(C)Cc2c(c(c(CC(O)=O)n2C1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H22ClNO2/c1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23/h3-11H,12-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261643

(CHEMBL4071061)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3ccco3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H18N4O2/c1-14-6-8-15(9-7-14)24-21(26)20-19(23-13-16-4-3-11-27-16)17(12-22)18-5-2-10-25(18)20/h3-4,6-9,11,13H,2,5,10H2,1H3,(H,24,26)/b23-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614349

(CHEMBL5289423)Show SMILES CC1(C)Cc2c(c(-c3ccc(Cl)s3)c(CC(O)=O)n2C1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50038649

(2-(6-(4-chlorophenyl)-2,2-dimethyl-7-phenyl-2,3-di...)Show SMILES CC1(C)Cc2c(c(c(CC(O)=O)n2C1)-c1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C23H22ClNO2/c1-23(2)13-19-22(15-6-4-3-5-7-15)21(16-8-10-17(24)11-9-16)18(12-20(26)27)25(19)14-23/h3-11H,12-14H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50614345

(CHEMBL5277078)Show SMILES Cc1c(c(c2C(=O)C(C)(C)Cn12)-c1ccccc1)-c1ccc(Cl)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50614349

(CHEMBL5289423)Show SMILES CC1(C)Cc2c(c(-c3ccc(Cl)s3)c(CC(O)=O)n2C1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261644
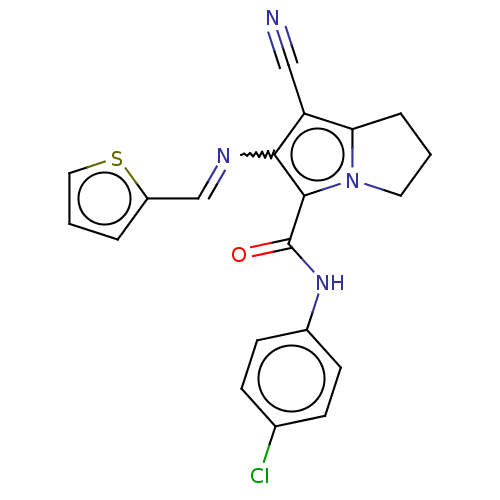
(CHEMBL4072244)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3cccs3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15ClN4OS/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261636

(CHEMBL4065185)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3ccco3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15ClN4O2/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50391240

(CHEBI:76228 | CHEMBL496240)Show InChI InChI=1S/C11H12N4O/c12-10-9(6-14-11(13)15-10)16-7-8-4-2-1-3-5-8/h1-6H,7H2,(H4,12,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614352

(CHEMBL5277274)Show SMILES COC1c2c(c(c(C)n2CC1(C)C)-c1ccc(Cl)cc1)-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261642

(CHEMBL4073566)Show SMILES O=C(Nc1ccccc1)c1c(N=Cc2ccc3ccccc3c2)c(C#N)c2CCCn12 Show InChI InChI=1S/C26H20N4O/c27-16-22-23-11-6-14-30(23)25(26(31)29-21-9-2-1-3-10-21)24(22)28-17-18-12-13-19-7-4-5-8-20(19)15-18/h1-5,7-10,12-13,15,17H,6,11,14H2,(H,29,31)/b28-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261637

(CHEMBL4092847)Show SMILES O=C(Nc1ccccc1)c1c(N=Cc2cccs2)c(C#N)c2CCCn12 Show InChI InChI=1S/C20H16N4OS/c21-12-16-17-9-4-10-24(17)19(18(16)22-13-15-8-5-11-26-15)20(25)23-14-6-2-1-3-7-14/h1-3,5-8,11,13H,4,9-10H2,(H,23,25)/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50504653
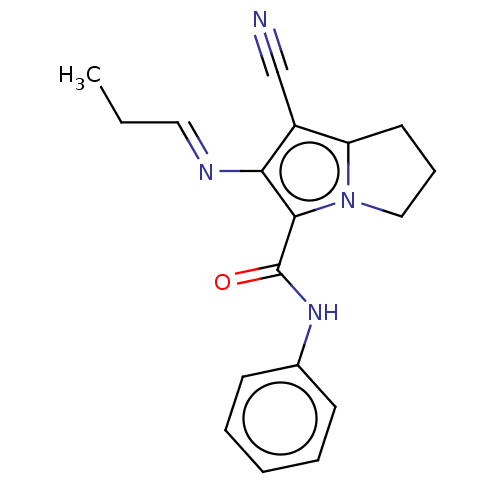
(CHEMBL4527978)Show InChI InChI=1S/C18H18N4O/c1-2-10-20-16-14(12-19)15-9-6-11-22(15)17(16)18(23)21-13-7-4-3-5-8-13/h3-5,7-8,10H,2,6,9,11H2,1H3,(H,21,23)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) incubated for 40 mins by ADP-glo assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50504651

(CHEMBL4471323)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H20N4O2S/c1-2-17-24(16(25)12-27-17)18-14(11-21)15-9-6-10-23(15)19(18)20(26)22-13-7-4-3-5-8-13/h3-5,7-8,17H,2,6,9-10,12H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) incubated for 40 mins by ADP-glo assay |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504639

(CHEMBL4557315)Show SMILES Oc1ccc(\C=N\c2c(C#N)c3CCCn3c2C(=O)Nc2ccc(Br)cc2)cc1 Show InChI InChI=1S/C22H17BrN4O2/c23-15-5-7-16(8-6-15)26-22(29)21-20(18(12-24)19-2-1-11-27(19)21)25-13-14-3-9-17(28)10-4-14/h3-10,13,28H,1-2,11H2,(H,26,29)/b25-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261641
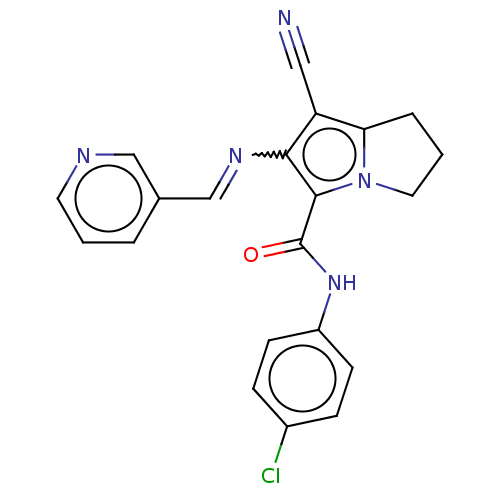
(CHEMBL4074351)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3cccnc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H16ClN5O/c22-15-5-7-16(8-6-15)26-21(28)20-19(25-13-14-3-1-9-24-12-14)17(11-23)18-4-2-10-27(18)20/h1,3,5-9,12-13H,2,4,10H2,(H,26,28)/b25-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614343

(CHEMBL597733)Show SMILES Cc1ccc(cc1)-c1c2CCCn2cc1-c1ccc(cc1)S(C)(=O)=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261635

(CHEMBL4091890)Show SMILES O=C(Nc1ccccc1)c1c(N=Cc2ccco2)c(C#N)c2CCCn12 Show InChI InChI=1S/C20H16N4O2/c21-12-16-17-9-4-10-24(17)19(18(16)22-13-15-8-5-11-26-15)20(25)23-14-6-2-1-3-7-14/h1-3,5-8,11,13H,4,9-10H2,(H,23,25)/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50614368
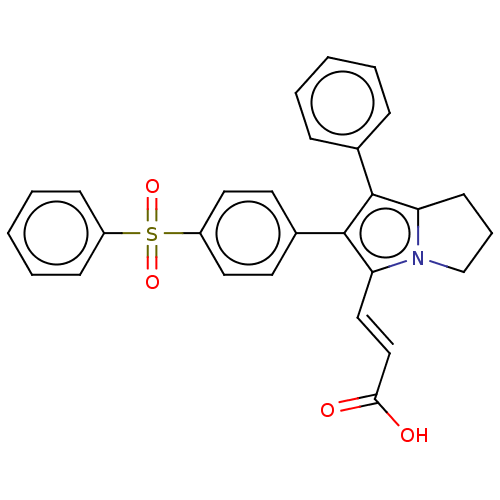
(CHEMBL5269149)Show SMILES OC(=O)\C=C\c1c(c(c2CCCn12)-c1ccccc1)-c1ccc(cc1)S(=O)(=O)c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50614342

(CHEMBL5279593) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614345

(CHEMBL5277078)Show SMILES Cc1c(c(c2C(=O)C(C)(C)Cn12)-c1ccccc1)-c1ccc(Cl)cc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50614350

(CHEMBL5274242) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50614350

(CHEMBL5274242) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50504640

(CHEMBL4528233)Show SMILES CCC1SCC(=O)N1c1c(C#N)c2CCCn2c1C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C20H19ClN4O2S/c1-2-17-25(16(26)11-28-17)18-14(10-22)15-4-3-9-24(15)19(18)20(27)23-13-7-5-12(21)6-8-13/h5-8,17H,2-4,9,11H2,1H3,(H,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Umm Al-Qura University
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 using arachidonic acid as substrate incubated for 2 mins by ELISA method |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111780
BindingDB Entry DOI: 10.7270/Q2B27ZJT |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM85511
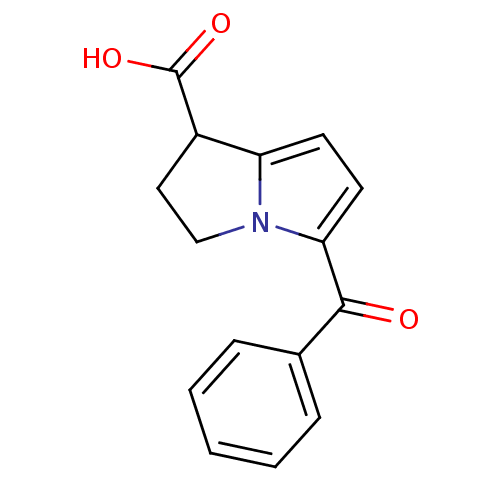
(CAS_74103-07-4 | KETOROLAC | Ketorolac tris salt |...)Show InChI InChI=1S/C15H13NO3/c17-14(10-4-2-1-3-5-10)13-7-6-12-11(15(18)19)8-9-16(12)13/h1-7,11H,8-9H2,(H,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data