 Found 697 hits with Last Name = 'pham' and Initial = 'at'
Found 697 hits with Last Name = 'pham' and Initial = 'at' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5'-nucleotidase
(Homo sapiens (Human)) | BDBM50527134

(CHEMBL4471306 | US20230295213, Compound a)Show SMILES C[C@H](Nc1cc(Cl)nc2n(ncc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O)c1ccccc1F |r| Show InChI InChI=1S/C20H24ClFN4O9P2/c1-10(11-4-2-3-5-13(11)22)24-14-6-16(21)25-19-12(14)7-23-26(19)20-18(28)17(27)15(35-20)8-34-37(32,33)9-36(29,30)31/h2-7,10,15,17-18,20,27-28H,8-9H2,1H3,(H,24,25)(H,32,33)(H2,29,30,31)/t10-,15+,17+,18+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602541
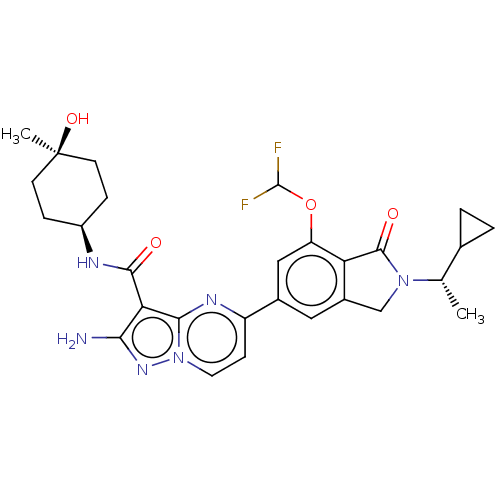
(CHEMBL5209268)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(OC(F)F)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@](C)(O)CC3)c2n1 |r,wU:30.32,1.1,33.37,(7.66,2.89,;6.89,1.55,;7.66,.22,;7.66,-1.32,;8.99,-.55,;5.35,1.55,;4.44,2.8,;2.98,2.32,;1.65,3.09,;.31,2.32,;.31,.78,;1.65,.01,;1.65,-1.53,;.32,-2.3,;.32,-3.84,;-1.02,-1.53,;2.98,.78,;4.44,.31,;4.84,-1.18,;-1.02,3.09,;-1.02,4.63,;-2.34,5.39,;-3.67,4.63,;-5.14,5.1,;-6.04,3.86,;-7.58,3.86,;-5.14,2.61,;-5.91,1.28,;-7.45,1.28,;-5.14,-.06,;-5.91,-1.39,;-5.14,-2.72,;-5.91,-4.06,;-7.45,-4.06,;-8.22,-5.39,;-8.99,-4.06,;-8.22,-2.72,;-7.45,-1.39,;-3.67,3.09,;-2.34,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
5'-nucleotidase
(Homo sapiens (Human)) | BDBM18136
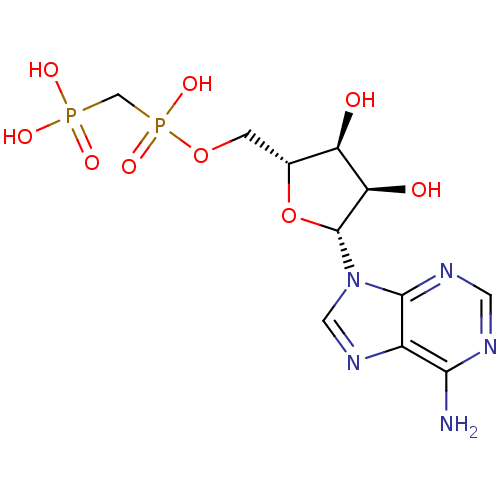
(ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)CP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H17N5O9P2/c12-9-6-10(14-2-13-9)16(3-15-6)11-8(18)7(17)5(25-11)1-24-27(22,23)4-26(19,20)21/h2-3,5,7-8,11,17-18H,1,4H2,(H,22,23)(H2,12,13,14)(H2,19,20,21)/t5-,7-,8-,11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CD73 |
J Med Chem 63: 3935-3955 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01713
BindingDB Entry DOI: 10.7270/Q2G1648T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602535
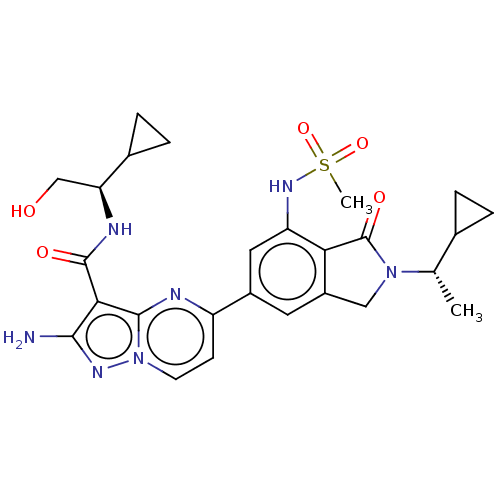
(CHEMBL5171421)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@@H](CO)C3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602525
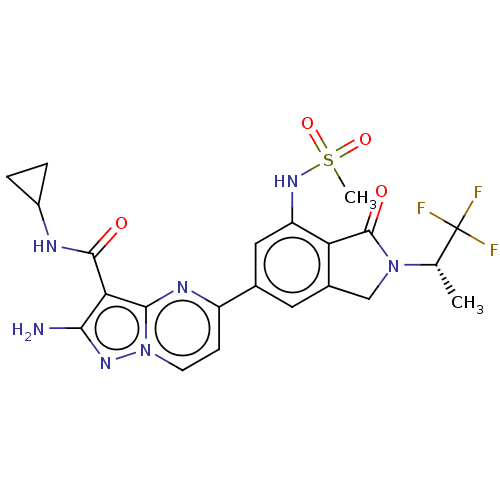
(CHEMBL5174699)Show SMILES C[C@H](N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602536
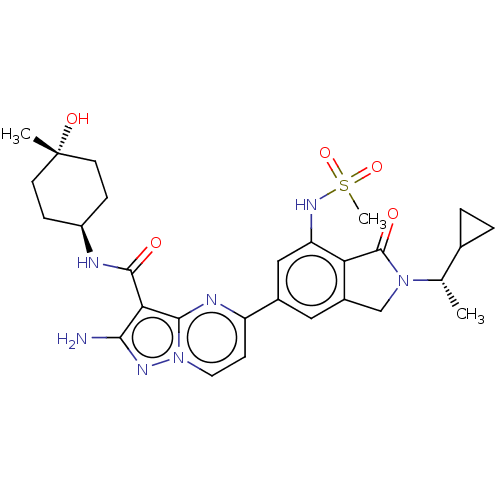
(CHEMBL5206549)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@](C)(O)CC3)c2n1 |r,wU:31.33,1.1,wD:34.38,(7.66,2.89,;6.89,1.55,;7.66,.22,;7.66,-1.32,;8.99,-.55,;5.35,1.55,;4.44,2.8,;2.98,2.32,;1.65,3.09,;.31,2.32,;.31,.78,;1.65,.01,;1.65,-1.53,;.32,-2.3,;.32,-3.84,;-1.02,-1.53,;.32,-.76,;2.98,.78,;4.44,.31,;4.84,-1.18,;-1.02,3.09,;-1.02,4.63,;-2.34,5.39,;-3.67,4.63,;-5.14,5.1,;-6.04,3.86,;-7.58,3.86,;-5.14,2.61,;-5.91,1.28,;-7.45,1.28,;-5.14,-.06,;-5.91,-1.39,;-5.14,-2.72,;-5.91,-4.06,;-7.45,-4.06,;-8.22,-5.39,;-8.99,-4.06,;-8.22,-2.72,;-7.45,-1.39,;-3.67,3.09,;-2.34,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602531
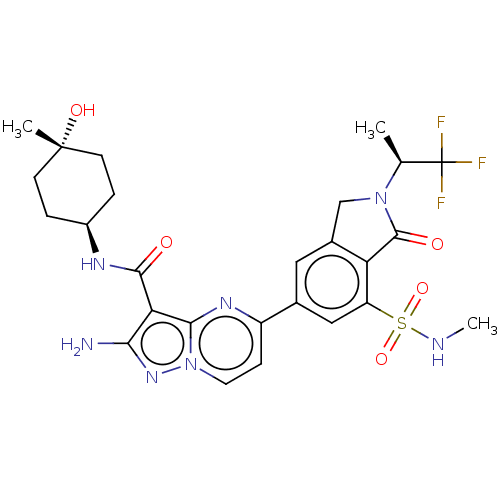
(CHEMBL5179088)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C(F)(F)F)C(=O)c12)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@](C)(O)CC3)c2n1 |r,wU:32.33,12.13,wD:35.38,(.21,-3.84,;.21,-2.3,;1.55,-1.53,;2.88,-2.3,;1.55,-3.07,;1.55,.01,;.21,.78,;.21,2.32,;1.54,3.09,;2.87,2.32,;4.34,2.8,;5.24,1.55,;6.78,1.55,;7.55,2.89,;7.55,.22,;9.09,.22,;6.78,-1.11,;8.1,-1.24,;4.34,.31,;4.74,-1.18,;2.87,.78,;-1.12,3.09,;-1.12,4.63,;-2.45,5.39,;-3.78,4.63,;-5.24,5.1,;-6.15,3.86,;-7.69,3.86,;-5.24,2.61,;-6.01,1.28,;-7.55,1.28,;-5.24,-.06,;-6.01,-1.39,;-5.24,-2.72,;-6.01,-4.06,;-7.55,-4.06,;-8.32,-5.39,;-9.09,-4.06,;-8.32,-2.72,;-7.55,-1.39,;-3.78,3.09,;-2.44,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546764
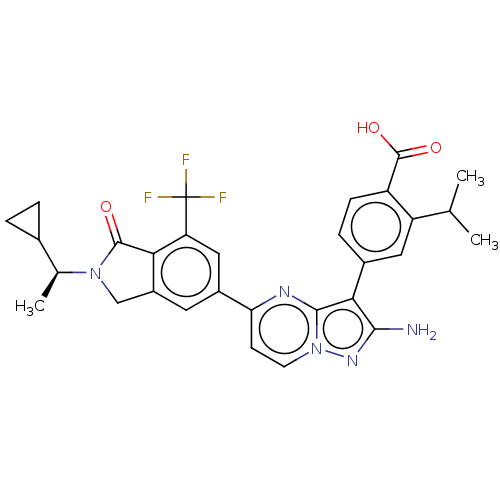
(CHEMBL4740423)Show SMILES CC(C)c1cc(ccc1C(O)=O)-c1c(N)nn2ccc(nc12)-c1cc2CN([C@@H](C)C3CC3)C(=O)c2c(c1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal His-tagged full-length human PI3K p120gamma expressed in Sf9 insect cells using phosphatidylinositol 4,5-bisphos... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01203
BindingDB Entry DOI: 10.7270/Q2K35Z80 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561856
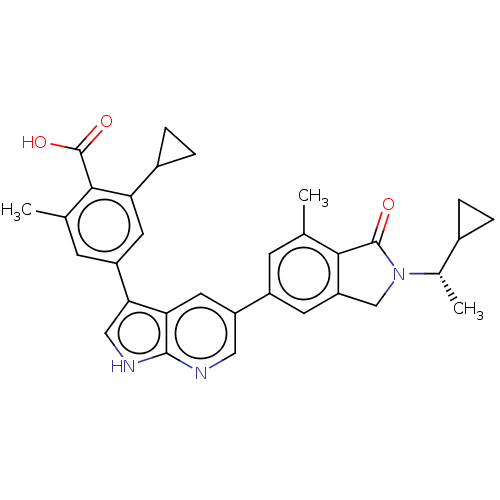
(CHEMBL4749505)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3cc(C)c(C(O)=O)c(c3)C3CC3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561885
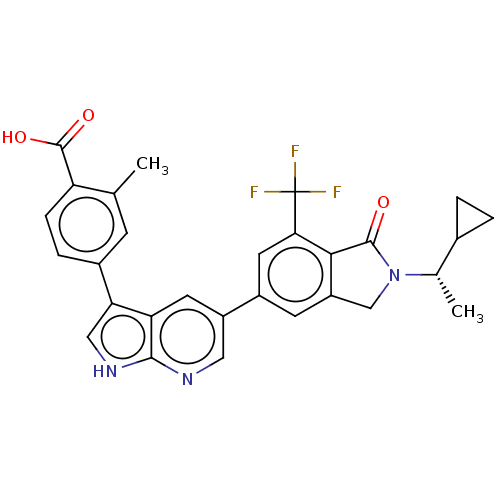
(CHEMBL4740643)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)C(F)(F)F)-c1cnc2[nH]cc(-c3ccc(C(O)=O)c(C)c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602504
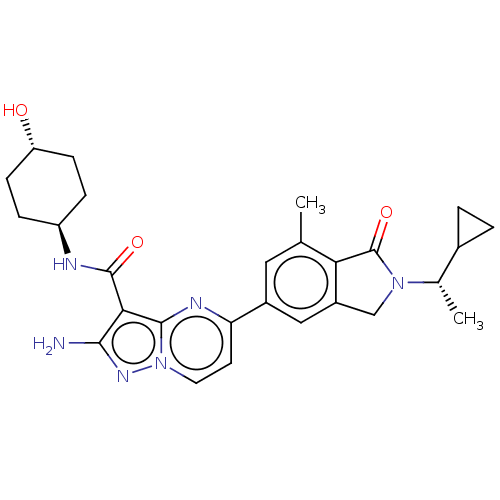
(CHEMBL5186057)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@H](O)CC3)c2n1 |r,wU:1.1,27.29,wD:30.33,(7.27,2.89,;6.5,1.55,;7.27,.22,;7.27,-1.32,;8.6,-.55,;4.96,1.55,;4.06,2.8,;2.59,2.32,;1.26,3.09,;-.07,2.32,;-.07,.78,;1.26,.01,;1.26,-1.53,;2.59,.78,;4.05,.31,;4.45,-1.18,;-1.41,3.09,;-1.41,4.63,;-2.73,5.39,;-4.06,4.63,;-5.52,5.1,;-6.43,3.86,;-7.97,3.86,;-5.52,2.61,;-6.29,1.28,;-7.83,1.28,;-5.52,-.06,;-6.29,-1.39,;-5.52,-2.72,;-6.29,-4.06,;-7.83,-4.06,;-8.6,-5.39,;-8.6,-2.72,;-7.83,-1.39,;-4.06,3.09,;-2.73,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602530
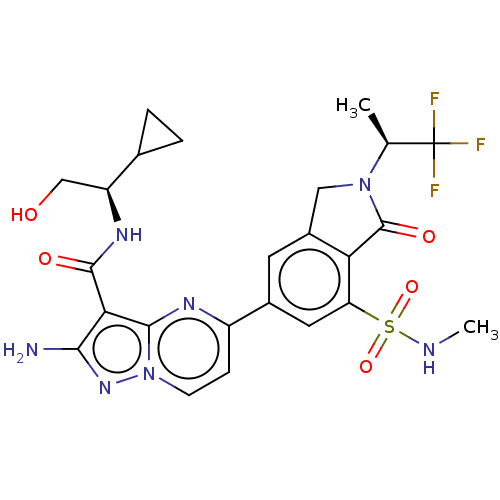
(CHEMBL5171703)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C(F)(F)F)C(=O)c12)-c1ccn2nc(N)c(C(=O)N[C@@H](CO)C3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546759
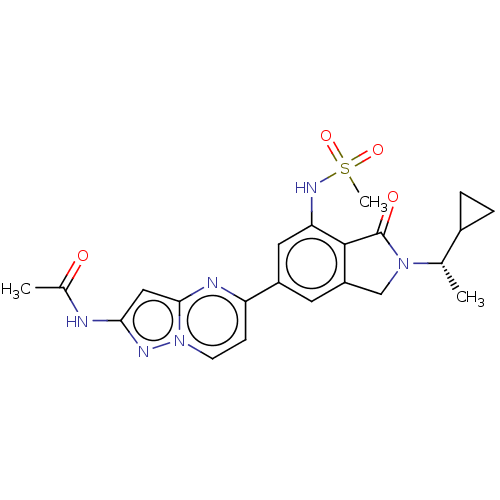
(CHEMBL4778393)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1ccn2nc(NC(C)=O)cc2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal His-tagged full-length human PI3K p120gamma expressed in Sf9 insect cells using phosphatidylinositol 4,5-bisphos... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01203
BindingDB Entry DOI: 10.7270/Q2K35Z80 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561859
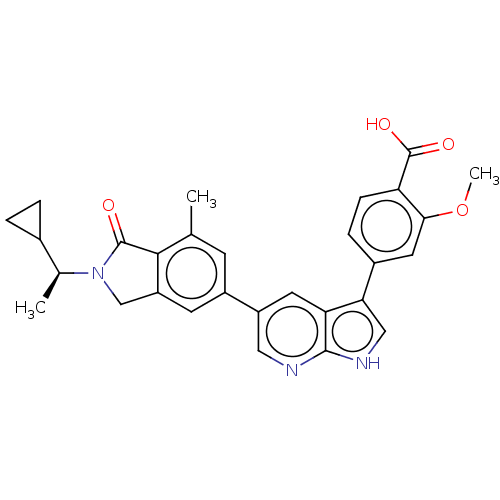
(CHEMBL4781463)Show SMILES COc1cc(ccc1C(O)=O)-c1c[nH]c2ncc(cc12)-c1cc2CN([C@@H](C)C3CC3)C(=O)c2c(C)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561884
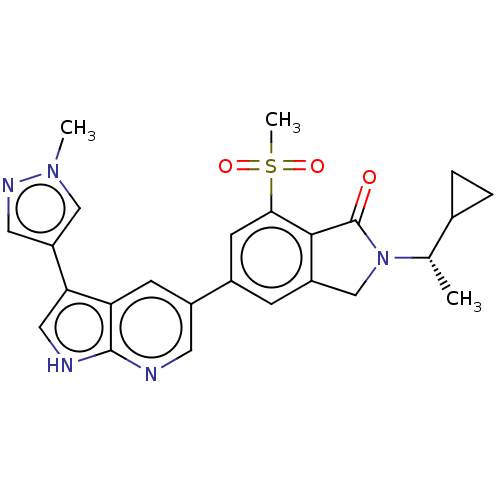
(CHEMBL4758027)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1cnc2[nH]cc(-c3cnn(C)c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561854
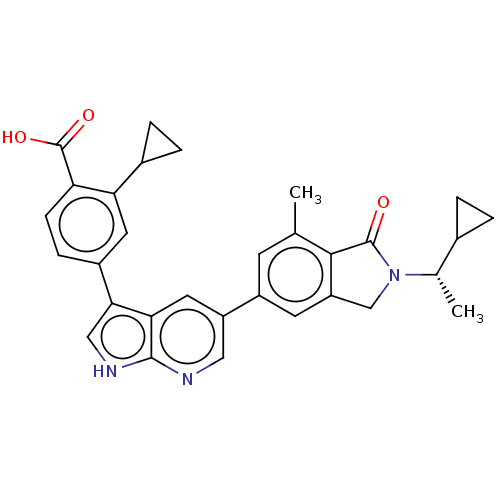
(CHEMBL4794110)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3ccc(C(O)=O)c(c3)C3CC3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561858

(CHEMBL4744413)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3ccc(C(O)=O)c(c3)C(F)(F)F)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602524
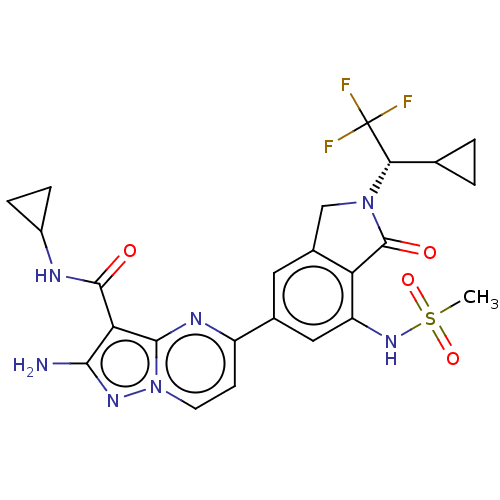
(CHEMBL5187986)Show SMILES CS(=O)(=O)Nc1cc(cc2CN([C@@H](C3CC3)C(F)(F)F)C(=O)c12)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602516
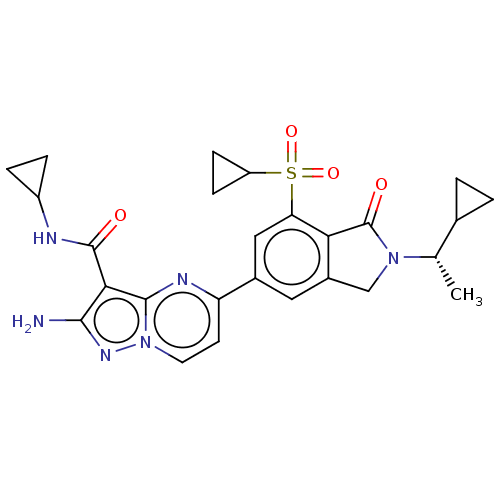
(CHEMBL5182329)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)C1CC1)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602512
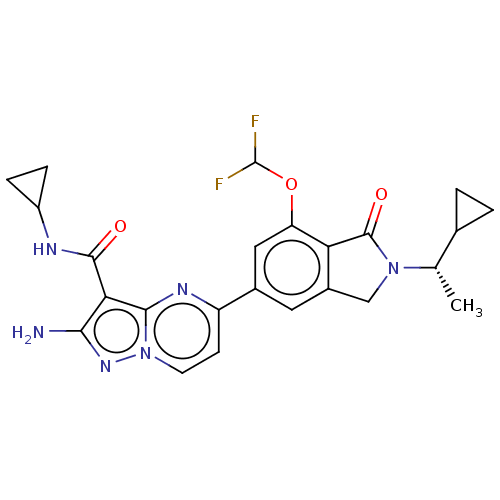
(CHEMBL5200339)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(OC(F)F)c2C1=O)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602497
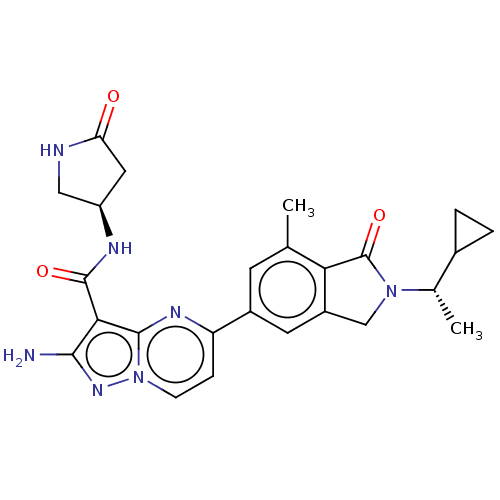
(CHEMBL5172570)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CNC(=O)C3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602495
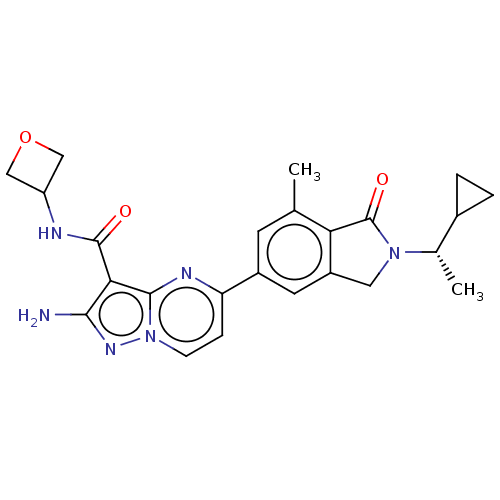
(CHEMBL5177379)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)NC3COC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602496
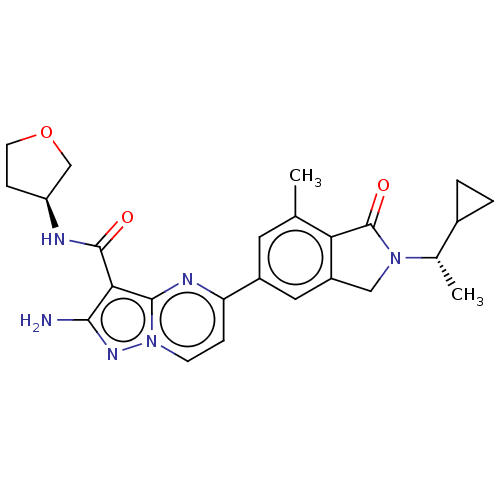
(CHEMBL5203565)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CCOC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546730
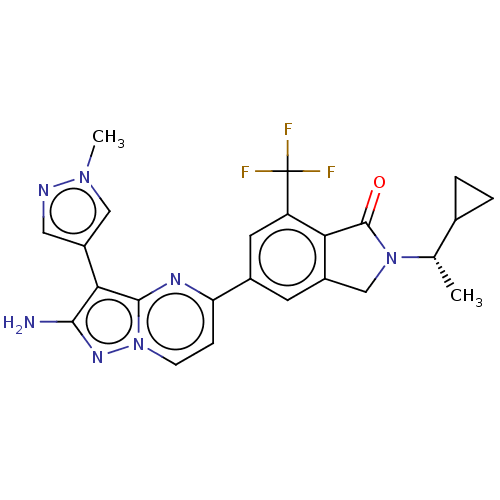
(CHEMBL4782083)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)C(F)(F)F)-c1ccn2nc(N)c(-c3cnn(C)c3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal His-tagged full-length human PI3K p120gamma expressed in Sf9 insect cells using phosphatidylinositol 4,5-bisphos... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01203
BindingDB Entry DOI: 10.7270/Q2K35Z80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602541

(CHEMBL5209268)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(OC(F)F)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@](C)(O)CC3)c2n1 |r,wU:30.32,1.1,33.37,(7.66,2.89,;6.89,1.55,;7.66,.22,;7.66,-1.32,;8.99,-.55,;5.35,1.55,;4.44,2.8,;2.98,2.32,;1.65,3.09,;.31,2.32,;.31,.78,;1.65,.01,;1.65,-1.53,;.32,-2.3,;.32,-3.84,;-1.02,-1.53,;2.98,.78,;4.44,.31,;4.84,-1.18,;-1.02,3.09,;-1.02,4.63,;-2.34,5.39,;-3.67,4.63,;-5.14,5.1,;-6.04,3.86,;-7.58,3.86,;-5.14,2.61,;-5.91,1.28,;-7.45,1.28,;-5.14,-.06,;-5.91,-1.39,;-5.14,-2.72,;-5.91,-4.06,;-7.45,-4.06,;-8.22,-5.39,;-8.99,-4.06,;-8.22,-2.72,;-7.45,-1.39,;-3.67,3.09,;-2.34,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602502
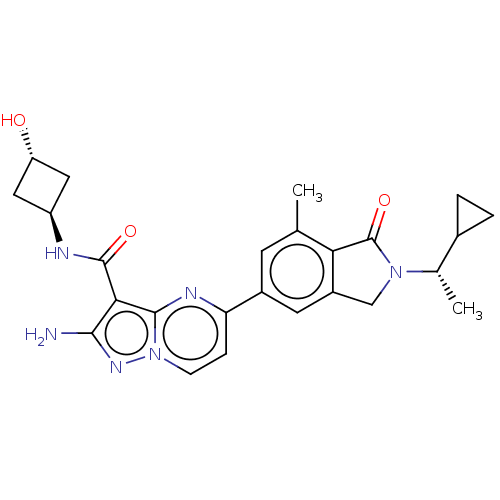
(CHEMBL5190157)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3C[C@H](O)C3)c2n1 |r,wU:27.29,1.1,wD:29.32,(7.04,2.51,;6.27,1.17,;7.04,-.16,;7.04,-1.7,;8.37,-.93,;4.73,1.17,;3.83,2.42,;2.36,1.95,;1.03,2.71,;-.3,1.94,;-.3,.4,;1.04,-.36,;1.04,-1.9,;2.36,.4,;3.83,-.07,;4.22,-1.56,;-1.63,2.71,;-1.63,4.25,;-2.96,5.01,;-4.29,4.25,;-5.75,4.72,;-6.66,3.48,;-8.2,3.48,;-5.75,2.23,;-6.52,.9,;-8.06,.9,;-5.75,-.44,;-6.52,-1.77,;-6.12,-3.28,;-7.6,-3.68,;-8.37,-5.01,;-8.01,-2.17,;-4.29,2.71,;-2.96,1.95,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602499
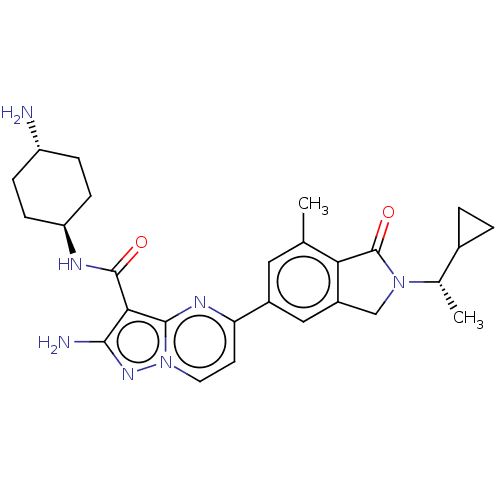
(CHEMBL5178231)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@H](N)CC3)c2n1 |r,wU:1.1,30.33,wD:27.29,(7.27,2.89,;6.5,1.55,;7.27,.22,;7.27,-1.32,;8.6,-.55,;4.96,1.55,;4.06,2.8,;2.59,2.32,;1.26,3.09,;-.07,2.32,;-.07,.78,;1.26,.01,;1.26,-1.53,;2.59,.78,;4.06,.31,;4.45,-1.18,;-1.41,3.09,;-1.41,4.63,;-2.73,5.39,;-4.06,4.63,;-5.52,5.1,;-6.43,3.86,;-7.97,3.86,;-5.52,2.61,;-6.29,1.28,;-7.83,1.28,;-5.52,-.06,;-6.29,-1.39,;-7.83,-1.39,;-8.6,-2.72,;-7.83,-4.06,;-8.6,-5.39,;-6.29,-4.06,;-5.52,-2.72,;-4.06,3.09,;-2.73,2.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602537
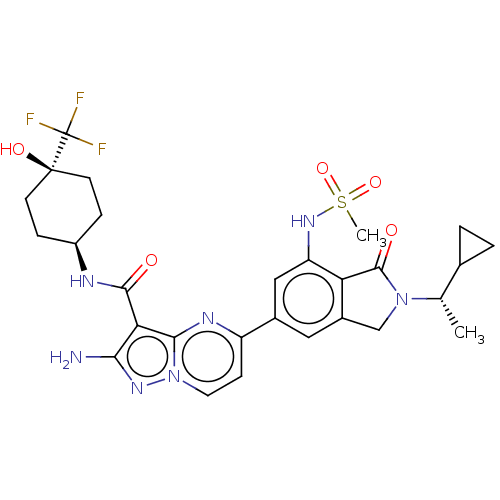
(CHEMBL5172719)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@](O)(CC3)C(F)(F)F)c2n1 |r,wU:31.33,1.1,34.37,(8.04,3.55,;7.27,2.22,;8.04,.89,;8.04,-.66,;9.37,.11,;5.73,2.22,;4.83,3.47,;3.36,2.99,;2.03,3.76,;.7,2.99,;.7,1.44,;2.03,.68,;2.03,-.86,;.7,-1.63,;.7,-3.17,;-.63,-.86,;.7,-.09,;3.36,1.45,;4.83,.97,;5.22,-.51,;-.64,3.76,;-.64,5.29,;-1.96,6.06,;-3.29,5.29,;-4.75,5.77,;-5.66,4.52,;-7.2,4.52,;-4.75,3.28,;-5.52,1.94,;-7.06,1.94,;-4.75,.61,;-5.52,-.72,;-4.75,-2.06,;-5.52,-3.39,;-7.06,-3.39,;-8.6,-3.39,;-7.83,-2.06,;-7.06,-.72,;-7.83,-4.73,;-7.06,-6.06,;-9.37,-4.73,;-8.6,-6.06,;-3.29,3.75,;-1.96,2.99,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546765
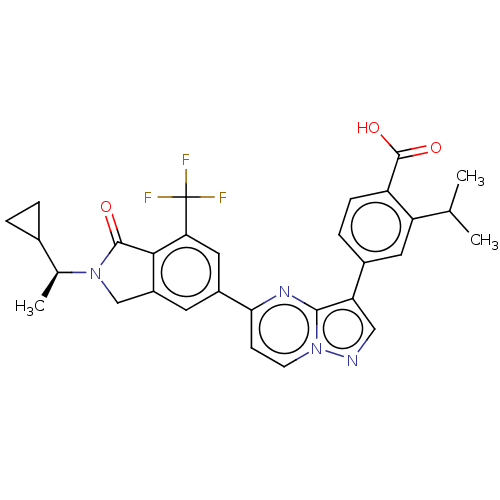
(CHEMBL4742062)Show SMILES CC(C)c1cc(ccc1C(O)=O)-c1cnn2ccc(nc12)-c1cc2CN([C@@H](C)C3CC3)C(=O)c2c(c1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal His-tagged full-length human PI3K p120gamma expressed in Sf9 insect cells using phosphatidylinositol 4,5-bisphos... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01203
BindingDB Entry DOI: 10.7270/Q2K35Z80 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602498
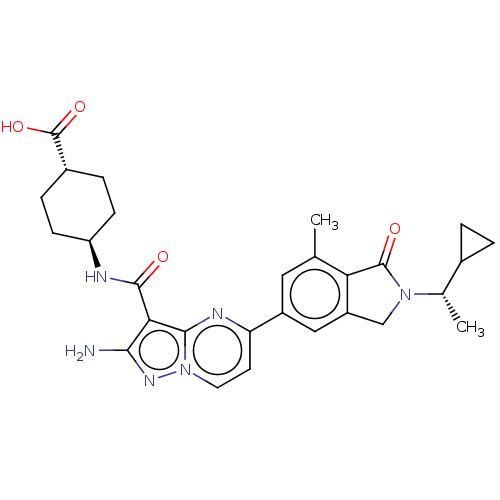
(CHEMBL5204993)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@@H](CC3)C(O)=O)c2n1 |r,wU:1.1,30.36,wD:27.29,(8.04,3.55,;7.27,2.22,;8.04,.88,;8.04,-.66,;9.37,.11,;5.73,2.22,;4.82,3.46,;3.36,2.99,;2.03,3.76,;.69,2.99,;.69,1.44,;2.03,.68,;2.03,-.86,;3.36,1.45,;4.82,.97,;5.22,-.52,;-.64,3.76,;-.64,5.29,;-1.96,6.06,;-3.29,5.29,;-4.76,5.77,;-5.66,4.52,;-7.2,4.52,;-4.76,3.27,;-5.53,1.94,;-7.07,1.94,;-4.76,.61,;-5.53,-.73,;-7.07,-.73,;-7.84,-2.06,;-7.07,-3.39,;-5.53,-3.39,;-4.76,-2.06,;-7.84,-4.73,;-9.37,-4.73,;-7.07,-6.06,;-3.29,3.75,;-1.96,2.99,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561853
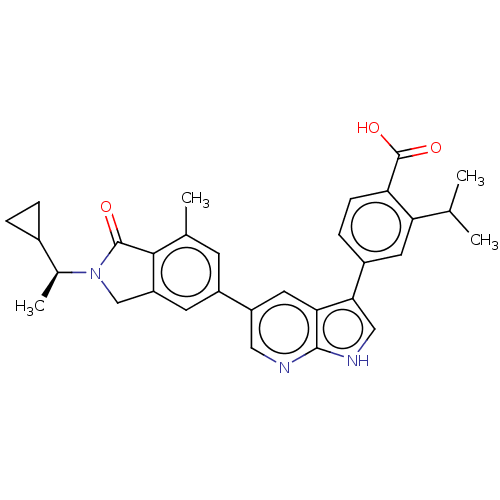
(CHEMBL4754968)Show SMILES CC(C)c1cc(ccc1C(O)=O)-c1c[nH]c2ncc(cc12)-c1cc2CN([C@@H](C)C3CC3)C(=O)c2c(C)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561889
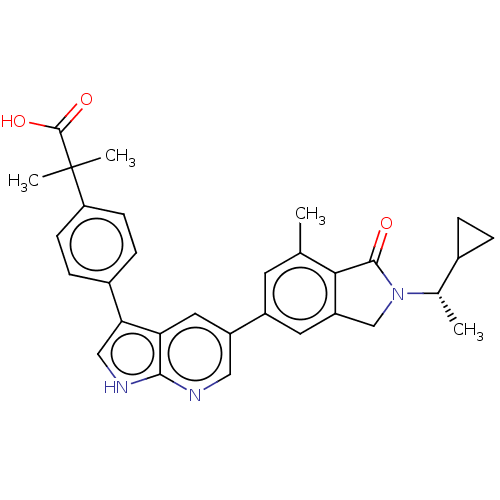
(CHEMBL4761831)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3ccc(cc3)C(C)(C)C(O)=O)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546758
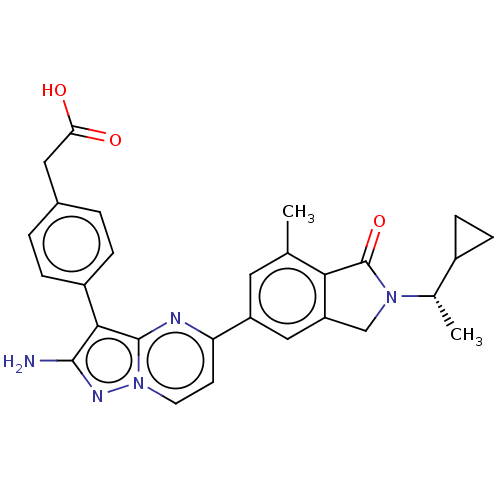
(CHEMBL4743121)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(-c3ccc(CC(O)=O)cc3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal His-tagged full-length human PI3K p120gamma expressed in Sf9 insect cells using phosphatidylinositol 4,5-bisphos... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01203
BindingDB Entry DOI: 10.7270/Q2K35Z80 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561855
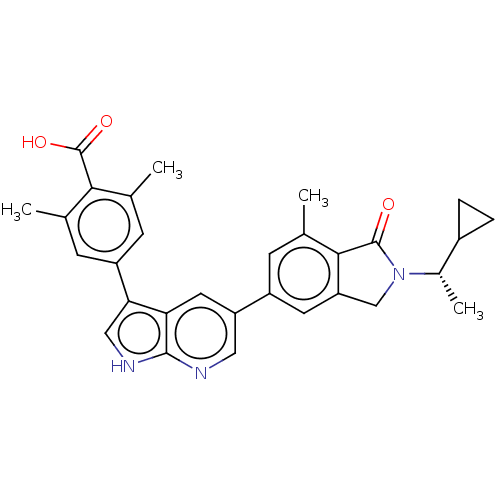
(CHEMBL4746279)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3cc(C)c(C(O)=O)c(C)c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546757
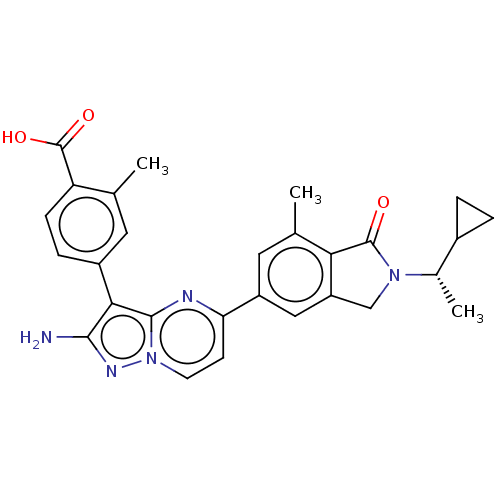
(CHEMBL4762555)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(-c3ccc(C(O)=O)c(C)c3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal His-tagged full-length human PI3K p120gamma expressed in Sf9 insect cells using phosphatidylinositol 4,5-bisphos... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01203
BindingDB Entry DOI: 10.7270/Q2K35Z80 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50546729
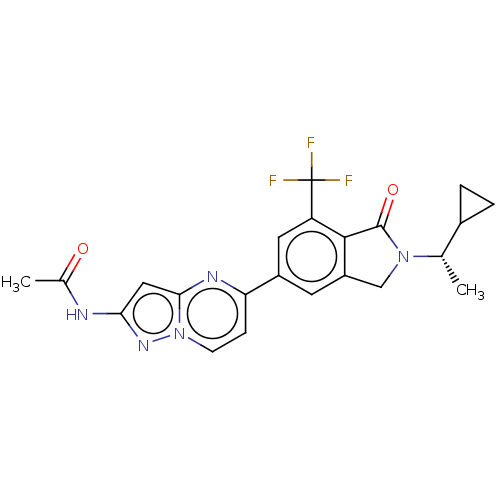
(CHEMBL4785086)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)C(F)(F)F)-c1ccn2nc(NC(C)=O)cc2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant N-terminal His-tagged full-length human PI3K p120gamma expressed in Sf9 insect cells using phosphatidylinositol 4,5-bisphos... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01203
BindingDB Entry DOI: 10.7270/Q2K35Z80 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602494
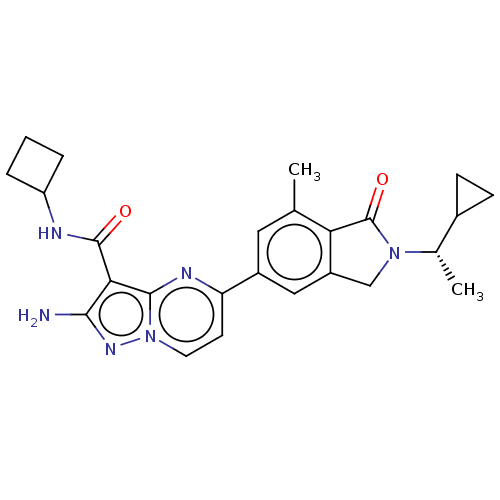
(CHEMBL5184295)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)NC3CCC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561876
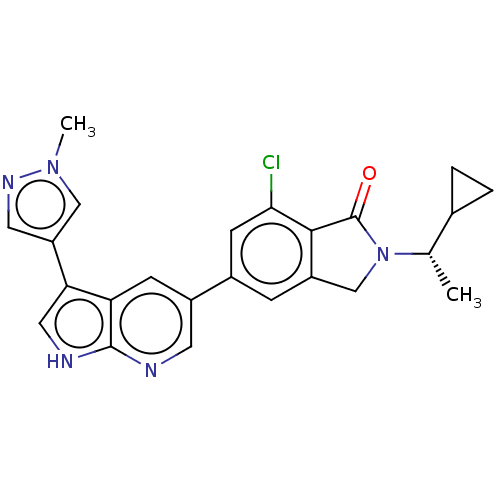
(CHEMBL4752613)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1cnc2[nH]cc(-c3cnn(C)c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561878
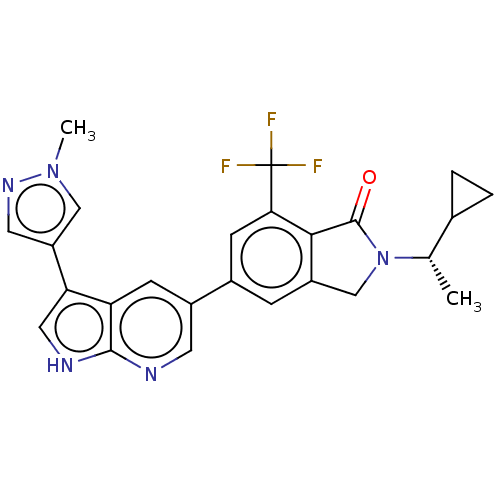
(CHEMBL4748899)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)C(F)(F)F)-c1cnc2[nH]cc(-c3cnn(C)c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602514
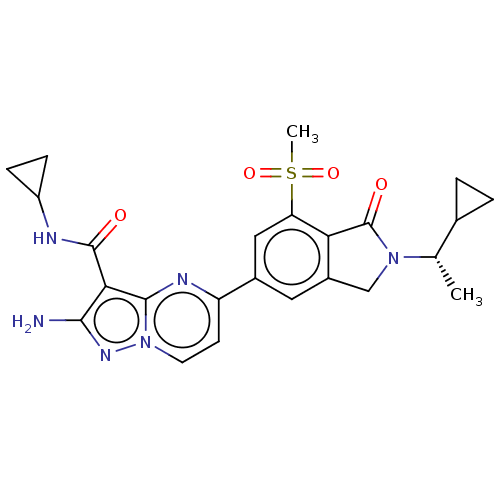
(CHEMBL5202602)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(C)(=O)=O)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602508
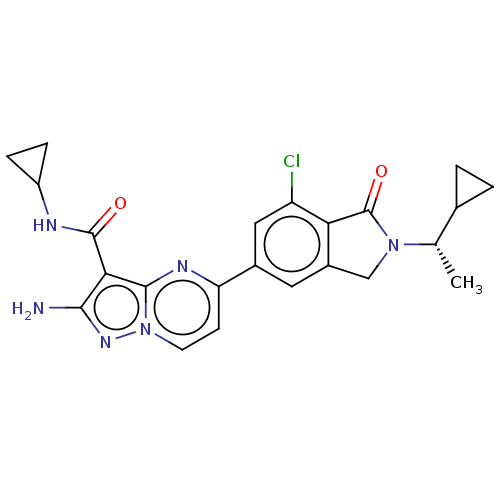
(CHEMBL5196851)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(Cl)c2C1=O)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561880
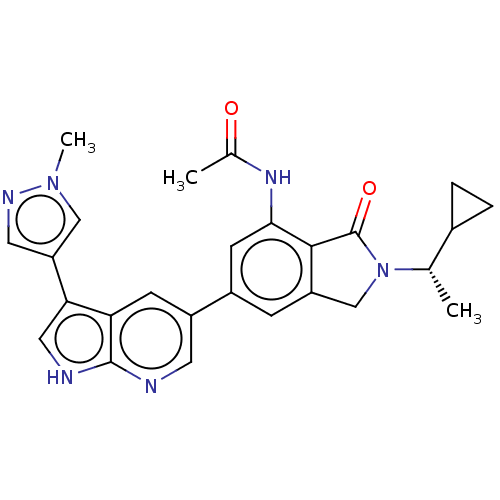
(CHEMBL4764811)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NC(C)=O)c2C1=O)-c1cnc2[nH]cc(-c3cnn(C)c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602533
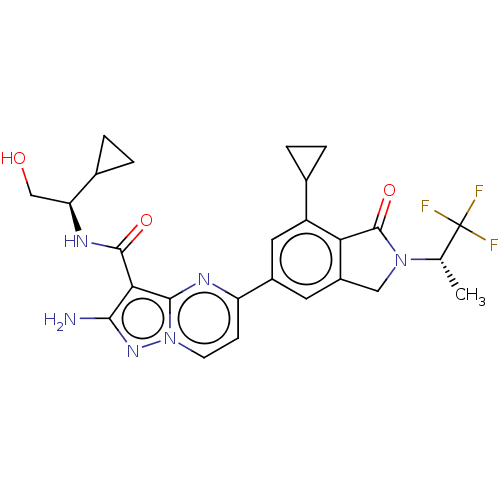
(CHEMBL5177397)Show SMILES C[C@H](N1Cc2cc(cc(C3CC3)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@@H](CO)C3CC3)c2n1)C(F)(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561851
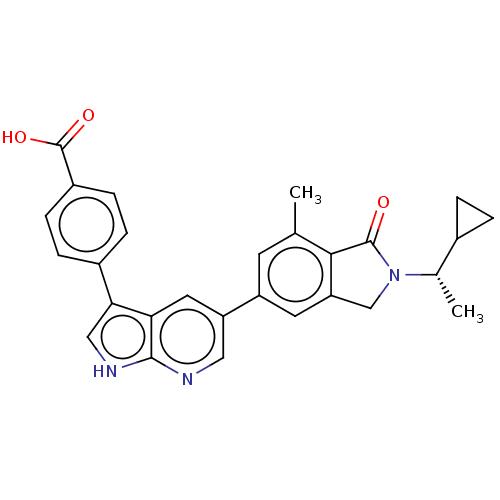
(CHEMBL4751253)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3ccc(cc3)C(O)=O)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561882
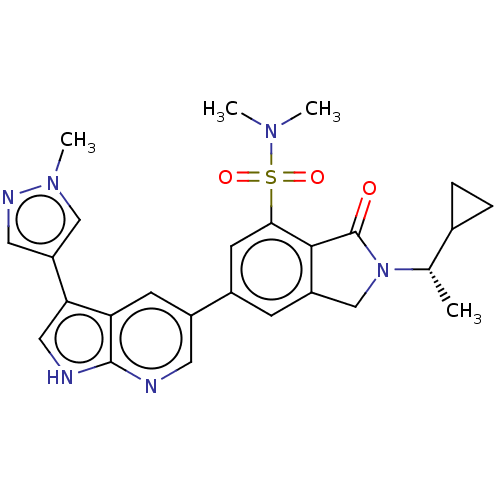
(CHEMBL4756678)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(c2C1=O)S(=O)(=O)N(C)C)-c1cnc2[nH]cc(-c3cnn(C)c3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561864
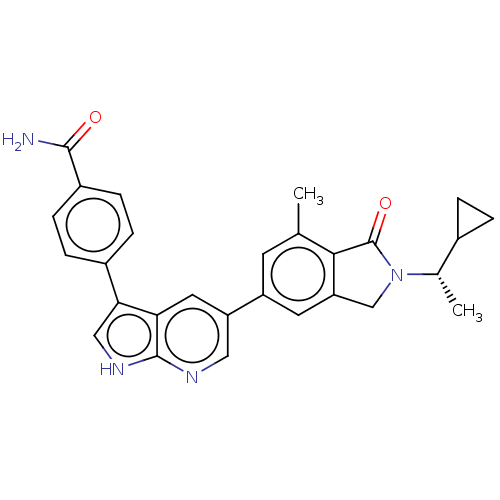
(CHEMBL4746409)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3ccc(cc3)C(N)=O)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
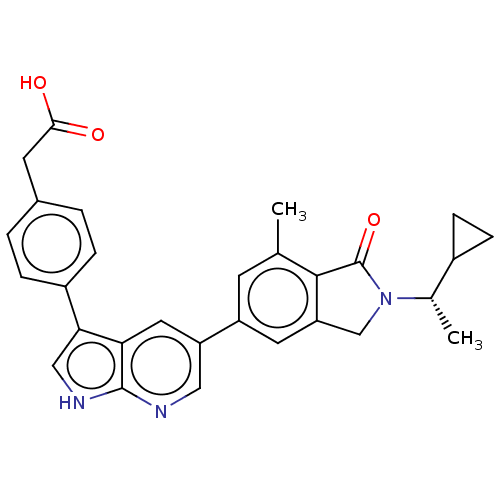
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561887
(CHEMBL4756242)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3ccc(CC(O)=O)cc3)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50561861
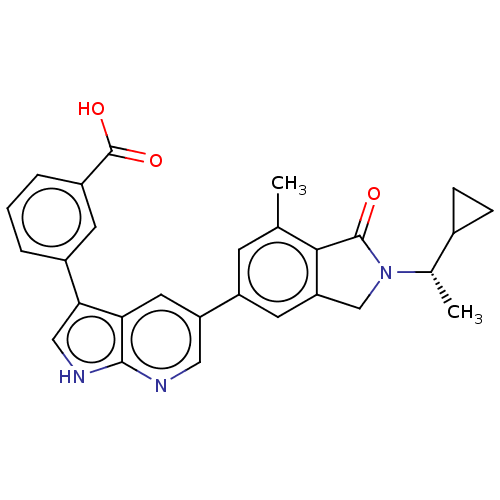
(CHEMBL4753736)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1cnc2[nH]cc(-c3cccc(c3)C(O)=O)c2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p120gamma (unknown origin) using phosphatidylinositol 4,5-bisphosphate as substrate in presence of ATP incubated for 60 mins by AD... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00387
BindingDB Entry DOI: 10.7270/Q2H70KHF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602519
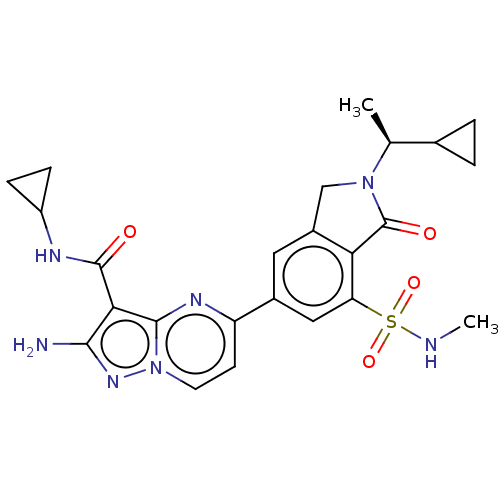
(CHEMBL5194953)Show SMILES CNS(=O)(=O)c1cc(cc2CN([C@@H](C)C3CC3)C(=O)c12)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50602520
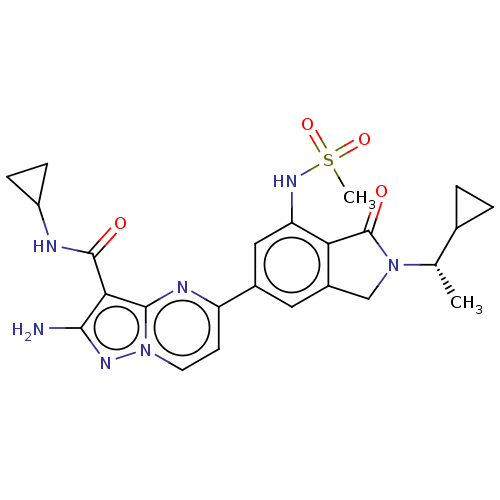
(CHEMBL5204743)Show SMILES C[C@@H](C1CC1)N1Cc2cc(cc(NS(C)(=O)=O)c2C1=O)-c1ccn2nc(N)c(C(=O)NC3CC3)c2n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01153
BindingDB Entry DOI: 10.7270/Q2KH0SCH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data