

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240973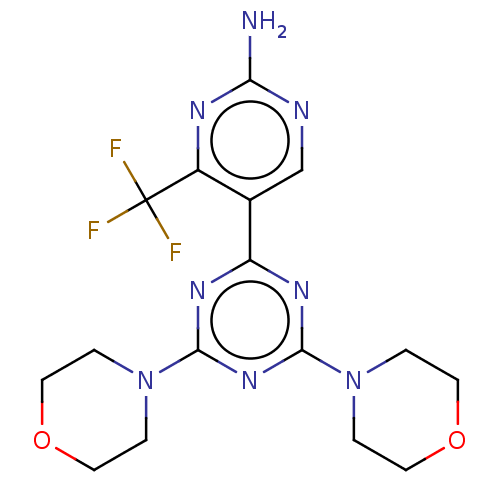 (CHEMBL4102855) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240989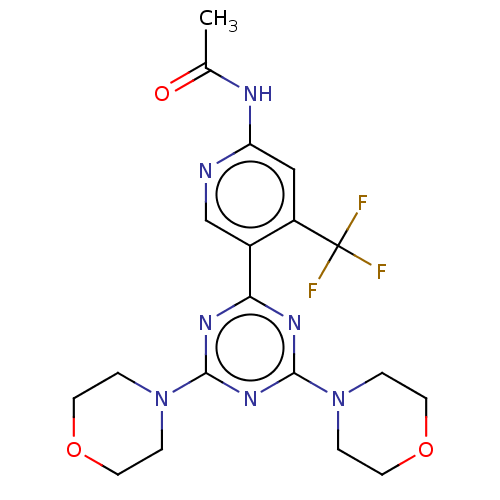 (CHEMBL4081904) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240991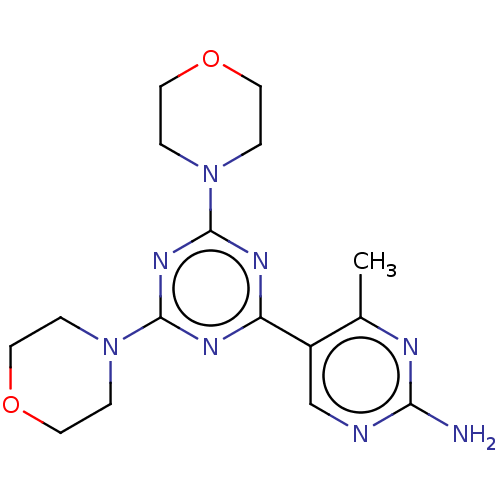 (CHEMBL4092165) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240975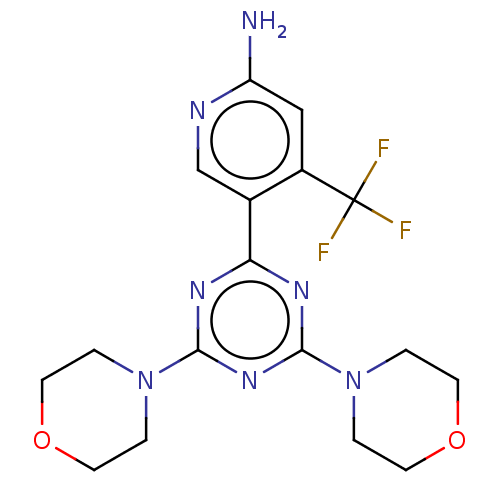 (CHEMBL4084907) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240992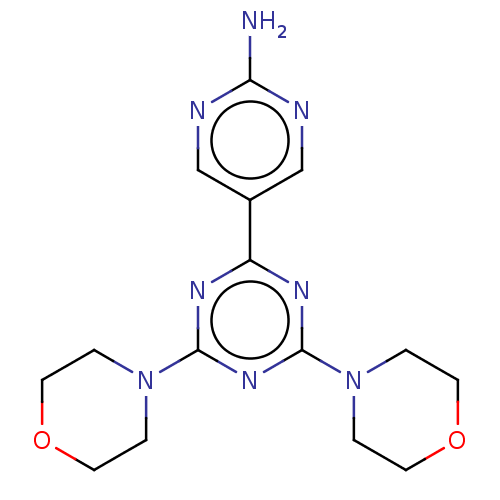 (CHEMBL4097282) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240977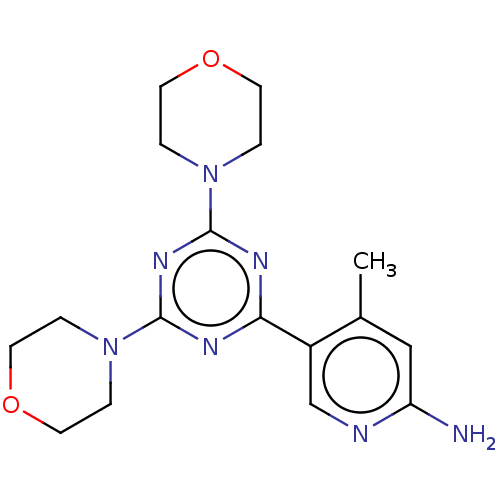 (CHEMBL4071150) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240975 (CHEMBL4084907) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240992 (CHEMBL4097282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240984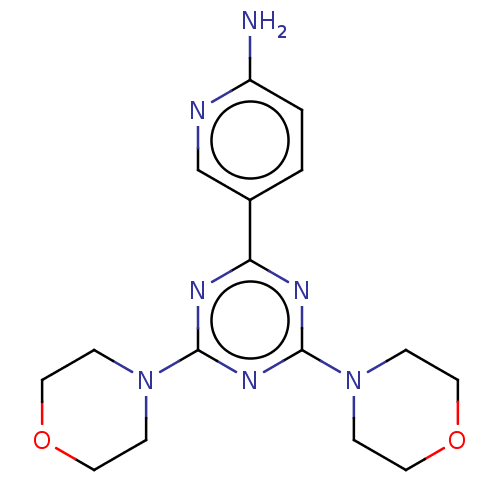 (CHEMBL4068426) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240984 (CHEMBL4068426) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240983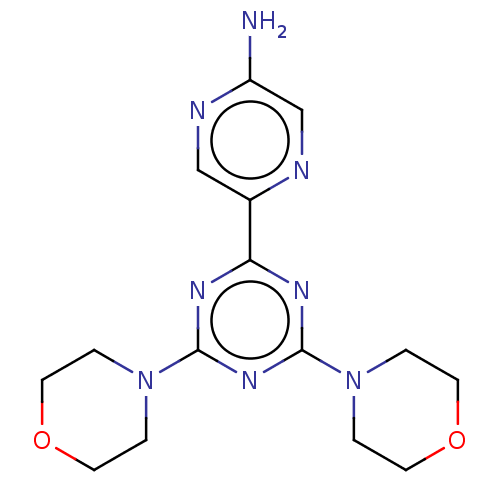 (CHEMBL4079258) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240973 (CHEMBL4102855) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240991 (CHEMBL4092165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240976 (CHEMBL4098203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240995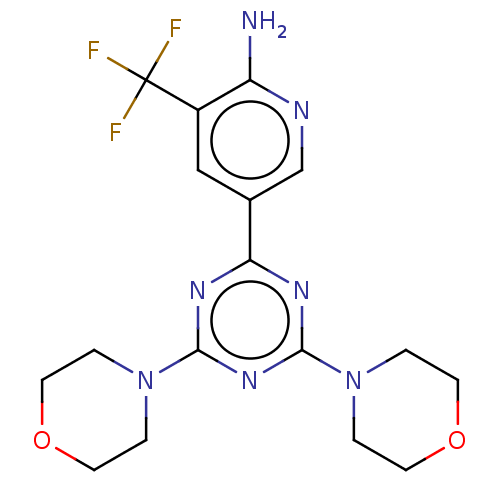 (CHEMBL4071370) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 284 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240985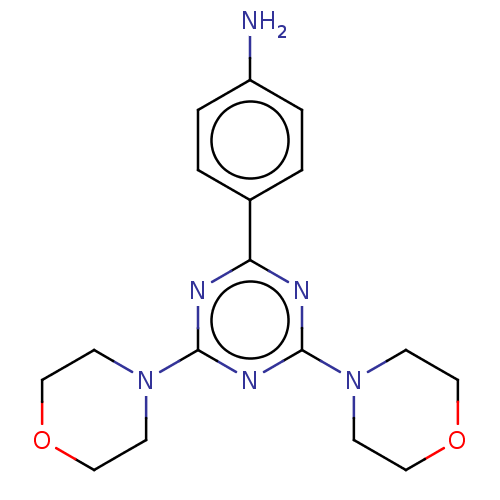 (CHEMBL4089567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240977 (CHEMBL4071150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 609 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240993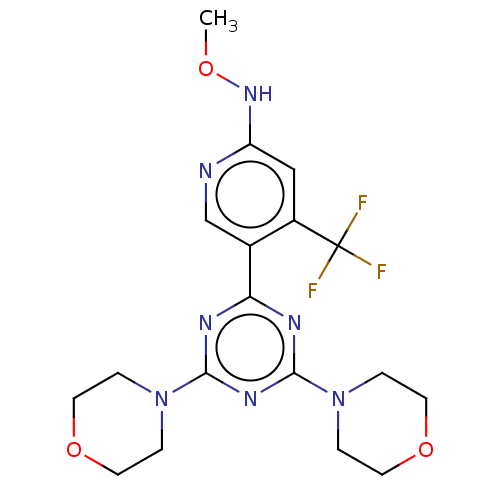 (CHEMBL4074098) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 689 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240983 (CHEMBL4079258) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 911 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240976 (CHEMBL4098203) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240989 (CHEMBL4081904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240985 (CHEMBL4089567) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240994 (CHEMBL4099904) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240993 (CHEMBL4074098) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240988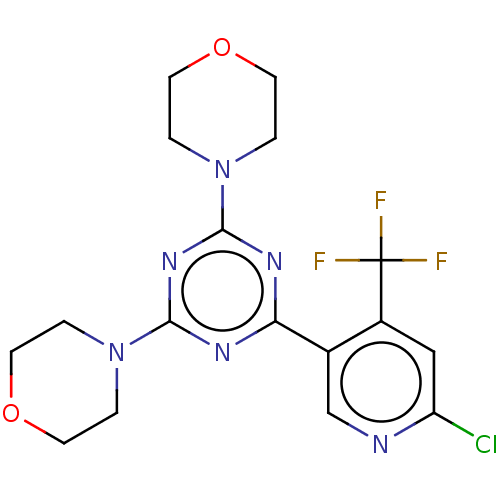 (CHEMBL4100862) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240988 (CHEMBL4100862) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human C-terminal GST-tagged mTOR (1360 to 2549 residues) expressed in baculovirus expression system using after 1 hr AlexaFluor647-labe... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50240987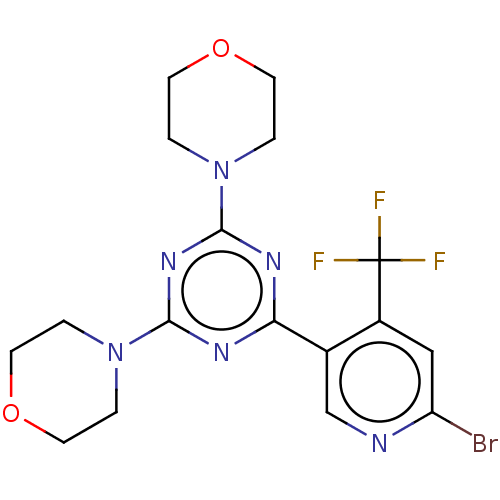 (CHEMBL4062723) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Displacement of [3H]baclofen from gamma-aminobutyric-acid B receptor | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50240995 (CHEMBL4071370) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged PI3K p110alpha/p85alpha expressed in baculovirus expression system after 1 hr using AlexaFluor647-labeled ... | J Med Chem 60: 7524-7538 (2017) Article DOI: 10.1021/acs.jmedchem.7b00930 BindingDB Entry DOI: 10.7270/Q2WM1GKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542881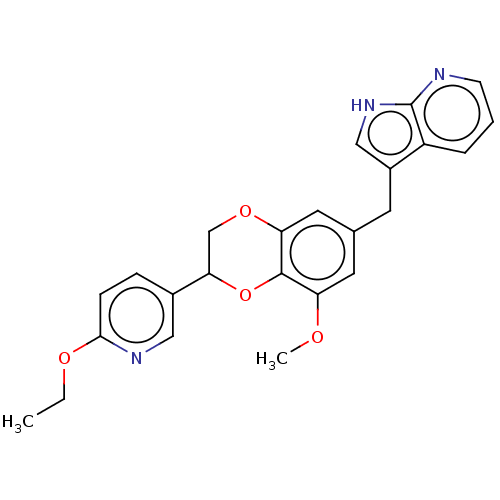 (3-((2-(6-ethoxypyridin-3-yl)-8-methoxy-2,3- dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542862 (4-(3-((2-(4-(difluoromethoxy)phenyl)-8-methoxy-2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542975 (3-(3-methoxy-4-((6-methoxypyridin-3-yl)methoxy)ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542847 (3-((2-(6-ethylpyridin-3-yl)-8-methoxy-2,3- dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542824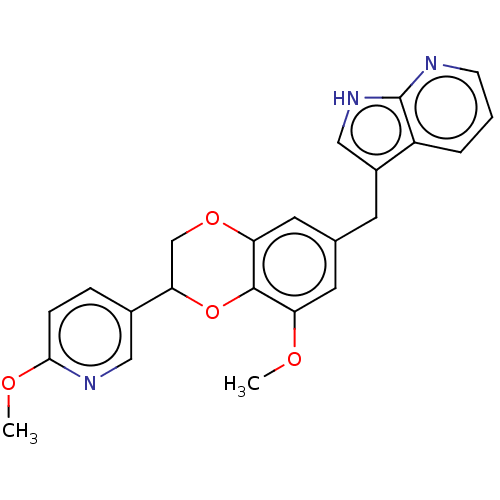 (3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542819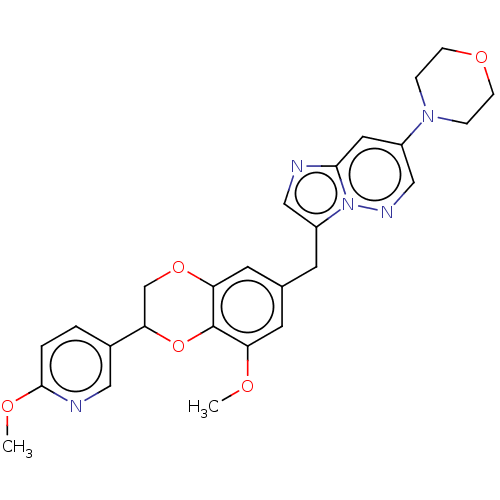 (4-(3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542818 (4-(3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542551 (3-((2-(4-methoxyphenyl)-2,3-dihydrobenzo[b][1,4]di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542814 (4-(3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542815 (6-methoxy-3-((8-methoxy-2-(6-methoxypyridin-3-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542791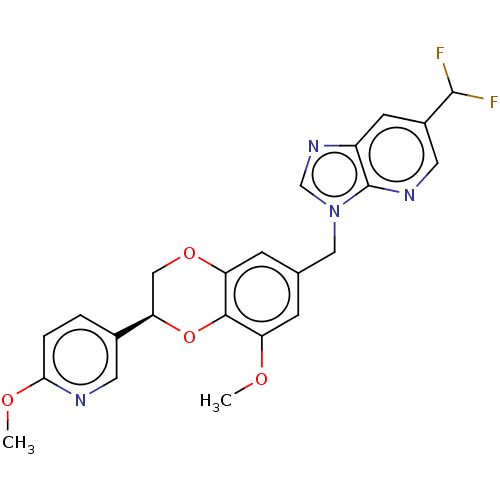 ((S)-6-(difluoromethyl)-3-((8-methoxy-2-(6-methoxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542813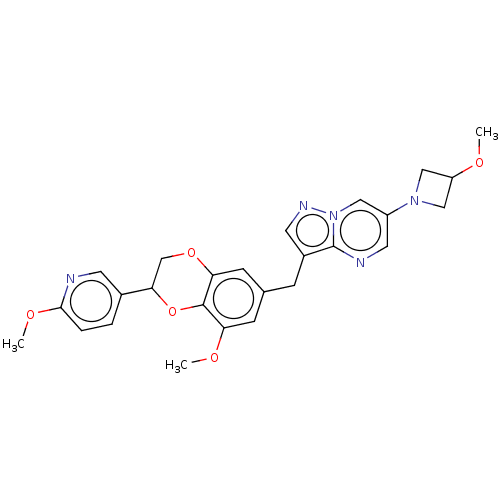 (3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542721 (4-(3-((2-(6-methoxypyridin-3-yl)-2,3- dihydrobenzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542519 ((R)-4-(3-((2-(4-methoxyphenyl)-2,3- imidazo[4,5-b]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542518 ((S)-4-(3-((2-(4-methoxyphenyl)-2,3- imidazo[4,5-b]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542974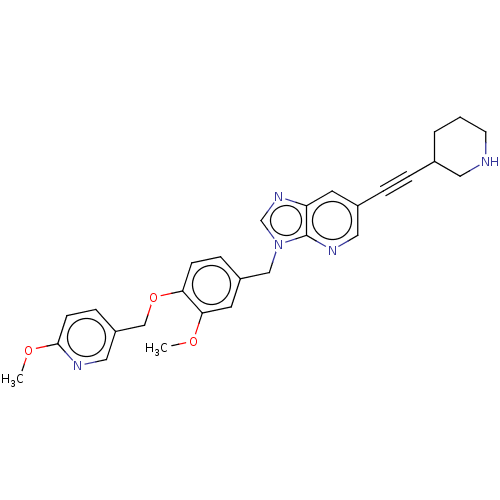 (3-(3-methoxy-4-((6-methoxypyridin-3-yl)methoxy)ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542516 (4-(1-((2-(4-methoxyphenyl)-2,3- dihydrobenzo[b][1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542816 (3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542820 (3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542848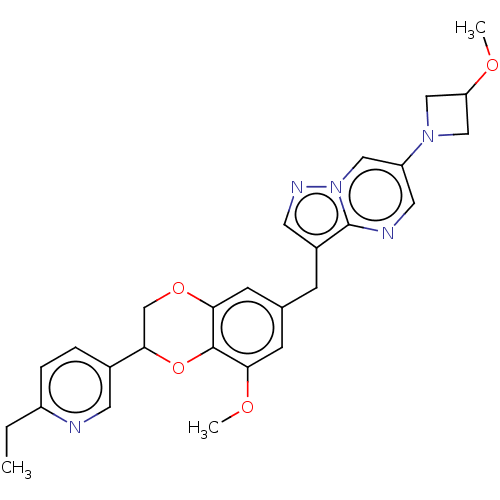 (3-((2-(6-ethylpyridin-3-yl)-8-methoxy-2,3- dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542753 (3-((8-methoxy-2-(6-methoxypyridin-3-yl)-2,3- dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM542777 (6-(1,3-dimethyl-1H-pyrazol-4-yl)-3-((8-methoxy-2-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description All assay reaction conditions for IC50 determinations were within the linear range with respect to time and enzyme concentration. In a 384 well polyp... | Citation and Details BindingDB Entry DOI: 10.7270/Q2QC06QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 400 total ) | Next | Last >> |