 Found 73 hits with Last Name = 'smart' and Initial = 'b'
Found 73 hits with Last Name = 'smart' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein cereblon
(Homo sapiens (Human)) | BDBM50586185
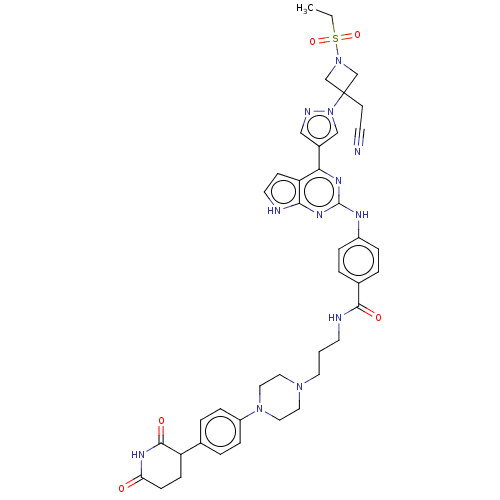
(CHEMBL5090416)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCN2CCN(CC2)c2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586179
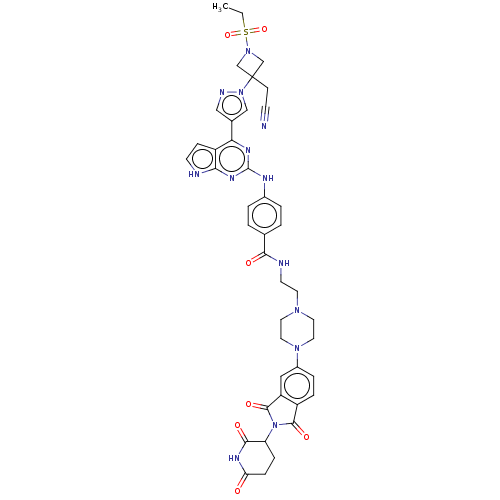
(CHEMBL4744617)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCN2CCN(CC2)c2ccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c3c2)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586184

(CHEMBL5078429)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCN2CCN(CC2)c2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586180

(CHEMBL5082689)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCNc2cccc(c2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586176
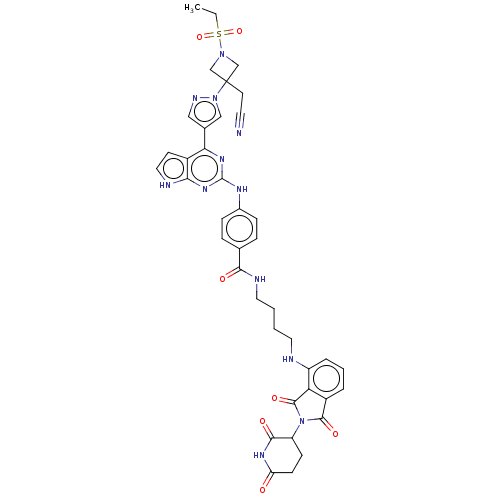
(CHEMBL4742130)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCNc2cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c23)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476682
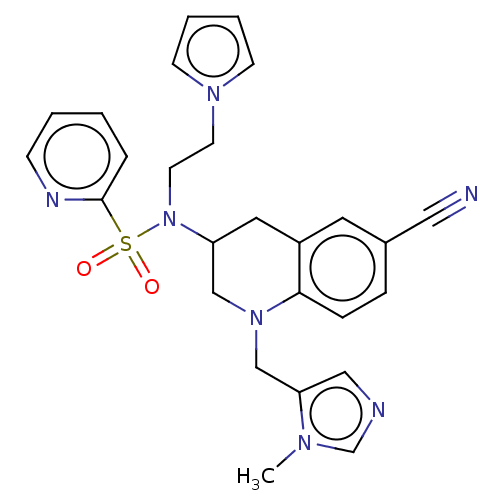
(CHEMBL428712 | PB-43)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CCn1cccc1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C26H27N7O2S/c1-30-20-28-17-24(30)19-32-18-23(15-22-14-21(16-27)7-8-25(22)32)33(13-12-31-10-4-5-11-31)36(34,35)26-6-2-3-9-29-26/h2-11,14,17,20,23H,12-13,15,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476678
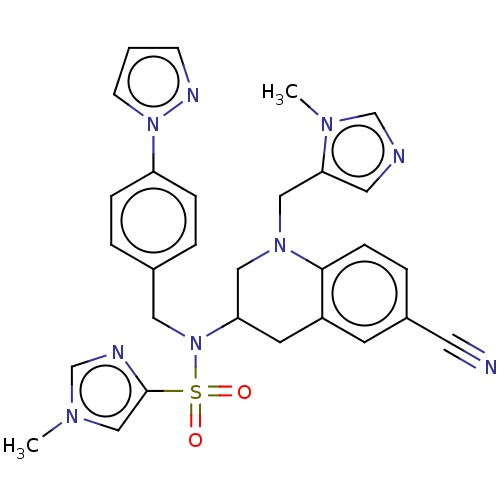
(CHEMBL395222)Show SMILES Cn1cnc(c1)S(=O)(=O)N(Cc1ccc(cc1)-n1cccn1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C29H29N9O2S/c1-34-19-29(32-21-34)41(39,40)38(16-22-4-7-25(8-5-22)37-11-3-10-33-37)26-13-24-12-23(14-30)6-9-28(24)36(17-26)18-27-15-31-20-35(27)2/h3-12,15,19-21,26H,13,16-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13328
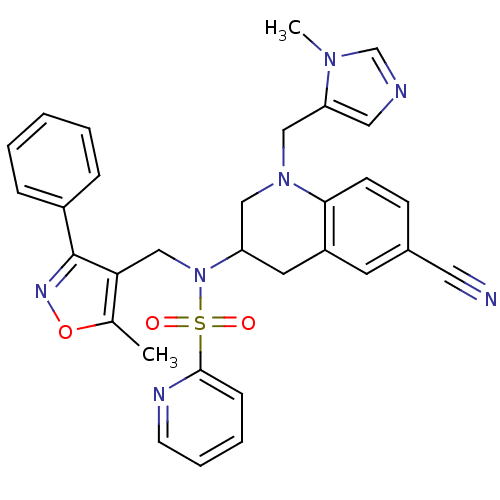
(N-{6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1...)Show SMILES Cc1onc(c1CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1ccccn1)-c1ccccc1 Show InChI InChI=1S/C31H29N7O3S/c1-22-28(31(35-41-22)24-8-4-3-5-9-24)20-38(42(39,40)30-10-6-7-13-34-30)26-15-25-14-23(16-32)11-12-29(25)37(18-26)19-27-17-33-21-36(27)2/h3-14,17,21,26H,15,18-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476685

(CHEMBL232104)Show SMILES COC(=O)N1CCC(CN(C2CN(Cc3cncn3C)c3ccc(cc3C2)C#N)S(=O)(=O)c2ccccn2)CC1 Show InChI InChI=1S/C28H33N7O4S/c1-32-20-30-16-25(32)19-34-18-24(14-23-13-22(15-29)6-7-26(23)34)35(40(37,38)27-5-3-4-10-31-27)17-21-8-11-33(12-9-21)28(36)39-2/h3-7,10,13,16,20-21,24H,8-9,11-12,14,17-19H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13324
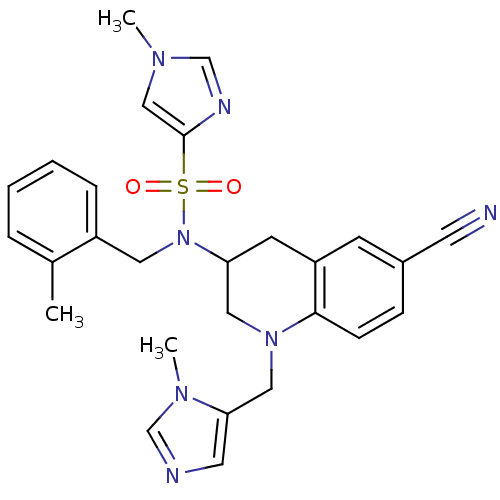
(1-Methyl-1H-imidazole-4-sulfonic Acid [6-Cyano-1-(...)Show SMILES Cc1ccccc1CN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cn(C)cn1 Show InChI InChI=1S/C27H29N7O2S/c1-20-6-4-5-7-22(20)14-34(37(35,36)27-17-31(2)19-30-27)24-11-23-10-21(12-28)8-9-26(23)33(15-24)16-25-13-29-18-32(25)3/h4-10,13,17-19,24H,11,14-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586177

(CHEMBL5090604)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCOc2cccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c23)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13327
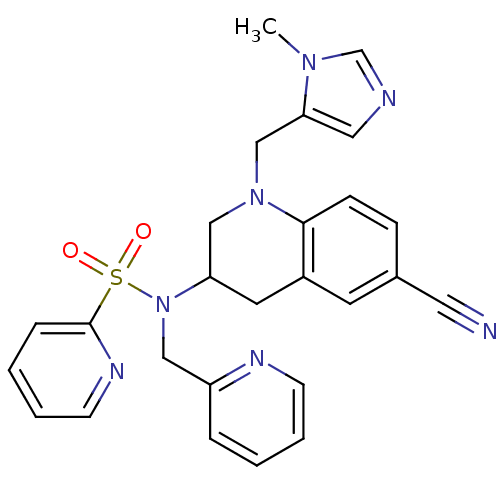
(N-{6-cyano-1-[(1-methyl-1H-imidazol-5-yl)methyl]-1...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1ccccn1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C26H25N7O2S/c1-31-19-28-15-24(31)18-32-17-23(13-21-12-20(14-27)8-9-25(21)32)33(16-22-6-2-4-10-29-22)36(34,35)26-7-3-5-11-30-26/h2-12,15,19,23H,13,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476680
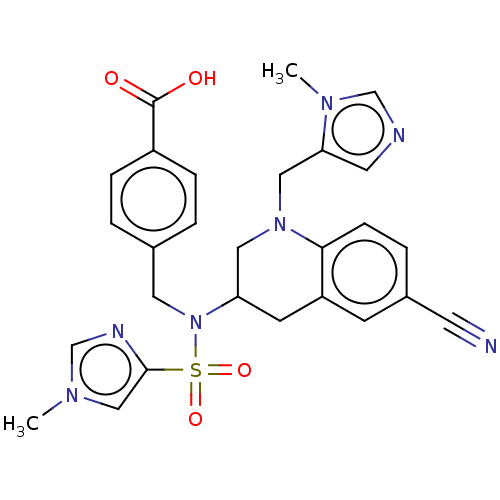
(CHEMBL234757)Show SMILES Cn1cnc(c1)S(=O)(=O)N(Cc1ccc(cc1)C(O)=O)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C27H27N7O4S/c1-31-16-26(30-18-31)39(37,38)34(13-19-3-6-21(7-4-19)27(35)36)23-10-22-9-20(11-28)5-8-25(22)33(14-23)15-24-12-29-17-32(24)2/h3-9,12,16-18,23H,10,13-15H2,1-2H3,(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13335

(N-tert-Butyl-2-[[6-cyano-1-(3-methyl-3H-imidazol-4...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC(=O)NC(C)(C)C)S(=O)(=O)c1nccn1C Show InChI InChI=1S/C25H32N8O3S/c1-25(2,3)29-23(34)16-33(37(35,36)24-28-8-9-30(24)4)20-11-19-10-18(12-26)6-7-22(19)32(14-20)15-21-13-27-17-31(21)5/h6-10,13,17,20H,11,14-16H2,1-5H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476679
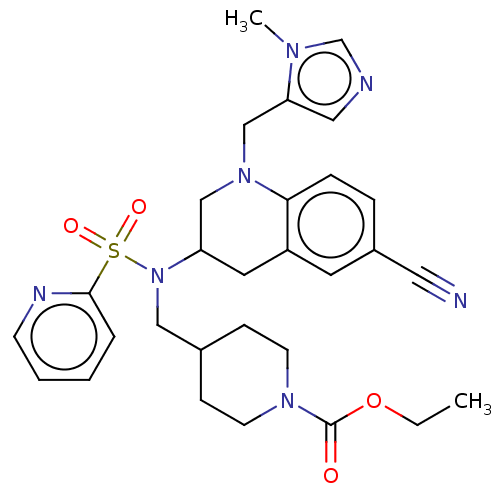
(CHEMBL232523)Show SMILES CCOC(=O)N1CCC(CN(C2CN(Cc3cncn3C)c3ccc(cc3C2)C#N)S(=O)(=O)c2ccccn2)CC1 Show InChI InChI=1S/C29H35N7O4S/c1-3-40-29(37)34-12-9-22(10-13-34)18-36(41(38,39)28-6-4-5-11-32-28)25-15-24-14-23(16-30)7-8-27(24)35(19-25)20-26-17-31-21-33(26)2/h4-8,11,14,17,21-22,25H,3,9-10,12-13,15,18-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476681
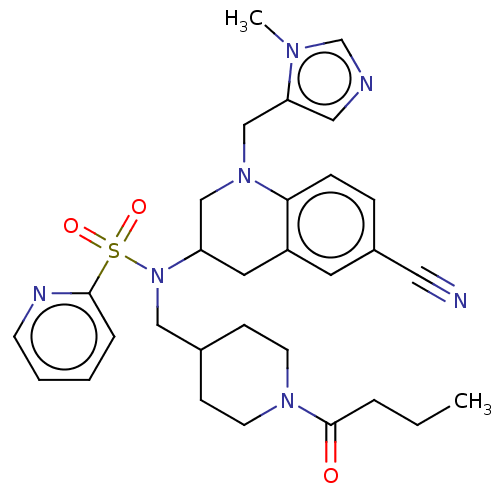
(CHEMBL234151 | PB-91)Show SMILES CCCC(=O)N1CCC(CN(C2CN(Cc3cncn3C)c3ccc(cc3C2)C#N)S(=O)(=O)c2ccccn2)CC1 Show InChI InChI=1S/C30H37N7O3S/c1-3-6-30(38)35-13-10-23(11-14-35)19-37(41(39,40)29-7-4-5-12-33-29)26-16-25-15-24(17-31)8-9-28(25)36(20-26)21-27-18-32-22-34(27)2/h4-5,7-9,12,15,18,22-23,26H,3,6,10-11,13-14,16,19-21H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476676

(CHEMBL231662)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC1CCN(CC1)C(=O)OC(C)(C)C)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C31H39N7O4S/c1-31(2,3)42-30(39)36-13-10-23(11-14-36)19-38(43(40,41)29-7-5-6-12-34-29)26-16-25-15-24(17-32)8-9-28(25)37(20-26)21-27-18-33-22-35(27)4/h5-9,12,15,18,22-23,26H,10-11,13-14,16,19-21H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476675

(CHEMBL233742)Show SMILES COCCCN(C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N)S(=O)(=O)c1cn(C)cn1 Show InChI InChI=1S/C23H29N7O3S/c1-27-15-23(26-17-27)34(31,32)30(7-4-8-33-3)20-10-19-9-18(11-24)5-6-22(19)29(13-20)14-21-12-25-16-28(21)2/h5-6,9,12,15-17,20H,4,7-8,10,13-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476677
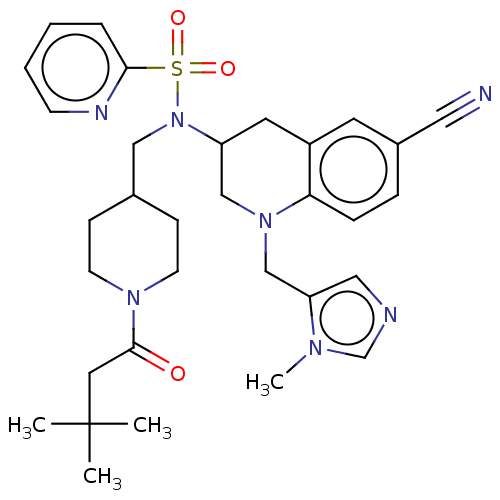
(CHEMBL394086 | PB-90)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC1CCN(CC1)C(=O)CC(C)(C)C)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C32H41N7O3S/c1-32(2,3)17-31(40)37-13-10-24(11-14-37)20-39(43(41,42)30-7-5-6-12-35-30)27-16-26-15-25(18-33)8-9-29(26)38(21-27)22-28-19-34-23-36(28)4/h5-9,12,15,19,23-24,27H,10-11,13-14,16-17,20-22H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13330

(N-[(6-chloro-2,1,3-benzothiadiazol-5-yl)methyl]-N-...)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(Cc1cc2nsnc2cc1Cl)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C27H23ClN8O2S2/c1-34-17-30-13-22(34)16-35-15-21(9-19-8-18(12-29)5-6-26(19)35)36(40(37,38)27-4-2-3-7-31-27)14-20-10-24-25(11-23(20)28)33-39-32-24/h2-8,10-11,13,17,21H,9,14-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586187
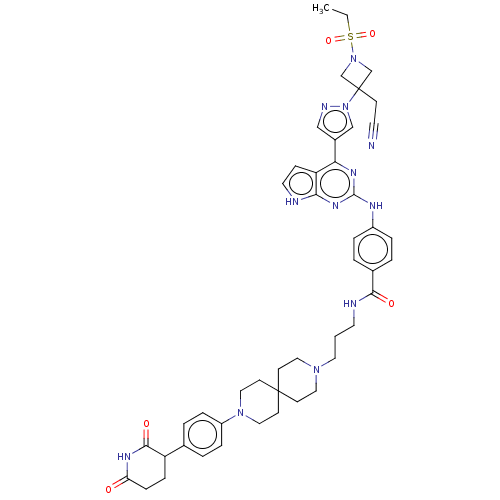
(CHEMBL5074539)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCN2CCC3(CC2)CCN(CC3)c2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476674
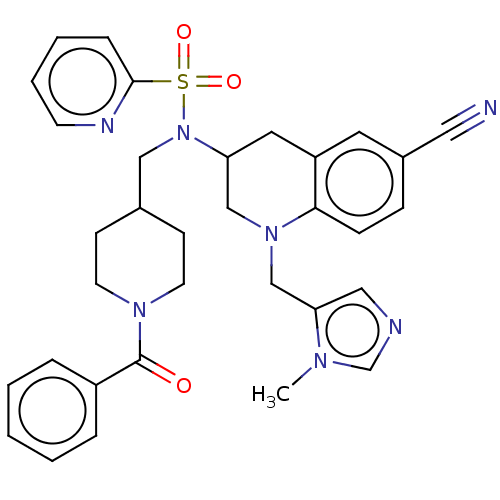
(CHEMBL394407 | PB-95)Show SMILES Cn1cncc1CN1CC(Cc2cc(ccc12)C#N)N(CC1CCN(CC1)C(=O)c1ccccc1)S(=O)(=O)c1ccccn1 Show InChI InChI=1S/C33H35N7O3S/c1-37-24-35-20-30(37)23-39-22-29(18-28-17-26(19-34)10-11-31(28)39)40(44(42,43)32-9-5-6-14-36-32)21-25-12-15-38(16-13-25)33(41)27-7-3-2-4-8-27/h2-11,14,17,20,24-25,29H,12-13,15-16,18,21-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586182

(CHEMBL5073737)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCOc2cccc(c2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586181
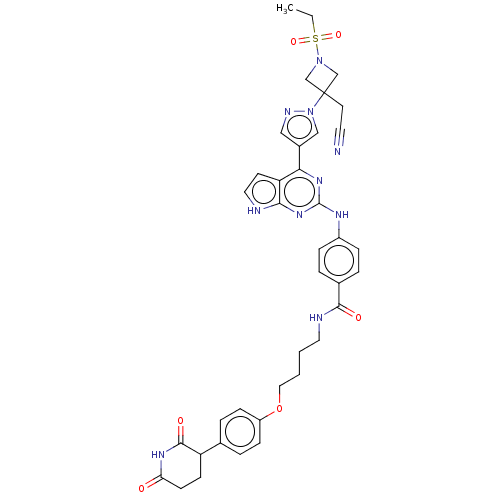
(CHEMBL5082136)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCCOc2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586178

(CHEMBL5092577)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NC2CCN(CC2)c2ccc3C(=O)N(C4CCC(=O)NC4=O)C(=O)c3c2)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein cereblon
(Homo sapiens (Human)) | BDBM50586186
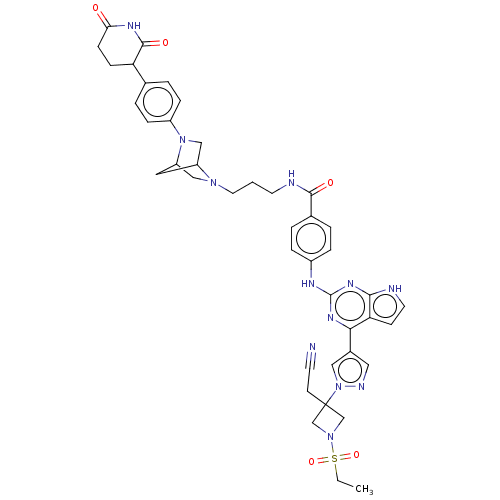
(CHEMBL5077200)Show SMILES CCS(=O)(=O)N1CC(CC#N)(C1)n1cc(cn1)-c1nc(Nc2ccc(cc2)C(=O)NCCCN2CC3CC2CN3c2ccc(cc2)C2CCC(=O)NC2=O)nc2[nH]ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to CRBN (unknown origin) measured by Fluorescent Polarization assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00650
BindingDB Entry DOI: 10.7270/Q2D79GBF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476684

(CHEMBL392237)Show SMILES CC(C)COC(=O)N1CCC(CN(C2CN(Cc3cncn3C)c3ccc(cc3C2)C#N)S(=O)(=O)c2ccccn2)CC1 Show InChI InChI=1S/C31H39N7O4S/c1-23(2)21-42-31(39)36-12-9-24(10-13-36)18-38(43(40,41)30-6-4-5-11-34-30)27-15-26-14-25(16-32)7-8-29(26)37(19-27)20-28-17-33-22-35(28)3/h4-8,11,14,17,22-24,27H,9-10,12-13,15,18-21H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50186585
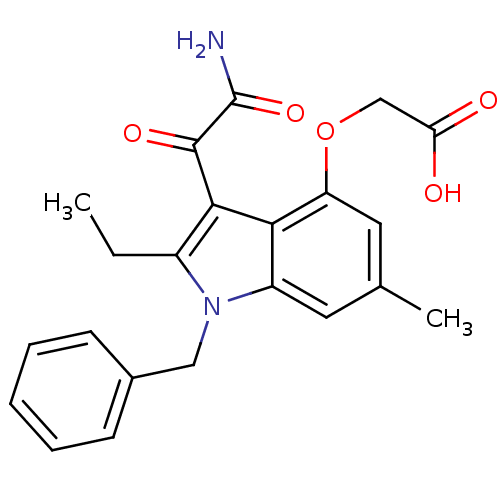
(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50186585

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50186585

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50186585

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G10 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Mus musculus) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G10 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Mus musculus) | BDBM50186585

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G10 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G10 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50476683
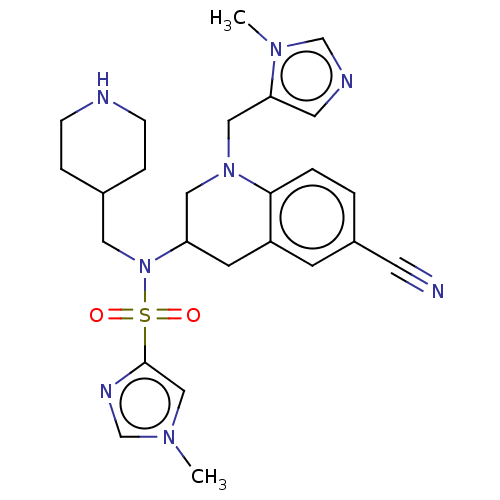
(CHEMBL234161)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CC1CCNCC1)C1CN(Cc2cncn2C)c2ccc(cc2C1)C#N Show InChI InChI=1S/C25H32N8O2S/c1-30-16-25(29-18-30)36(34,35)33(13-19-5-7-27-8-6-19)22-10-21-9-20(11-26)3-4-24(21)32(14-22)15-23-12-28-17-31(23)2/h3-4,9,12,16-19,22,27H,5-8,10,13-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of protein farnesyltransferase |
J Med Chem 50: 4585-605 (2007)
Article DOI: 10.1021/jm0703340
BindingDB Entry DOI: 10.7270/Q2RX9FT9 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50186585

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Mus musculus) | BDBM50186586
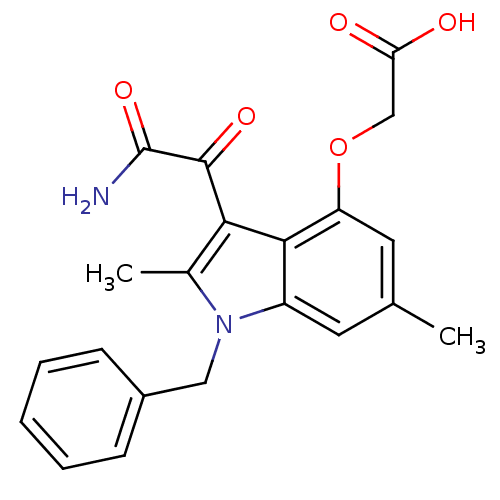
(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2,6-dimethyl-1...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-12-8-15-19(16(9-12)28-11-17(24)25)18(20(26)21(22)27)13(2)23(15)10-14-6-4-3-5-7-14/h3-9H,10-11H2,1-2H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50186586

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2,6-dimethyl-1...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-12-8-15-19(16(9-12)28-11-17(24)25)18(20(26)21(22)27)13(2)23(15)10-14-6-4-3-5-7-14/h3-9H,10-11H2,1-2H3,(H2,22,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Mus musculus) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G1B |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Mus musculus) | BDBM50186585

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G1B |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50186586

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2,6-dimethyl-1...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-12-8-15-19(16(9-12)28-11-17(24)25)18(20(26)21(22)27)13(2)23(15)10-14-6-4-3-5-7-14/h3-9H,10-11H2,1-2H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G2A |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50186586

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2,6-dimethyl-1...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-12-8-15-19(16(9-12)28-11-17(24)25)18(20(26)21(22)27)13(2)23(15)10-14-6-4-3-5-7-14/h3-9H,10-11H2,1-2H3,(H2,22,27)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Group IIE secretory phospholipase A2
(Mus musculus) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G2E |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data