 Found 336 hits with Last Name = 'bajrami' and Initial = 'b'
Found 336 hits with Last Name = 'bajrami' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572983

(CHEMBL4848846)Show SMILES C[C@@H](C(=O)Nc1ccc(C)c(c1)S(=O)(=O)NCCc1ccccn1)n1ncc(Cl)c(Cl)c1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572968
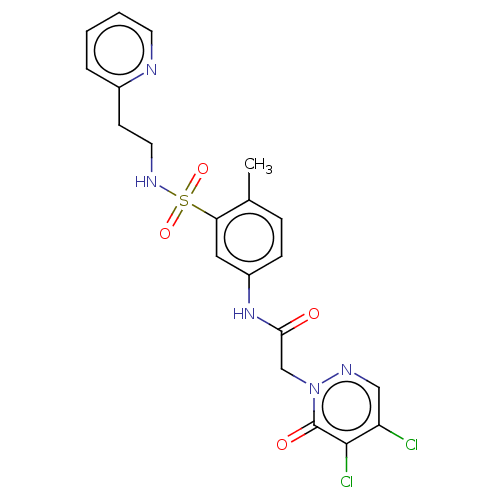
(CHEMBL4862851)Show SMILES Cc1ccc(NC(=O)Cn2ncc(Cl)c(Cl)c2=O)cc1S(=O)(=O)NCCc1ccccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572964

(CHEMBL4867592)Show SMILES Cc1ccc(NC(=O)Cn2ncc(Cl)c(Cl)c2=O)cc1S(=O)(=O)N1CCCCCC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572989

(CHEMBL4846332)Show SMILES Cc1ccc(NC(=O)Cn2ncc(Cl)cc2=O)cc1S(=O)(=O)NCCc1ccccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572991
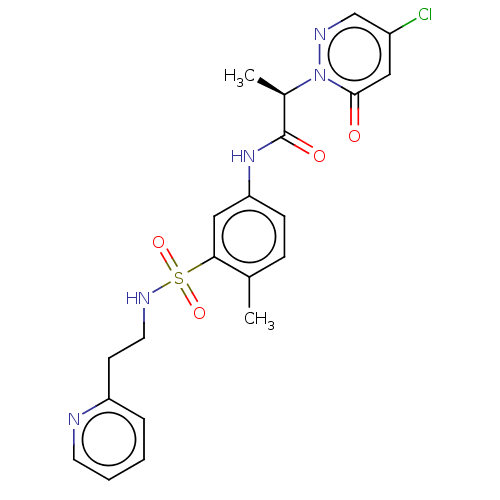
(CHEMBL4858967)Show SMILES C[C@H](C(=O)Nc1ccc(C)c(c1)S(=O)(=O)NCCc1ccccn1)n1ncc(Cl)cc1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572990

(CHEMBL4859105)Show SMILES C[C@@H](C(=O)Nc1ccc(C)c(c1)S(=O)(=O)NCCc1ccccn1)n1ncc(Cl)cc1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50553436
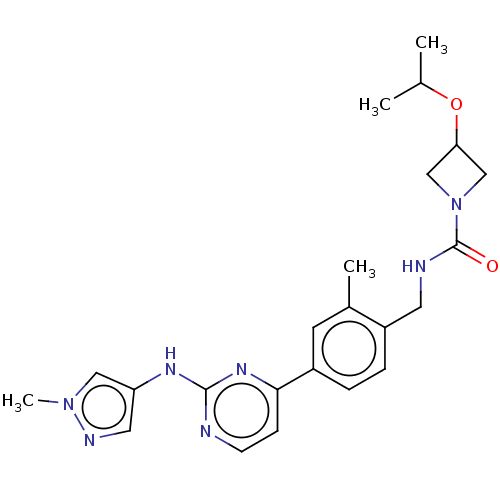
(CHEMBL4744041)Show SMILES CC(C)OC1CN(C1)C(=O)NCc1ccc(cc1C)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Time-dependent inhibition of CYP3A4 in human liver microsomes using testosterone in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00702
BindingDB Entry DOI: 10.7270/Q2BK1H1M |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572988
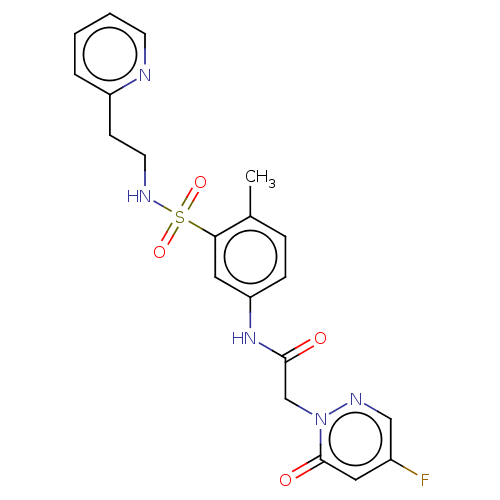
(CHEMBL4855695)Show SMILES Cc1ccc(NC(=O)Cn2ncc(F)cc2=O)cc1S(=O)(=O)NCCc1ccccn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50572984
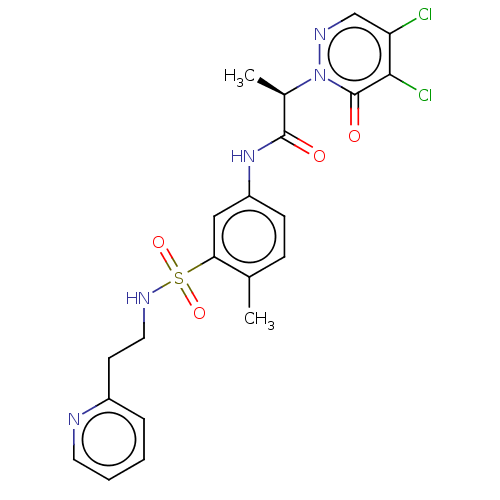
(CHEMBL4874198)Show SMILES C[C@H](C(=O)Nc1ccc(C)c(c1)S(=O)(=O)NCCc1ccccn1)n1ncc(Cl)c(Cl)c1=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of human PRMT5 assessed as initial binding constant by LC-MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00507
BindingDB Entry DOI: 10.7270/Q26977DH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583942
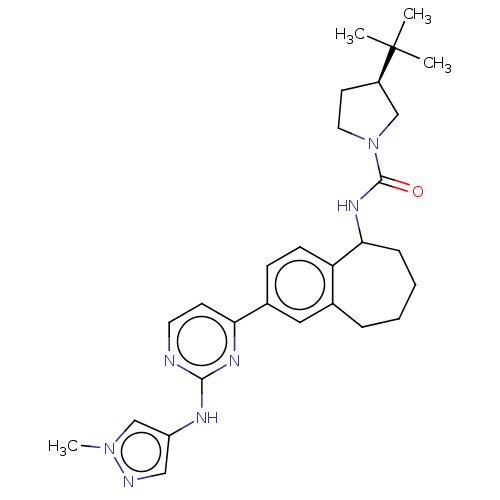
(CHEMBL5088454)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3C(CCCCc3c2)NC(=O)N2CC[C@H](C2)C(C)(C)C)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50553434
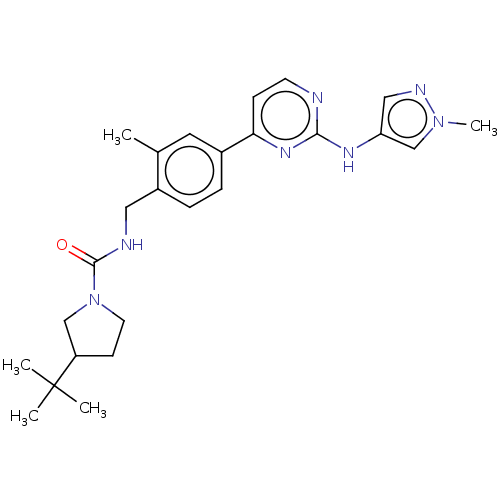
(CHEMBL4782313)Show SMILES Cc1cc(ccc1CNC(=O)N1CCC(C1)C(C)(C)C)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length N-terminal His6-tagged BTK expressed in baculovirus infected Sf21 insect cells using fluorescein-labeled ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00702
BindingDB Entry DOI: 10.7270/Q2BK1H1M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50553431
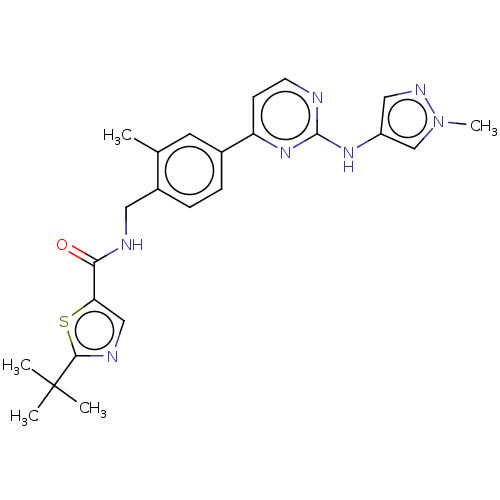
(CHEMBL4754065)Show SMILES Cc1cc(ccc1CNC(=O)c1cnc(s1)C(C)(C)C)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length N-terminal His6-tagged BTK expressed in baculovirus infected Sf21 insect cells using fluorescein-labeled ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00702
BindingDB Entry DOI: 10.7270/Q2BK1H1M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324380
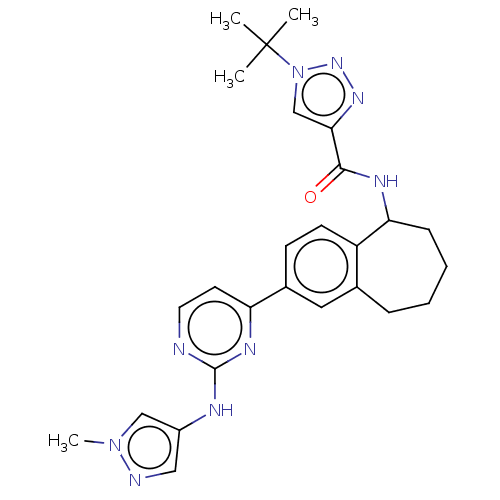
(1-(tert-butyl)-N- (2-(2-((1- methyl-1H-pyrazol-4- ...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3C(CCCCc3c2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 Show InChI InChI=1S/C26H31N9O/c1-26(2,3)35-16-23(32-33-35)24(36)30-22-8-6-5-7-17-13-18(9-10-20(17)22)21-11-12-27-25(31-21)29-19-14-28-34(4)15-19/h9-16,22H,5-8H2,1-4H3,(H,30,36)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596438
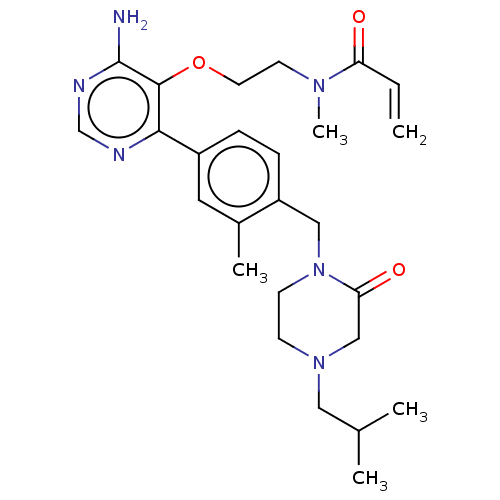
(CHEMBL5188786)Show SMILES CC(C)CN1CCN(Cc2ccc(cc2C)-c2ncnc(N)c2OCCN(C)C(=O)C=C)C(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583944

(CHEMBL5089878)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3C(CCCCc3c2)NC(=O)c2cnc(s2)C(C)(C)C)cn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324294
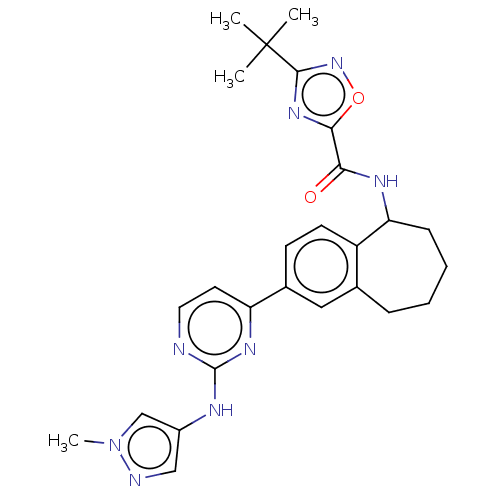
(3-(tert-butyl)-N-(2-(2-((1-methyl-1H-pyrazol-4-yl)...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3C(CCCCc3c2)NC(=O)c2nc(no2)C(C)(C)C)cn1 Show InChI InChI=1S/C26H30N8O2/c1-26(2,3)24-32-23(36-33-24)22(35)30-21-8-6-5-7-16-13-17(9-10-19(16)21)20-11-12-27-25(31-20)29-18-14-28-34(4)15-18/h9-15,21H,5-8H2,1-4H3,(H,30,35)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324290
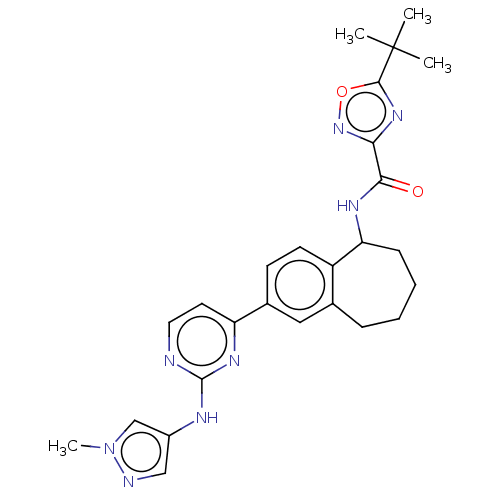
(5-(tert-butyl)-N-(2-(2-((1-methyl-1H-pyrazol-4-yl)...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3C(CCCCc3c2)NC(=O)c2noc(n2)C(C)(C)C)cn1 Show InChI InChI=1S/C26H30N8O2/c1-26(2,3)24-32-22(33-36-24)23(35)30-21-8-6-5-7-16-13-17(9-10-19(16)21)20-11-12-27-25(31-20)29-18-14-28-34(4)15-18/h9-15,21H,5-8H2,1-4H3,(H,30,35)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50553426
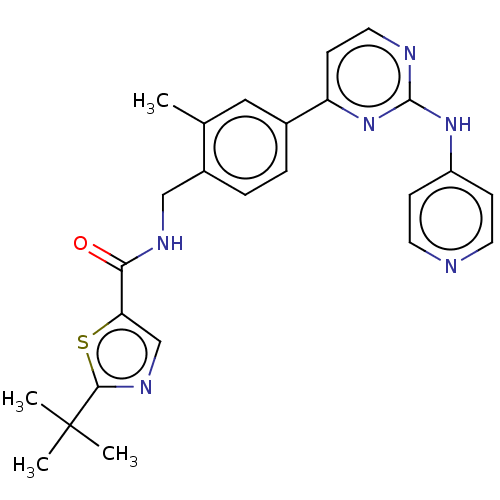
(CHEMBL4746275)Show SMILES Cc1cc(ccc1CNC(=O)c1cnc(s1)C(C)(C)C)-c1ccnc(Nc2ccncc2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length N-terminal His6-tagged BTK expressed in baculovirus infected Sf21 insect cells using fluorescein-labeled ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00702
BindingDB Entry DOI: 10.7270/Q2BK1H1M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596418
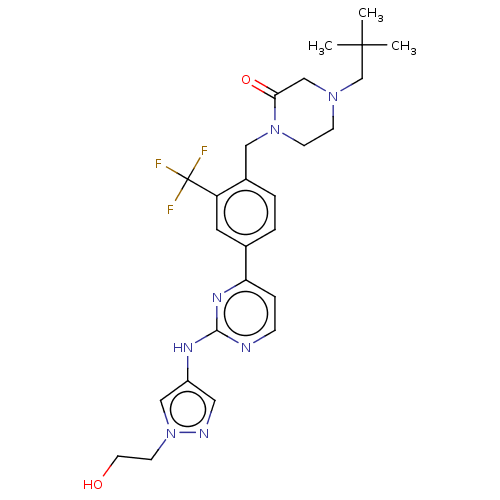
(CHEMBL5192152)Show SMILES CC(C)(C)CN1CCN(Cc2ccc(cc2C(F)(F)F)-c2ccnc(Nc3cnn(CCO)c3)n2)C(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50553435
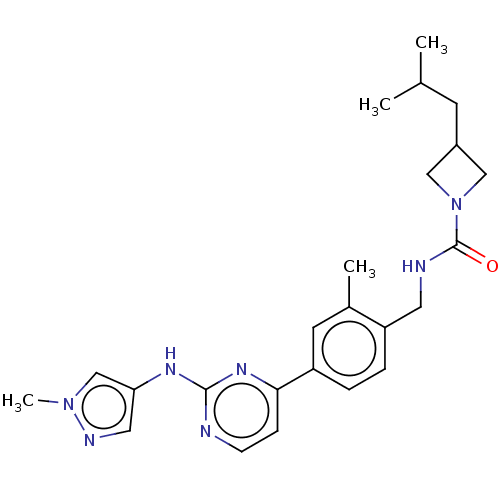
(CHEMBL4796367)Show SMILES CC(C)CC1CN(C1)C(=O)NCc1ccc(cc1C)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length N-terminal His6-tagged BTK expressed in baculovirus infected Sf21 insect cells using fluorescein-labeled ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00702
BindingDB Entry DOI: 10.7270/Q2BK1H1M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583938

(CHEMBL5090290)Show SMILES CC(C)OC1CN(C1)C(=O)N[C@@H]1CCCCc2cc(ccc12)-c1ccnc(Nc2cnn(C)c2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596435
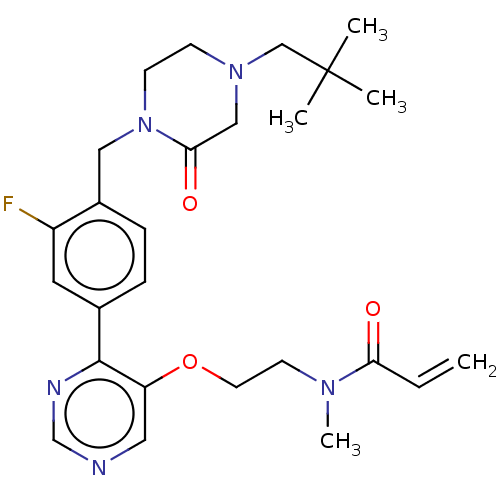
(CHEMBL5173641)Show SMILES CN(CCOc1cncnc1-c1ccc(CN2CCN(CC(C)(C)C)CC2=O)c(F)c1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596423
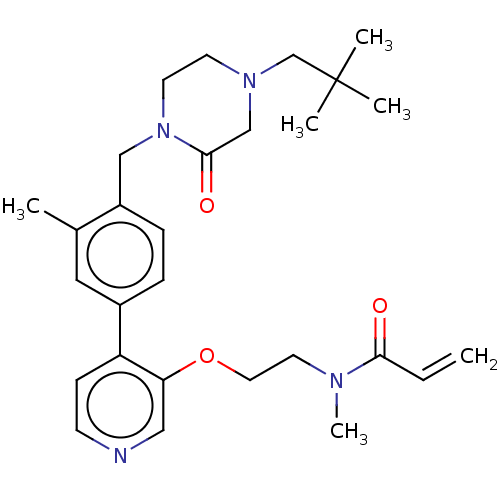
(CHEMBL5188750)Show SMILES CN(CCOc1cnccc1-c1ccc(CN2CCN(CC(C)(C)C)CC2=O)c(C)c1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324288
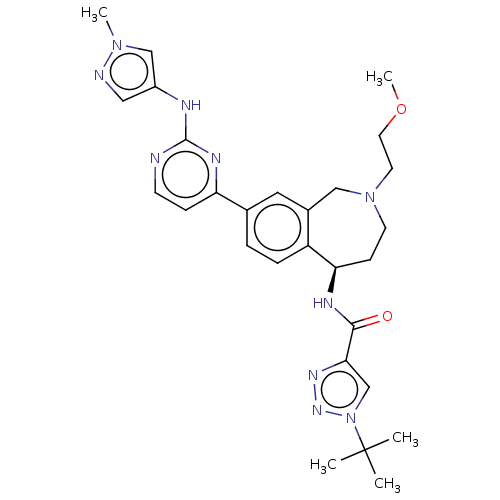
((R)-1-(tert-butyl)-N-(2-(2-methoxyethyl)-8-(2-((1-...)Show SMILES COCCN1CC[C@@H](NC(=O)c2cn(nn2)C(C)(C)C)c2ccc(cc2C1)-c1ccnc(Nc2cnn(C)c2)n1 |r| Show InChI InChI=1S/C28H36N10O2/c1-28(2,3)38-18-25(34-35-38)26(39)32-24-9-11-37(12-13-40-5)16-20-14-19(6-7-22(20)24)23-8-10-29-27(33-23)31-21-15-30-36(4)17-21/h6-8,10,14-15,17-18,24H,9,11-13,16H2,1-5H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583947
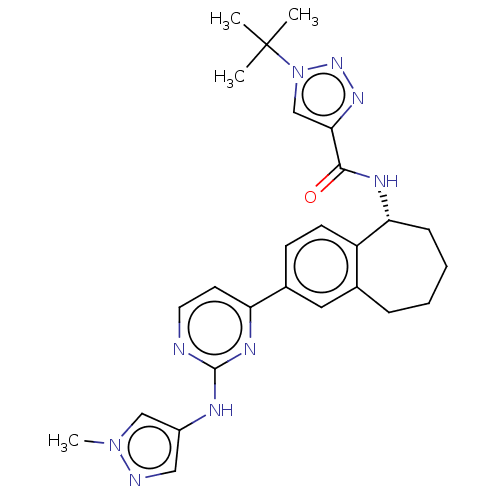
(CHEMBL5085931)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCCCc3c2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583946
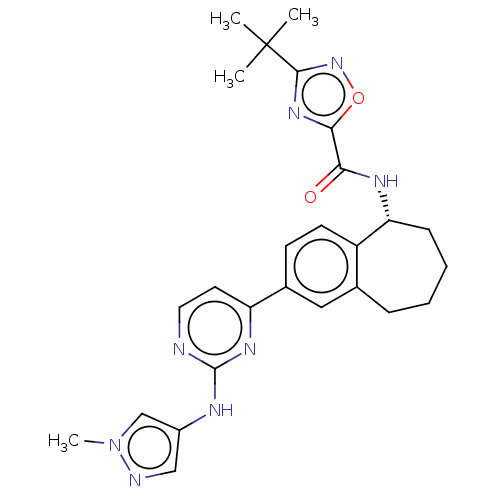
(CHEMBL5076817)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCCCc3c2)NC(=O)c2nc(no2)C(C)(C)C)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583945
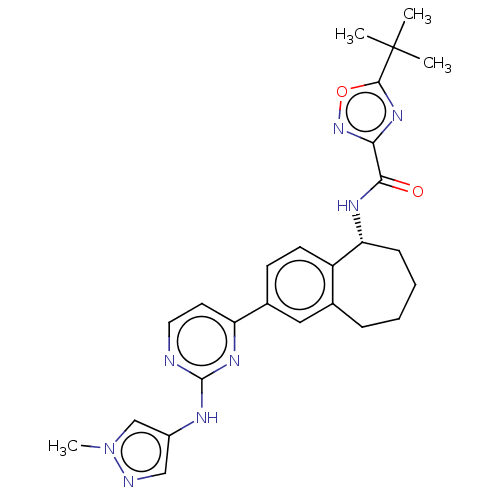
(CHEMBL5080861)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCCCc3c2)NC(=O)c2noc(n2)C(C)(C)C)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324337
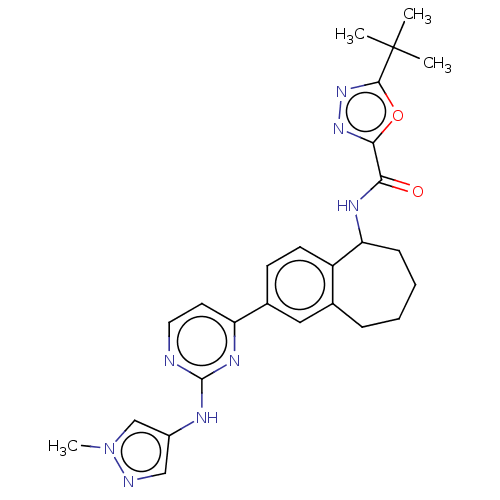
(5-(tert-butyl)-N-(2- (2-((1- methyl-1H-pyrazol-4- ...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3C(CCCCc3c2)NC(=O)c2nnc(o2)C(C)(C)C)cn1 Show InChI InChI=1S/C26H30N8O2/c1-26(2,3)24-33-32-23(36-24)22(35)30-21-8-6-5-7-16-13-17(9-10-19(16)21)20-11-12-27-25(31-20)29-18-14-28-34(4)15-18/h9-15,21H,5-8H2,1-4H3,(H,30,35)(H,27,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324293
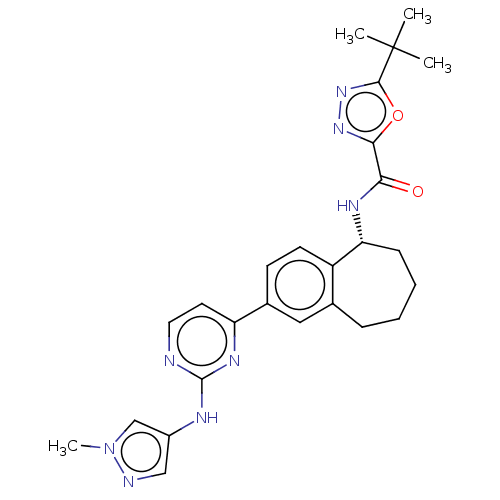
((R)-5-(tert-butyl)-N-(2-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCCCc3c2)NC(=O)c2nnc(o2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C26H30N8O2/c1-26(2,3)24-33-32-23(36-24)22(35)30-21-8-6-5-7-16-13-17(9-10-19(16)21)20-11-12-27-25(31-20)29-18-14-28-34(4)15-18/h9-15,21H,5-8H2,1-4H3,(H,30,35)(H,27,29,31)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324284
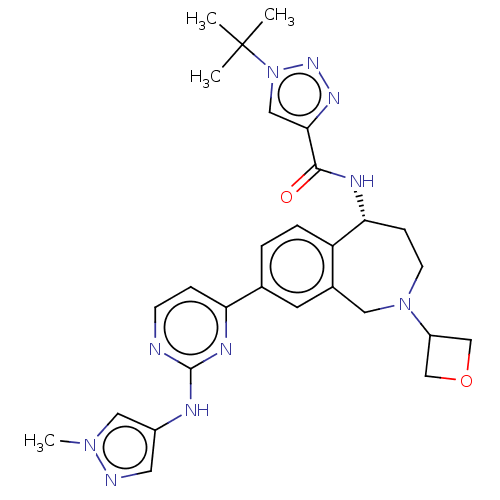
((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(Cc3c2)C2COC2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C28H34N10O2/c1-28(2,3)38-15-25(34-35-38)26(39)32-24-8-10-37(21-16-40-17-21)13-19-11-18(5-6-22(19)24)23-7-9-29-27(33-23)31-20-12-30-36(4)14-20/h5-7,9,11-12,14-15,21,24H,8,10,13,16-17H2,1-4H3,(H,32,39)(H,29,31,33)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583941
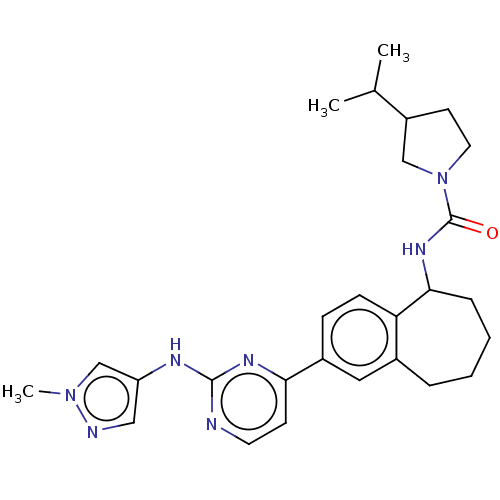
(CHEMBL5087183)Show SMILES CC(C)C1CCN(C1)C(=O)NC1CCCCc2cc(ccc12)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM324285
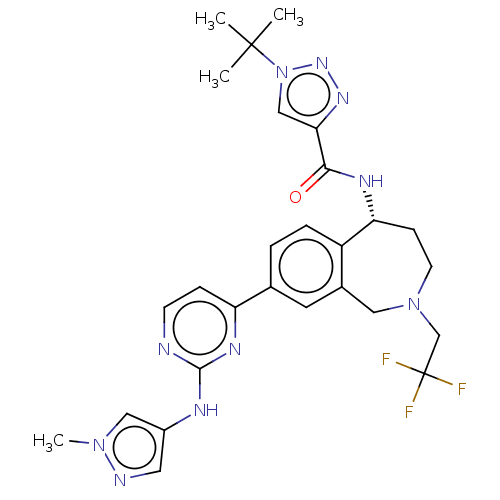
((R)-1-(tert-butyl)-N-(8-(2-((1-methyl-1H-pyrazol-4...)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CCN(CC(F)(F)F)Cc3c2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| Show InChI InChI=1S/C27H31F3N10O/c1-26(2,3)40-15-23(36-37-40)24(41)34-22-8-10-39(16-27(28,29)30)13-18-11-17(5-6-20(18)22)21-7-9-31-25(35-21)33-19-12-32-38(4)14-19/h5-7,9,11-12,14-15,22H,8,10,13,16H2,1-4H3,(H,34,41)(H,31,33,35)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596424
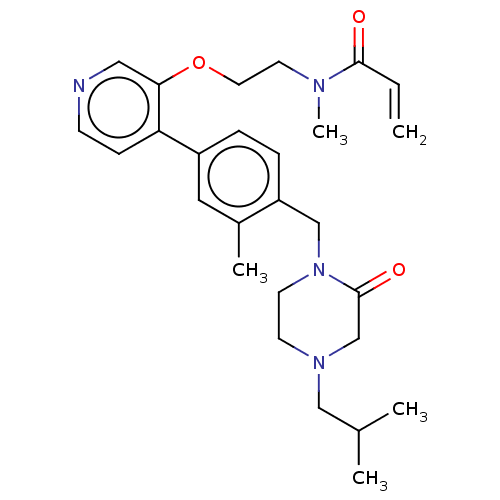
(CHEMBL5197926)Show SMILES CC(C)CN1CCN(Cc2ccc(cc2C)-c2ccncc2OCCN(C)C(=O)C=C)C(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596437
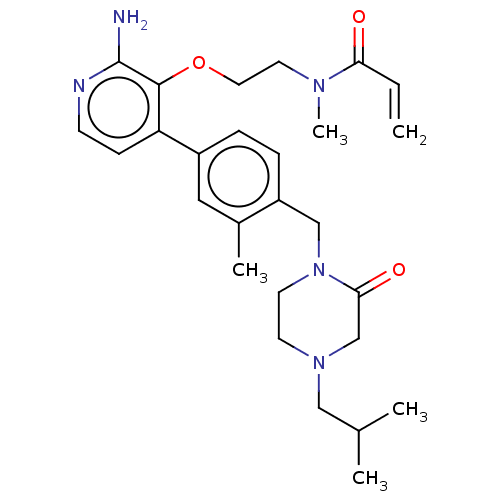
(CHEMBL5170448)Show SMILES CC(C)CN1CCN(Cc2ccc(cc2C)-c2ccnc(N)c2OCCN(C)C(=O)C=C)C(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583932
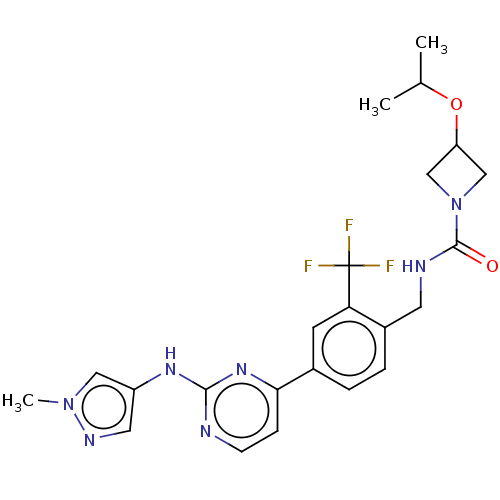
(CHEMBL5073223)Show SMILES CC(C)OC1CN(C1)C(=O)NCc1ccc(cc1C(F)(F)F)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583937
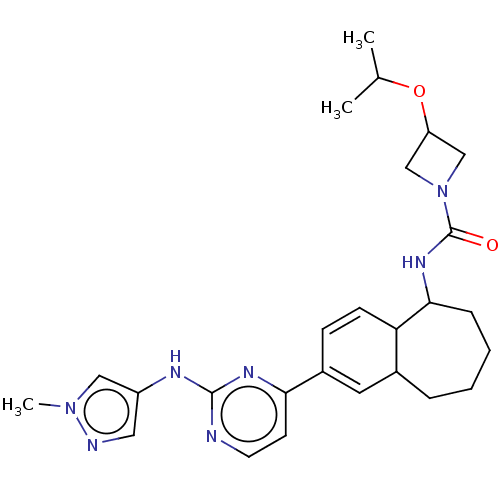
(CHEMBL5091935)Show SMILES CC(C)OC1CN(C1)C(=O)NC1CCCCC2C=C(C=CC12)c1ccnc(Nc2cnn(C)c2)n1 |c:18,20| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583950

(CHEMBL5094998)Show SMILES CN1CC[C@@H](NC(=O)c2cn(nn2)C(C)(C)C)c2ccc(cc2C1)-c1ccnc(Nc2cnn(C)c2)n1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583948
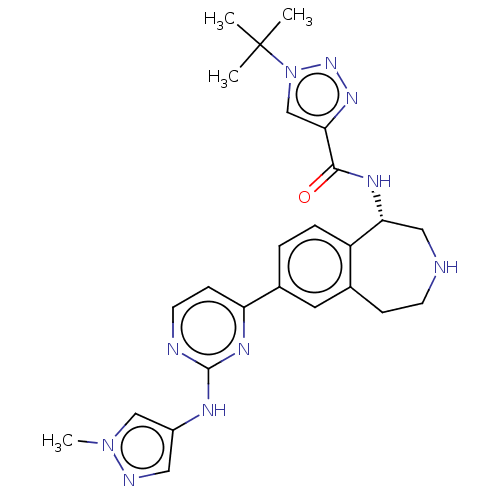
(CHEMBL5081318)Show SMILES Cn1cc(Nc2nccc(n2)-c2ccc3[C@@H](CNCCc3c2)NC(=O)c2cn(nn2)C(C)(C)C)cn1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596431
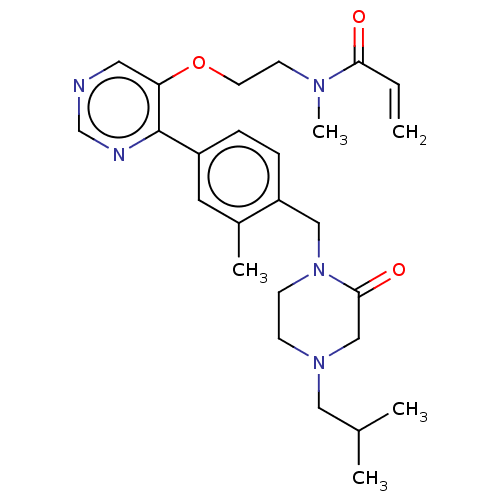
(CHEMBL5197765)Show SMILES CC(C)CN1CCN(Cc2ccc(cc2C)-c2ncncc2OCCN(C)C(=O)C=C)C(=O)C1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596430

(CHEMBL5186635)Show SMILES CN(CCOc1cncnc1-c1ccc(CN2CCN(CC3(C)CC3)CC2=O)c(C)c1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244495
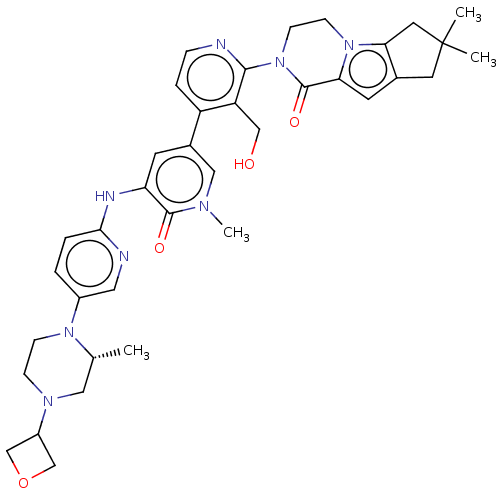
(CHEMBL4066176)Show SMILES C[C@@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50557487
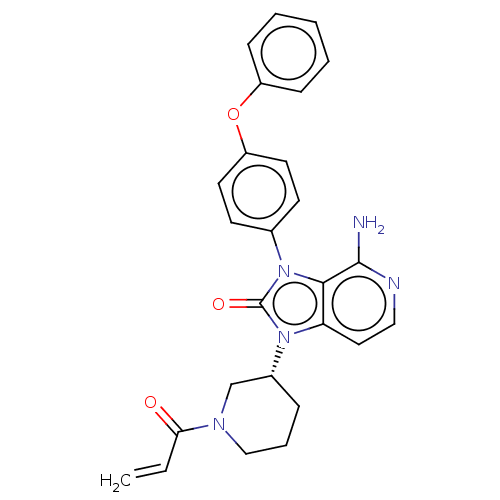
(Tolebrutinib)Show SMILES Nc1nccc2n([C@@H]3CCCN(C3)C(=O)C=C)c(=O)n(-c3ccc(Oc4ccccc4)cc3)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
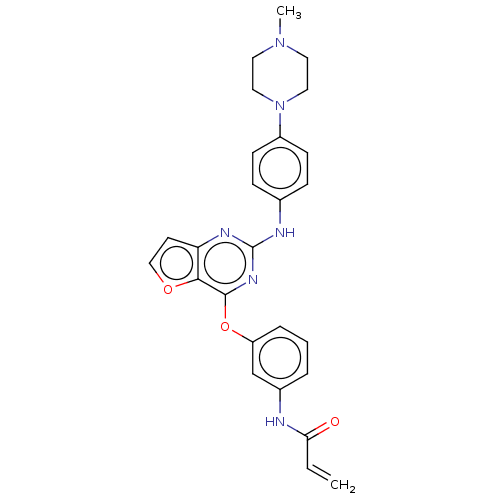
(Homo sapiens (Human)) | BDBM50369724
(HM71224 | Ly3337641 | Poseltinib)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3occc3n2)cc1 Show InChI InChI=1S/C26H26N6O3/c1-3-23(33)27-19-5-4-6-21(17-19)35-25-24-22(11-16-34-24)29-26(30-25)28-18-7-9-20(10-8-18)32-14-12-31(2)13-15-32/h3-11,16-17H,1,12-15H2,2H3,(H,27,33)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
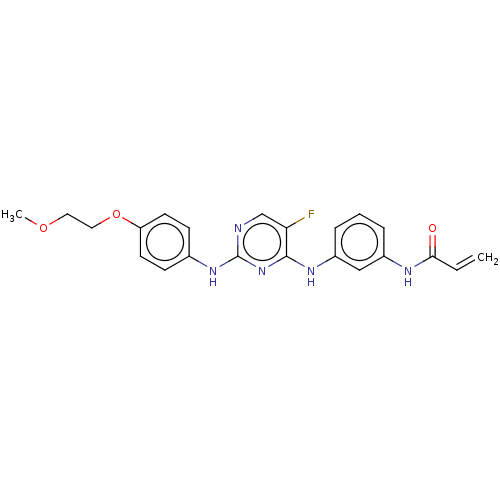
(Homo sapiens (Human)) | BDBM50161162
(AVL-292 | CC-292 | Spebrutinib | US10596172, Compo...)Show SMILES COCCOc1ccc(Nc2ncc(F)c(Nc3cccc(NC(=O)C=C)c3)n2)cc1 Show InChI InChI=1S/C19H18ClN3O/c20-13-9-11-7-8-21-14-6-5-10-3-1-2-4-12(10)16(14)15(11)18-17(13)22-19(24)23-18/h1-4,9,14,16,21H,5-8H2,(H2,22,23,24)/t14-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50459553
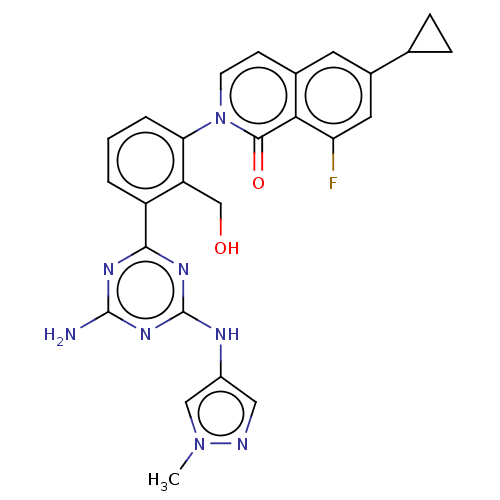
(CHEMBL4209441)Show SMILES Cn1cc(Nc2nc(N)nc(n2)-c2cccc(c2CO)-n2ccc3cc(cc(F)c3c2=O)C2CC2)cn1 Show InChI InChI=1S/C26H23FN8O2/c1-34-12-17(11-29-34)30-26-32-23(31-25(28)33-26)18-3-2-4-21(19(18)13-36)35-8-7-15-9-16(14-5-6-14)10-20(27)22(15)24(35)37/h2-4,7-12,14,36H,5-6,13H2,1H3,(H3,28,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194720
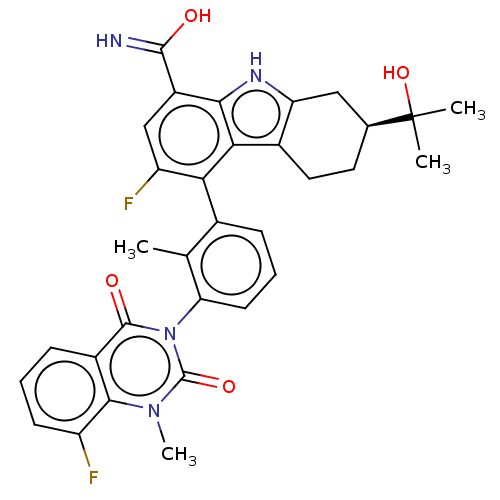
(CHEMBL3900554)Show SMILES [H][C@@]1(CCc2c(C1)[nH]c1c(cc(F)c(-c3cccc(c3C)-n3c(=O)n(C)c4c(F)cccc4c3=O)c21)C(O)=N)C(C)(C)O |r,wU:1.0,(7.64,3.3,;7.24,1.81,;5.84,2.47,;4.58,1.59,;4.71,.05,;6.1,-.6,;7.37,.28,;4.84,-1.48,;3.3,-1.34,;2.9,-2.83,;1.41,-3.22,;.33,-2.13,;-1.16,-2.53,;.73,-.64,;-.36,.45,;.05,1.93,;-1.04,3.03,;-2.53,2.63,;-2.93,1.15,;-1.85,.05,;-2.25,-1.43,;-4.42,.75,;-4.82,-.73,;-3.74,-1.83,;-6.31,-1.13,;-6.71,-2.62,;-7.4,-.04,;-8.89,-.43,;-9.29,-1.92,;-9.97,.66,;-9.57,2.15,;-8.08,2.54,;-6.99,1.45,;-5.51,1.84,;-5.1,3.33,;2.22,-.25,;3.99,-3.92,;5.48,-3.53,;3.58,-5.41,;8.77,1.67,;8.91,3.21,;8.63,.14,;10.3,1.53,)| Show InChI InChI=1S/C32H30F2N4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-21(33)28(19)37(4)31(38)41)25-22(34)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-10,14,16,36,42H,11-13H2,1-4H3,(H2,35,39)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50583930
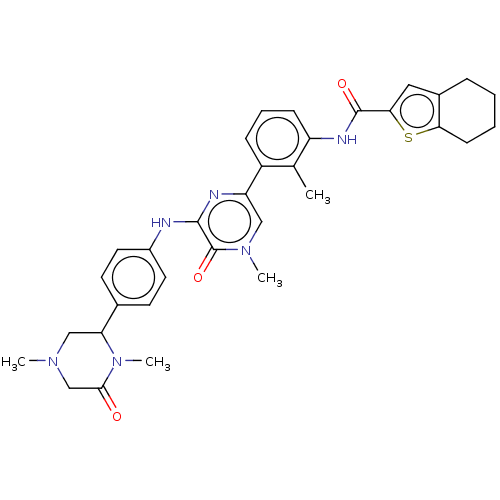
(CHEMBL5069979)Show SMILES CN1CC(N(C)C(=O)C1)c1ccc(Nc2nc(cn(C)c2=O)-c2cccc(NC(=O)c3cc4CCCCc4s3)c2C)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50553436

(CHEMBL4744041)Show SMILES CC(C)OC1CN(C1)C(=O)NCc1ccc(cc1C)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human BTK using fluorescein-labeled polyGAT peptide as substrate incubated for 30 mins by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00926
BindingDB Entry DOI: 10.7270/Q23B641C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50553436

(CHEMBL4744041)Show SMILES CC(C)OC1CN(C1)C(=O)NCc1ccc(cc1C)-c1ccnc(Nc2cnn(C)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length N-terminal His6-tagged BTK expressed in baculovirus infected Sf21 insect cells using fluorescein-labeled ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00702
BindingDB Entry DOI: 10.7270/Q2BK1H1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50596419

(CHEMBL5189116)Show SMILES Cc1cc(ccc1CN1CCN(CC(C)(C)C)CC1=O)-c1ccnc(Nc2cnn(CCO)c2)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128549
BindingDB Entry DOI: 10.7270/Q2C251FV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data