 Found 64 hits with Last Name = 'metwaly' and Initial = 'am'
Found 64 hits with Last Name = 'metwaly' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50030474
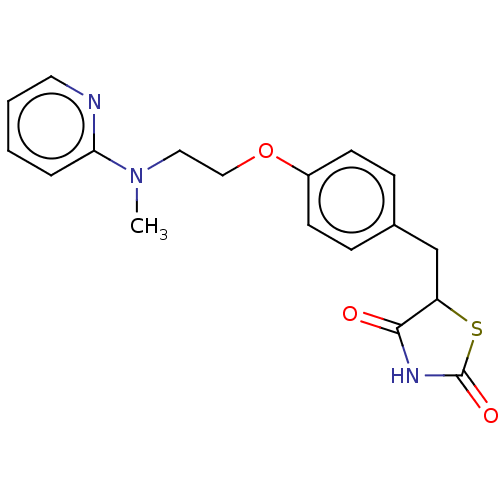
(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50030474

(Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,15H,10-12H2,1H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211660

(CHEMBL3915254)Show SMILES Cc1nc2ccccc2n(CC(=O)Nc2ccc(cc2)S(=O)(=O)NC(=S)NC2CCCCC2)c1=O Show InChI InChI=1S/C24H27N5O4S2/c1-16-23(31)29(21-10-6-5-9-20(21)25-16)15-22(30)26-18-11-13-19(14-12-18)35(32,33)28-24(34)27-17-7-3-2-4-8-17/h5-6,9-14,17H,2-4,7-8,15H2,1H3,(H,26,30)(H2,27,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269543

(CHEMBL4068985)Show SMILES COc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)S(=O)(=O)NC(=O)NC2CCCCC2)nc2ccccc2c1=O Show InChI InChI=1S/C30H31N5O6S2/c1-41-23-15-13-22(14-16-23)35-28(37)25-9-5-6-10-26(25)33-30(35)42-19-27(36)31-21-11-17-24(18-12-21)43(39,40)34-29(38)32-20-7-3-2-4-8-20/h5-6,9-18,20H,2-4,7-8,19H2,1H3,(H,31,36)(H2,32,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269553
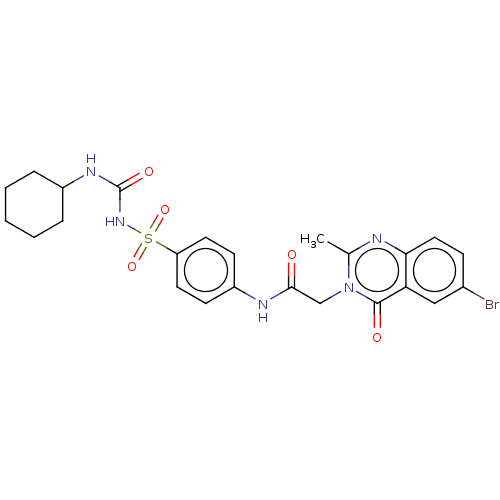
(CHEMBL4104828)Show SMILES Cc1nc2ccc(Br)cc2c(=O)n1CC(=O)Nc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C24H26BrN5O5S/c1-15-26-21-12-7-16(25)13-20(21)23(32)30(15)14-22(31)27-18-8-10-19(11-9-18)36(34,35)29-24(33)28-17-5-3-2-4-6-17/h7-13,17H,2-6,14H2,1H3,(H,27,31)(H2,28,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211669
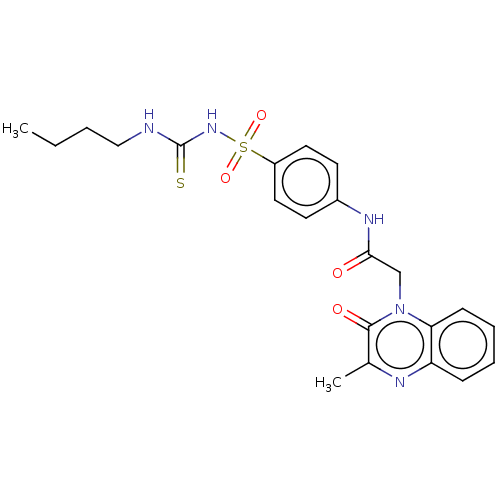
(CHEMBL3943220)Show SMILES CCCCNC(=S)NS(=O)(=O)c1ccc(NC(=O)Cn2c3ccccc3nc(C)c2=O)cc1 Show InChI InChI=1S/C22H25N5O4S2/c1-3-4-13-23-22(32)26-33(30,31)17-11-9-16(10-12-17)25-20(28)14-27-19-8-6-5-7-18(19)24-15(2)21(27)29/h5-12H,3-4,13-14H2,1-2H3,(H,25,28)(H2,23,26,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269548

(CHEMBL4069917)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccc(NC(=O)CSc2nc3ccccc3c(=O)n2-c2ccc(OC)cc2)cc1 Show InChI InChI=1S/C28H29N5O6S2/c1-3-4-17-29-27(36)32-41(37,38)22-15-9-19(10-16-22)30-25(34)18-40-28-31-24-8-6-5-7-23(24)26(35)33(28)20-11-13-21(39-2)14-12-20/h5-16H,3-4,17-18H2,1-2H3,(H,30,34)(H2,29,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269511

(CHEMBL4072706)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)S(=O)(=O)NC(=O)Nc2ccccc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H25N5O5S2/c1-20-11-15-23(16-12-20)35-28(37)25-9-5-6-10-26(25)33-30(35)41-19-27(36)31-22-13-17-24(18-14-22)42(39,40)34-29(38)32-21-7-3-2-4-8-21/h2-18H,19H2,1H3,(H,31,36)(H2,32,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269510

(CHEMBL4085687)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)S(=O)(=O)NC(=O)NC2CCCCC2)nc2ccccc2c1=O Show InChI InChI=1S/C30H31N5O5S2/c1-20-11-15-23(16-12-20)35-28(37)25-9-5-6-10-26(25)33-30(35)41-19-27(36)31-22-13-17-24(18-14-22)42(39,40)34-29(38)32-21-7-3-2-4-8-21/h5-6,9-18,21H,2-4,7-8,19H2,1H3,(H,31,36)(H2,32,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269552
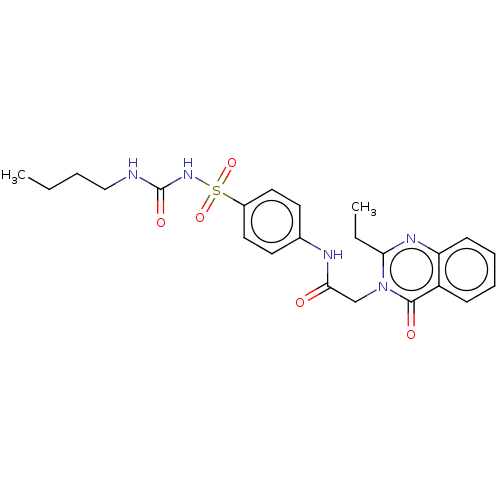
(CHEMBL4080597)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccc(NC(=O)Cn2c(CC)nc3ccccc3c2=O)cc1 Show InChI InChI=1S/C23H27N5O5S/c1-3-5-14-24-23(31)27-34(32,33)17-12-10-16(11-13-17)25-21(29)15-28-20(4-2)26-19-9-7-6-8-18(19)22(28)30/h6-13H,3-5,14-15H2,1-2H3,(H,25,29)(H2,24,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269542
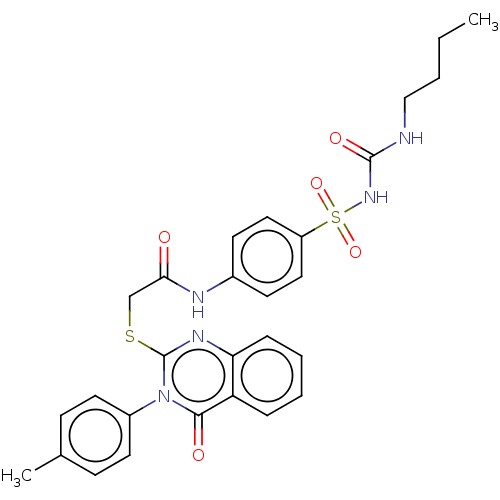
(CHEMBL4093428)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccc(NC(=O)CSc2nc3ccccc3c(=O)n2-c2ccc(C)cc2)cc1 Show InChI InChI=1S/C28H29N5O5S2/c1-3-4-17-29-27(36)32-40(37,38)22-15-11-20(12-16-22)30-25(34)18-39-28-31-24-8-6-5-7-23(24)26(35)33(28)21-13-9-19(2)10-14-21/h5-16H,3-4,17-18H2,1-2H3,(H,30,34)(H2,29,32,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 434 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549123
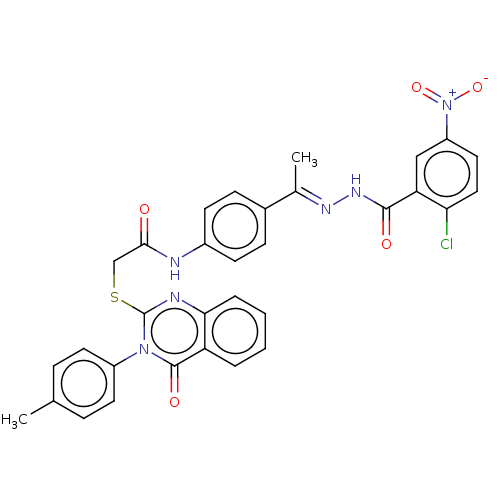
(CHEMBL4779272)Show SMILES C\C(=N/NC(=O)c1cc(ccc1Cl)[N+]([O-])=O)c1ccc(NC(=O)CSc2nc3ccccc3c(=O)n2-c2ccc(C)cc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 452 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269549
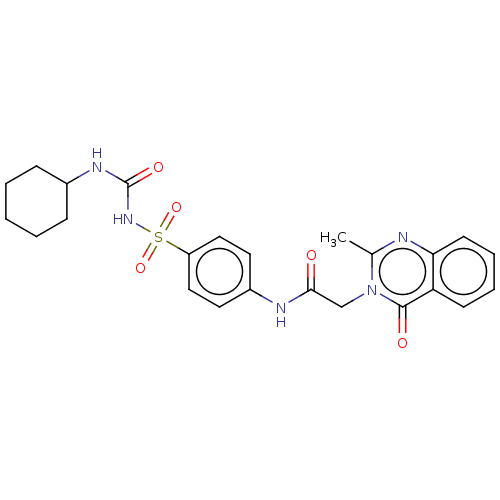
(CHEMBL4097121)Show SMILES Cc1nc2ccccc2c(=O)n1CC(=O)Nc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C24H27N5O5S/c1-16-25-21-10-6-5-9-20(21)23(31)29(16)15-22(30)26-18-11-13-19(14-12-18)35(33,34)28-24(32)27-17-7-3-2-4-8-17/h5-6,9-14,17H,2-4,7-8,15H2,1H3,(H,26,30)(H2,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 464 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211676
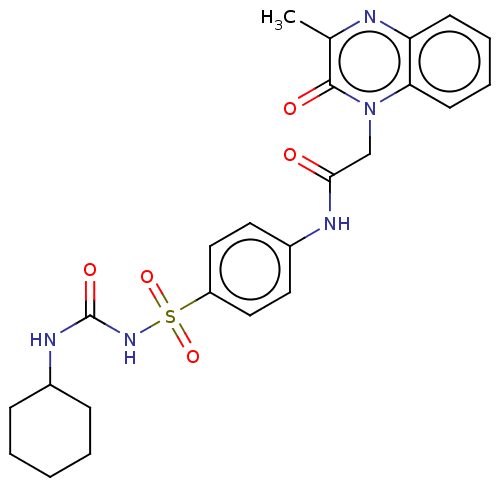
(CHEMBL3899514)Show SMILES Cc1nc2ccccc2n(CC(=O)Nc2ccc(cc2)S(=O)(=O)NC(=O)NC2CCCCC2)c1=O Show InChI InChI=1S/C24H27N5O5S/c1-16-23(31)29(21-10-6-5-9-20(21)25-16)15-22(30)26-18-11-13-19(14-12-18)35(33,34)28-24(32)27-17-7-3-2-4-8-17/h5-6,9-14,17H,2-4,7-8,15H2,1H3,(H,26,30)(H2,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 482 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211656
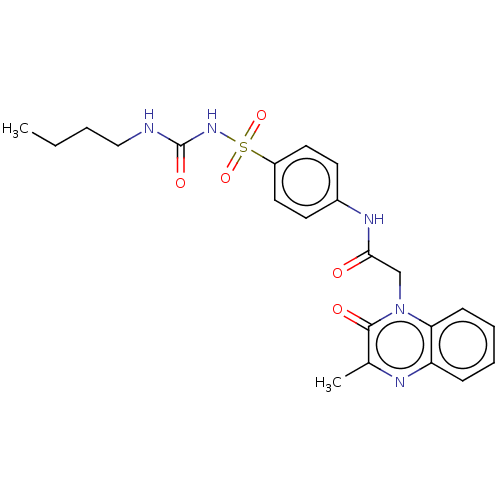
(CHEMBL3959627)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccc(NC(=O)Cn2c3ccccc3nc(C)c2=O)cc1 Show InChI InChI=1S/C22H25N5O5S/c1-3-4-13-23-22(30)26-33(31,32)17-11-9-16(10-12-17)25-20(28)14-27-19-8-6-5-7-18(19)24-15(2)21(27)29/h5-12H,3-4,13-14H2,1-2H3,(H,25,28)(H2,23,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 491 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269551
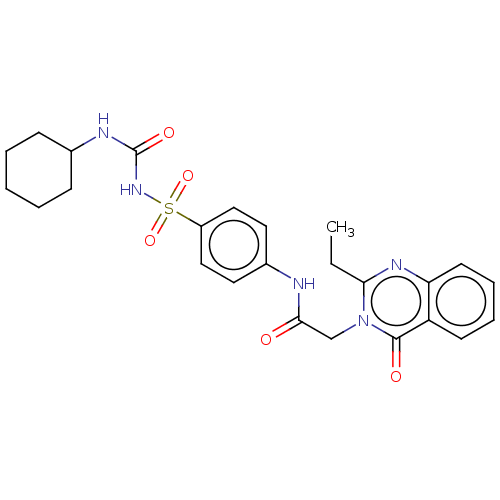
(CHEMBL4088467)Show SMILES CCc1nc2ccccc2c(=O)n1CC(=O)Nc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C25H29N5O5S/c1-2-22-28-21-11-7-6-10-20(21)24(32)30(22)16-23(31)26-18-12-14-19(15-13-18)36(34,35)29-25(33)27-17-8-4-3-5-9-17/h6-7,10-15,17H,2-5,8-9,16H2,1H3,(H,26,31)(H2,27,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468489
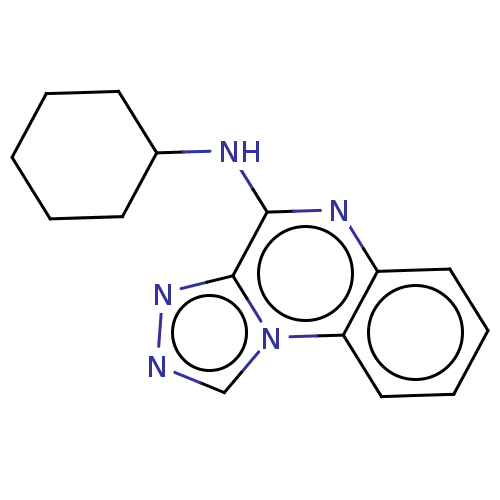
(CHEMBL1305588)Show InChI InChI=1S/C15H17N5/c1-2-6-11(7-3-1)17-14-15-19-16-10-20(15)13-9-5-4-8-12(13)18-14/h4-5,8-11H,1-3,6-7H2,(H,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549119

(CHEMBL4763393)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)C(=O)Nc2ccc(Cl)cc2)nc2ccccc2c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 684 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549115

(CHEMBL4784161)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)S(=O)(=O)Nc2nc(C)cc(C)n2)nc2ccccc2c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 707 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549116
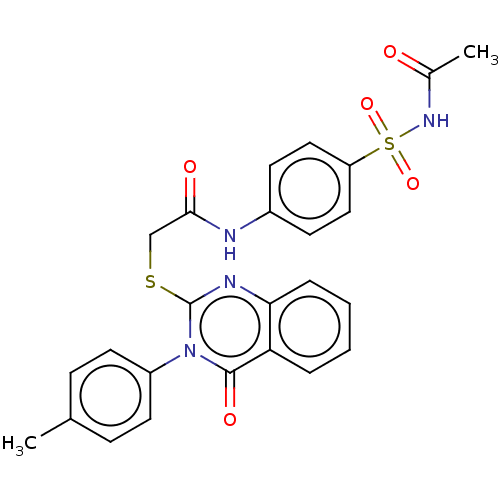
(CHEMBL4781567)Show SMILES CC(=O)NS(=O)(=O)c1ccc(NC(=O)CSc2nc3ccccc3c(=O)n2-c2ccc(C)cc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468484

(CHEMBL4288104)Show SMILES COc1cccc(NC(=O)CSc2nnc3c4nncn4c4ccccc4n23)c1 Show InChI InChI=1S/C19H15N7O2S/c1-28-13-6-4-5-12(9-13)21-16(27)10-29-19-24-23-18-17-22-20-11-25(17)14-7-2-3-8-15(14)26(18)19/h2-9,11H,10H2,1H3,(H,21,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468485

(CHEMBL4286850)Show SMILES Fc1ccc(NC(=O)CSc2nnc3c4nncn4c4ccccc4n23)cc1 Show InChI InChI=1S/C18H12FN7OS/c19-11-5-7-12(8-6-11)21-15(27)9-28-18-24-23-17-16-22-20-10-25(16)13-3-1-2-4-14(13)26(17)18/h1-8,10H,9H2,(H,21,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269550
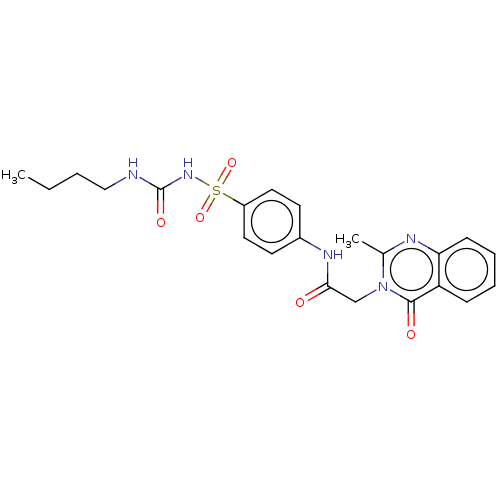
(CHEMBL4089408)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccc(NC(=O)Cn2c(C)nc3ccccc3c2=O)cc1 Show InChI InChI=1S/C22H25N5O5S/c1-3-4-13-23-22(30)26-33(31,32)17-11-9-16(10-12-17)25-20(28)14-27-15(2)24-19-8-6-5-7-18(19)21(27)29/h5-12H,3-4,13-14H2,1-2H3,(H,25,28)(H2,23,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549122

(CHEMBL4794044)Show SMILES C\C(=N/NC(N)=S)c1ccc(NC(=O)CSc2nc3ccccc3c(=O)n2-c2ccc(C)cc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549124
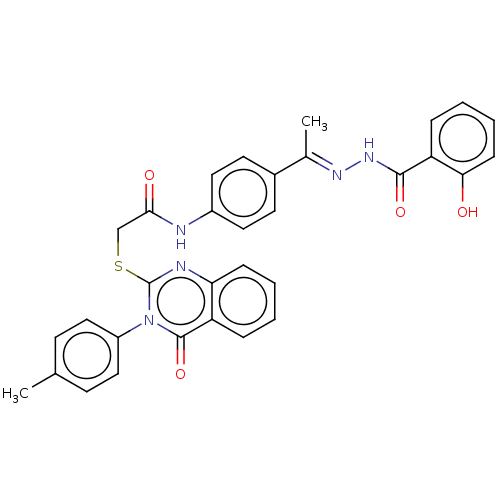
(CHEMBL4777885)Show SMILES C\C(=N/NC(=O)c1ccccc1O)c1ccc(NC(=O)CSc2nc3ccccc3c(=O)n2-c2ccc(C)cc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468490
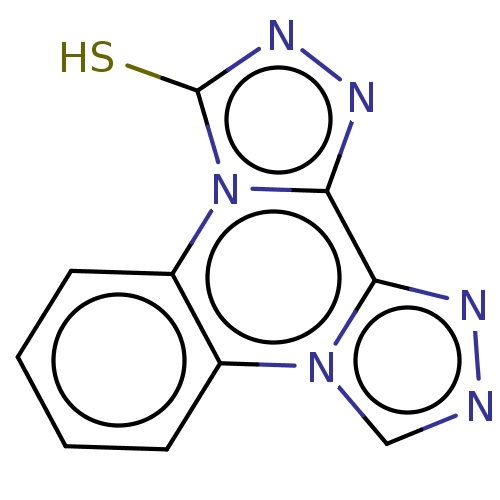
(CHEMBL4291398)Show InChI InChI=1S/C10H6N6S/c17-10-14-13-9-8-12-11-5-15(8)6-3-1-2-4-7(6)16(9)10/h1-5H,(H,14,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549117
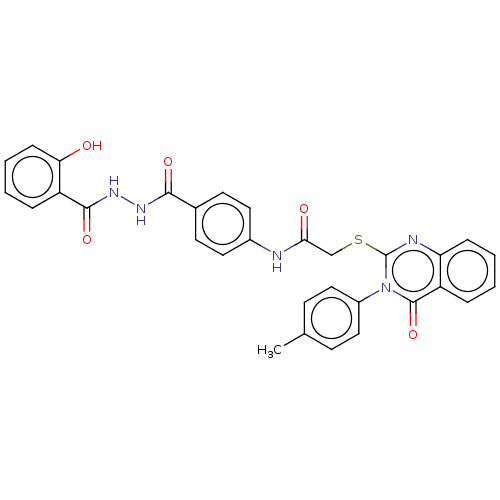
(CHEMBL4749461)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)C(=O)NNC(=O)c2ccccc2O)nc2ccccc2c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211677
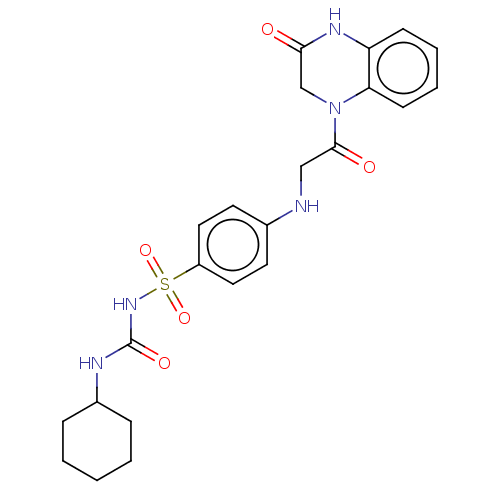
(CHEMBL3977442)Show SMILES O=C(NC1CCCCC1)NS(=O)(=O)c1ccc(NCC(=O)N2CC(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C23H27N5O5S/c29-21-15-28(20-9-5-4-8-19(20)26-21)22(30)14-24-16-10-12-18(13-11-16)34(32,33)27-23(31)25-17-6-2-1-3-7-17/h4-5,8-13,17,24H,1-3,6-7,14-15H2,(H,26,29)(H2,25,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211659
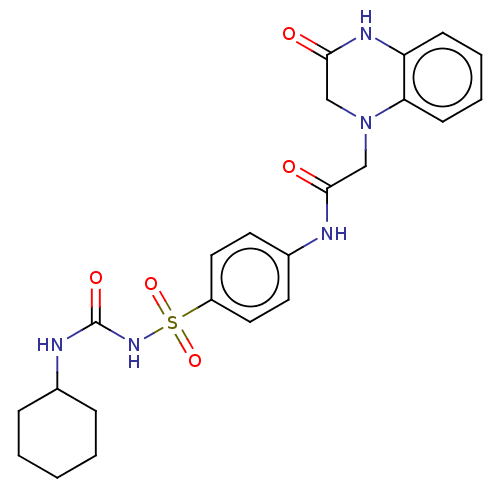
(CHEMBL3949291)Show SMILES O=C(CN1CC(=O)Nc2ccccc12)Nc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C23H27N5O5S/c29-21(14-28-15-22(30)26-19-8-4-5-9-20(19)28)24-17-10-12-18(13-11-17)34(32,33)27-23(31)25-16-6-2-1-3-7-16/h4-5,8-13,16H,1-3,6-7,14-15H2,(H,24,29)(H,26,30)(H2,25,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468486

(CHEMBL4281274)Show InChI InChI=1S/C16H13N7O/c24-16(18-11-6-2-1-3-7-11)22-20-14-15-21-17-10-23(15)13-9-5-4-8-12(13)19-14/h1-10H,(H,19,20)(H2,18,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549118
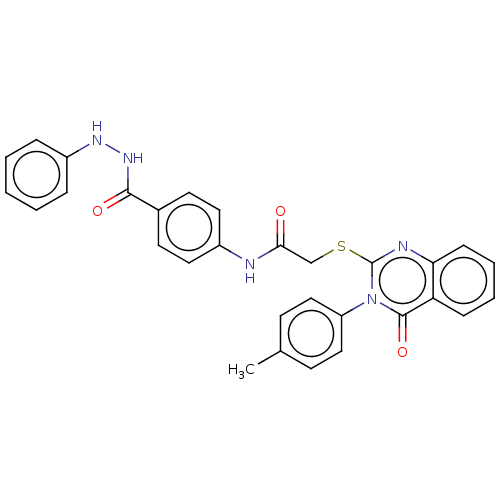
(CHEMBL4753486)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)C(=O)NNc2ccccc2)nc2ccccc2c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468482
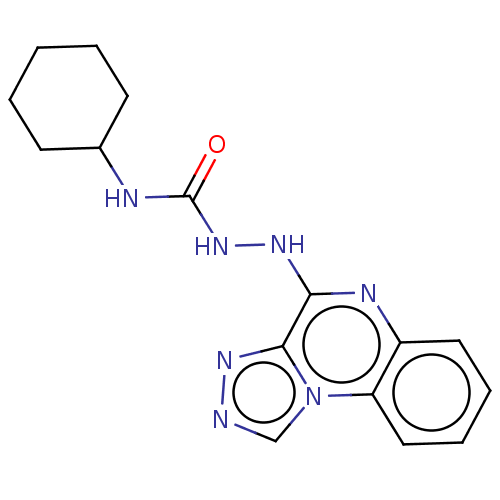
(CHEMBL4291890)Show InChI InChI=1S/C16H19N7O/c24-16(18-11-6-2-1-3-7-11)22-20-14-15-21-17-10-23(15)13-9-5-4-8-12(13)19-14/h4-5,8-11H,1-3,6-7H2,(H,19,20)(H2,18,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211658
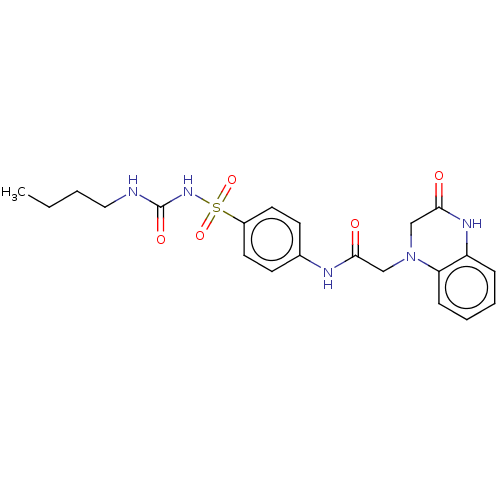
(CHEMBL3986626)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccc(NC(=O)CN2CC(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H25N5O5S/c1-2-3-12-22-21(29)25-32(30,31)16-10-8-15(9-11-16)23-19(27)13-26-14-20(28)24-17-6-4-5-7-18(17)26/h4-11H,2-3,12-14H2,1H3,(H,23,27)(H,24,28)(H2,22,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50211657
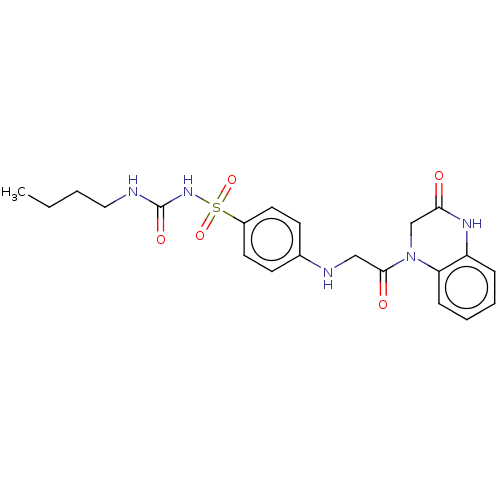
(CHEMBL3941555)Show SMILES CCCCNC(=O)NS(=O)(=O)c1ccc(NCC(=O)N2CC(=O)Nc3ccccc23)cc1 Show InChI InChI=1S/C21H25N5O5S/c1-2-3-12-22-21(29)25-32(30,31)16-10-8-15(9-11-16)23-13-20(28)26-14-19(27)24-17-6-4-5-7-18(17)26/h4-11,23H,2-3,12-14H2,1H3,(H,24,27)(H2,22,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma Green from recombinant human N-terminal GST-tagged PPARgamma-LBD by fluorescence polarization assay |
Bioorg Med Chem 25: 1496-1513 (2017)
Article DOI: 10.1016/j.bmc.2017.01.015
BindingDB Entry DOI: 10.7270/Q2H13463 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549120
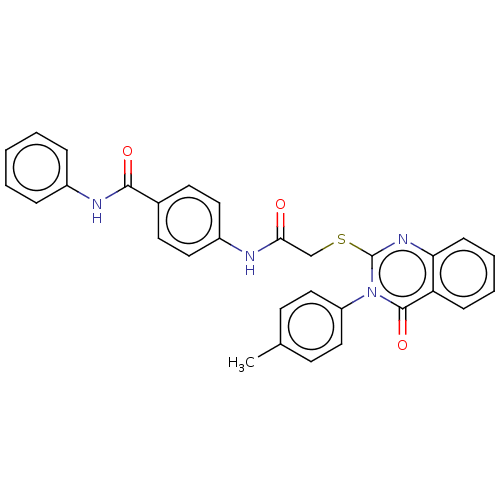
(CHEMBL4760160)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)C(=O)Nc2ccccc2)nc2ccccc2c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549121
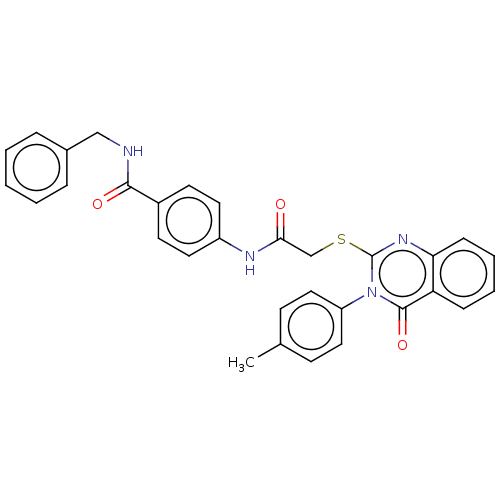
(CHEMBL4749856)Show SMILES Cc1ccc(cc1)-n1c(SCC(=O)Nc2ccc(cc2)C(=O)NCc2ccccc2)nc2ccccc2c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269512
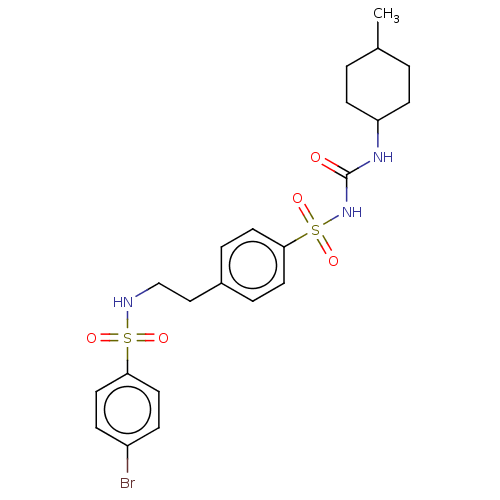
(CHEMBL491147)Show SMILES CC1CCC(CC1)NC(=O)NS(=O)(=O)c1ccc(CCNS(=O)(=O)c2ccc(Br)cc2)cc1 |(5.02,-22.18,;3.69,-21.41,;2.35,-22.18,;1.02,-21.41,;1.02,-19.87,;2.35,-19.1,;3.69,-19.87,;-.31,-19.1,;-.31,-17.56,;1.02,-16.79,;-1.65,-16.79,;-1.65,-15.25,;-.11,-15.25,;-3.19,-15.25,;-1.65,-13.71,;-.31,-12.94,;-.31,-11.4,;-1.65,-10.63,;-1.65,-9.09,;-.31,-8.32,;-.31,-6.78,;1.02,-6.01,;1.79,-7.35,;.25,-4.68,;2.35,-5.24,;2.35,-3.7,;3.69,-2.93,;5.02,-3.7,;6.35,-2.93,;5.02,-5.24,;3.69,-6.01,;-2.98,-11.4,;-2.98,-12.94,)| Show InChI InChI=1S/C22H28BrN3O5S2/c1-16-2-8-19(9-3-16)25-22(27)26-33(30,31)21-10-4-17(5-11-21)14-15-24-32(28,29)20-12-6-18(23)7-13-20/h4-7,10-13,16,19,24H,2-3,8-9,14-15H2,1H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50549125
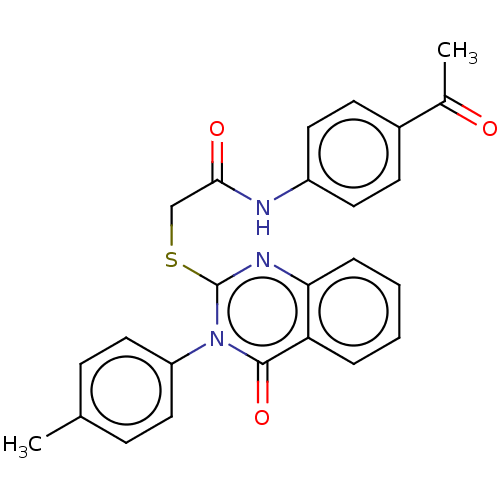
(CHEMBL4755652)Show SMILES CC(=O)c1ccc(NC(=O)CSc2nc3ccccc3c(=O)n2-c2ccc(C)cc2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human VEGFR2 incubated for 2.5 hrs by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115872
BindingDB Entry DOI: 10.7270/Q2KH0RZQ |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468480
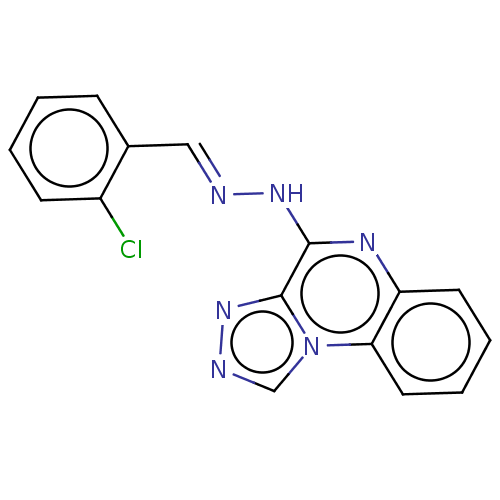
(CHEMBL4282332)Show InChI InChI=1S/C16H11ClN6/c17-12-6-2-1-5-11(12)9-18-21-15-16-22-19-10-23(16)14-8-4-3-7-13(14)20-15/h1-10H,(H,20,21)/b18-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468479
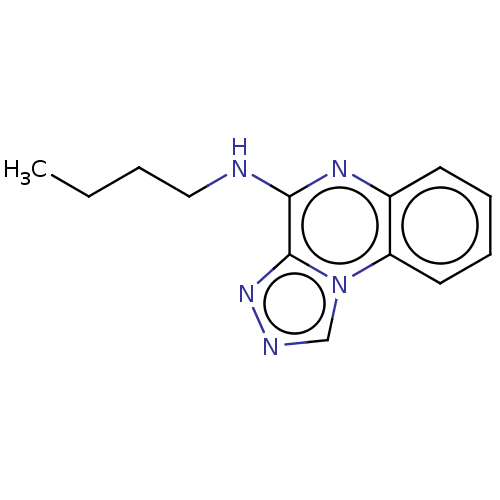
(CHEMBL4280810)Show InChI InChI=1S/C13H15N5/c1-2-3-8-14-12-13-17-15-9-18(13)11-7-5-4-6-10(11)16-12/h4-7,9H,2-3,8H2,1H3,(H,14,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50015825
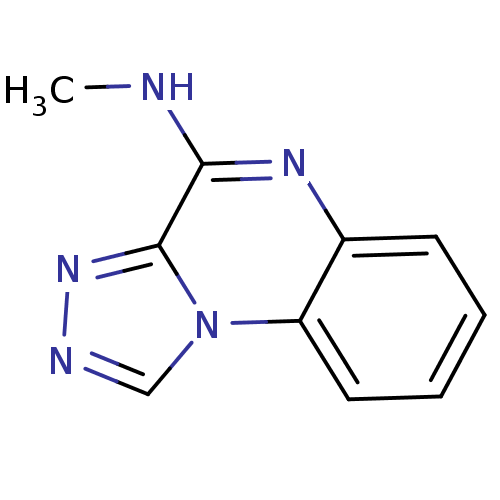
(CHEMBL73133 | Methyl-[1,2,4]triazolo[4,3-a]quinoxa...)Show InChI InChI=1S/C10H9N5/c1-11-9-10-14-12-6-15(10)8-5-3-2-4-7(8)13-9/h2-6H,1H3,(H,11,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468483
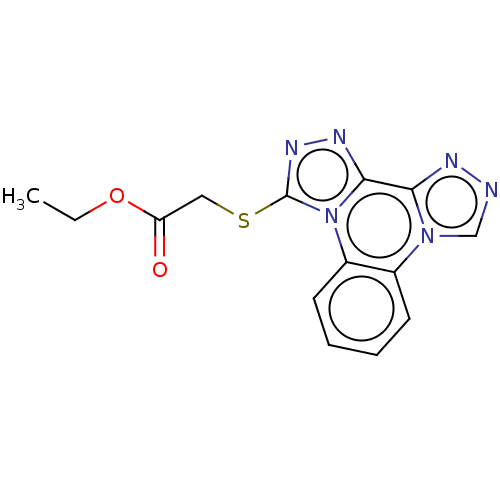
(CHEMBL4288069)Show InChI InChI=1S/C14H12N6O2S/c1-2-22-11(21)7-23-14-18-17-13-12-16-15-8-19(12)9-5-3-4-6-10(9)20(13)14/h3-6,8H,2,7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269524
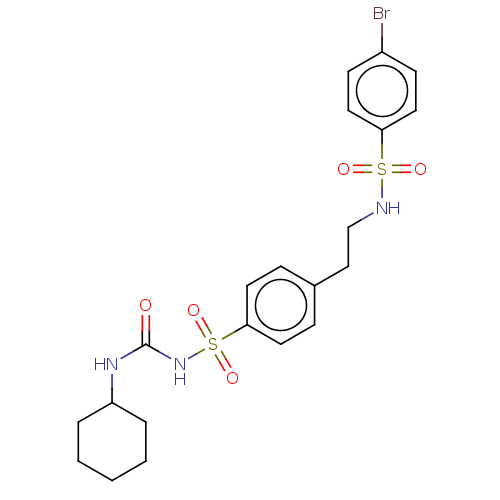
(CHEMBL491501)Show SMILES Brc1ccc(cc1)S(=O)(=O)NCCc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C21H26BrN3O5S2/c22-17-8-12-19(13-9-17)31(27,28)23-15-14-16-6-10-20(11-7-16)32(29,30)25-21(26)24-18-4-2-1-3-5-18/h6-13,18,23H,1-5,14-15H2,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269514
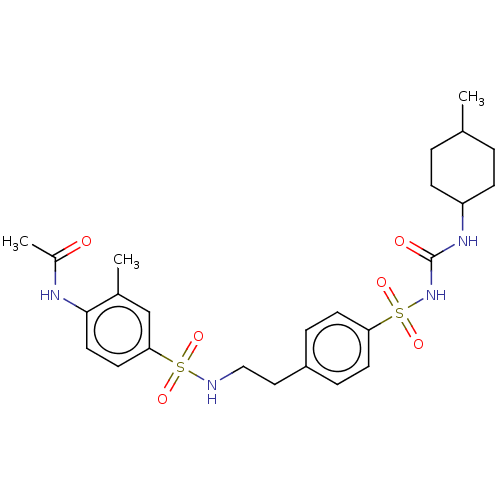
(CHEMBL491924)Show SMILES CC1CCC(CC1)NC(=O)NS(=O)(=O)c1ccc(CCNS(=O)(=O)c2ccc(NC(C)=O)c(C)c2)cc1 |(4.41,-17.91,;3.08,-17.14,;3.08,-15.6,;1.75,-14.83,;.41,-15.6,;.41,-17.14,;1.75,-17.91,;-.92,-14.83,;-.92,-13.29,;.41,-12.52,;-2.26,-12.52,;-2.26,-10.98,;-.72,-10.98,;-3.8,-10.98,;-2.26,-9.44,;-.92,-8.67,;-.92,-7.13,;-2.26,-6.36,;-2.26,-4.82,;-.92,-4.05,;-.92,-2.51,;.41,-1.74,;1.18,-3.07,;-.36,-.4,;1.75,-.97,;1.75,.57,;3.08,1.34,;4.41,.57,;5.75,1.34,;7.08,.57,;8.41,1.34,;7.08,-.97,;4.41,-.97,;5.75,-1.74,;3.08,-1.74,;-3.59,-7.13,;-3.59,-8.67,)| Show InChI InChI=1S/C25H34N4O6S2/c1-17-4-8-21(9-5-17)28-25(31)29-37(34,35)22-10-6-20(7-11-22)14-15-26-36(32,33)23-12-13-24(18(2)16-23)27-19(3)30/h6-7,10-13,16-17,21,26H,4-5,8-9,14-15H2,1-3H3,(H,27,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468488

(CHEMBL4290204)Show InChI InChI=1S/C16H10Cl2N6/c17-11-4-3-5-12(18)10(11)8-19-22-15-16-23-20-9-24(16)14-7-2-1-6-13(14)21-15/h1-9H,(H,21,22)/b19-8+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha
(Homo sapiens (Human)) | BDBM50468481
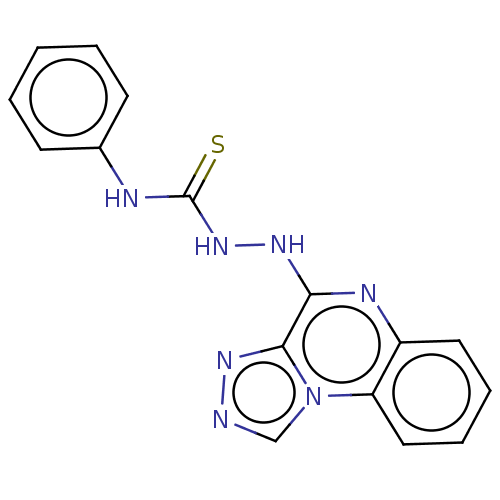
(CHEMBL4276735)Show InChI InChI=1S/C16H13N7S/c24-16(18-11-6-2-1-3-7-11)22-20-14-15-21-17-10-23(15)13-9-5-4-8-12(13)19-14/h1-10H,(H,19,20)(H2,18,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Al-Azhar University
Curated by ChEMBL
| Assay Description
Inhibition of human topoisomerase 2 using supercoiled pHOT1 DNA as substrate after 30 mins by agarose gel electrophoresis |
Eur J Med Chem 155: 117-134 (2018)
Article DOI: 10.1016/j.ejmech.2018.06.004
BindingDB Entry DOI: 10.7270/Q2TT4TN9 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269523
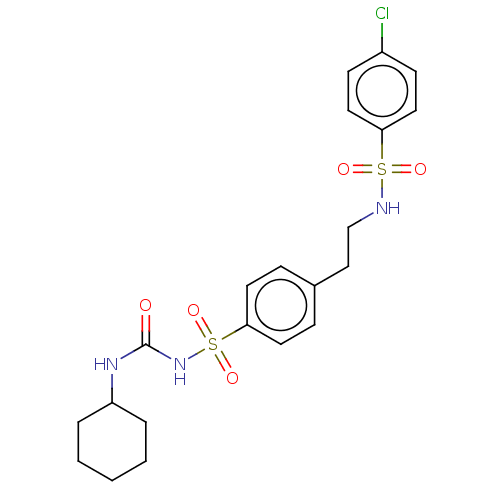
(CHEMBL491500)Show SMILES Clc1ccc(cc1)S(=O)(=O)NCCc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C21H26ClN3O5S2/c22-17-8-12-19(13-9-17)31(27,28)23-15-14-16-6-10-20(11-7-16)32(29,30)25-21(26)24-18-4-2-1-3-5-18/h6-13,18,23H,1-5,14-15H2,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50269522
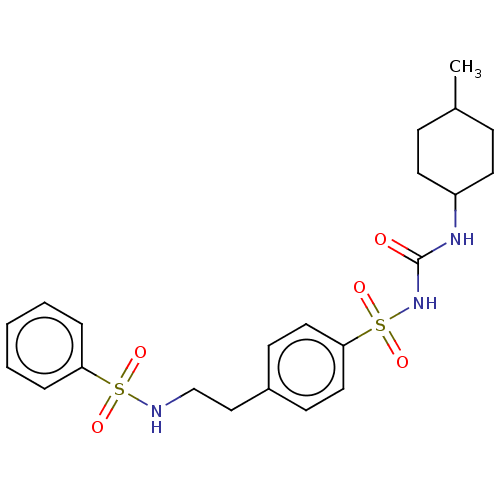
(CHEMBL491324)Show SMILES CC1CCC(CC1)NC(=O)NS(=O)(=O)c1ccc(CCNS(=O)(=O)c2ccccc2)cc1 |(4.45,-33.33,;3.11,-32.56,;1.78,-33.33,;.45,-32.56,;.45,-31.02,;1.78,-30.25,;3.11,-31.02,;-.89,-30.25,;-.89,-28.71,;.45,-27.94,;-2.22,-27.94,;-2.22,-26.4,;-3.76,-26.4,;-.68,-26.4,;-2.22,-24.86,;-.89,-24.09,;-.89,-22.55,;-2.22,-21.78,;-2.22,-20.24,;-.89,-19.47,;-.89,-17.93,;.45,-17.16,;-.32,-15.83,;1.22,-18.49,;1.78,-16.39,;1.78,-14.85,;3.11,-14.08,;4.45,-14.85,;4.45,-16.39,;3.11,-17.16,;-3.55,-22.55,;-3.55,-24.09,)| Show InChI InChI=1S/C22H29N3O5S2/c1-17-7-11-19(12-8-17)24-22(26)25-32(29,30)21-13-9-18(10-14-21)15-16-23-31(27,28)20-5-3-2-4-6-20/h2-6,9-10,13-14,17,19,23H,7-8,11-12,15-16H2,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Chemistry Department, Faculty of Pharmacy (Boys), Al-Azhar University, Cairo 11884, Egypt.
Curated by ChEMBL
| Assay Description
Displacement of fluormone-PPARgamma green from GST-tagged PPARgamma-LBD (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem 25: 4723-4744 (2017)
Article DOI: 10.1016/j.bmc.2017.07.015
BindingDB Entry DOI: 10.7270/Q2DZ0BTW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data