

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2208 ((6S)-3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2204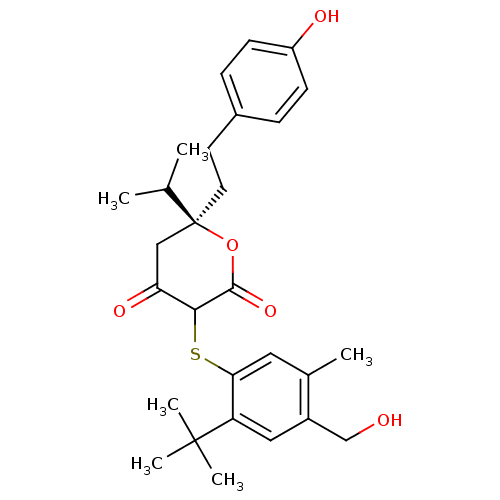 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2206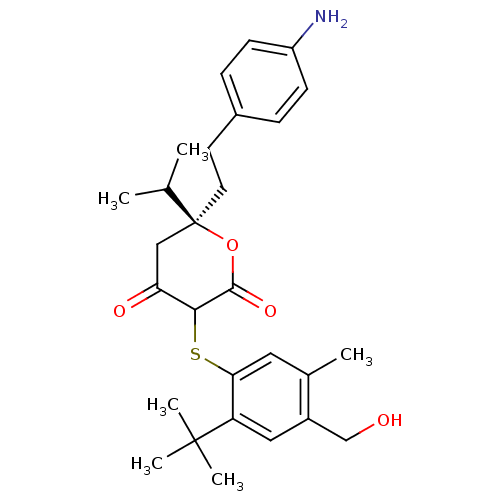 ((6S)-6-[2-(4-aminophenyl)ethyl]-3-{[2-tert-butyl-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2533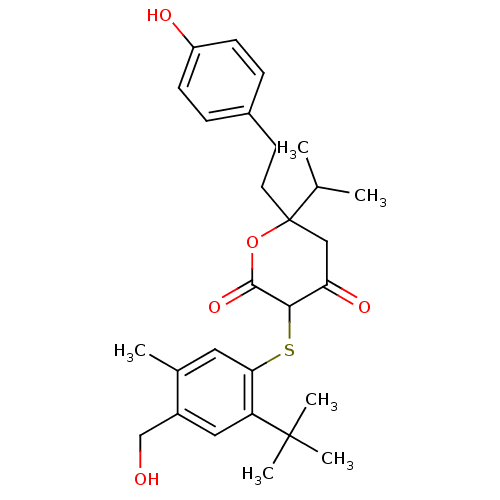 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2536 (6-Alkyl-6-phenethyldihydropyrone 13y | 6-[2-(4-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM448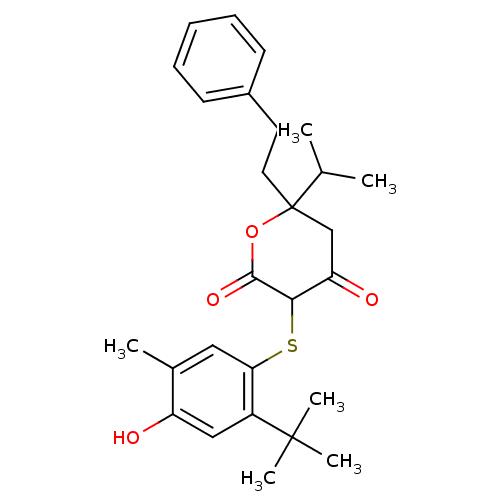 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2166 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18.4 | -45.9 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2207 ((6R)-6-[2-(4-aminophenyl)ethyl]-3-{[2-tert-butyl-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2205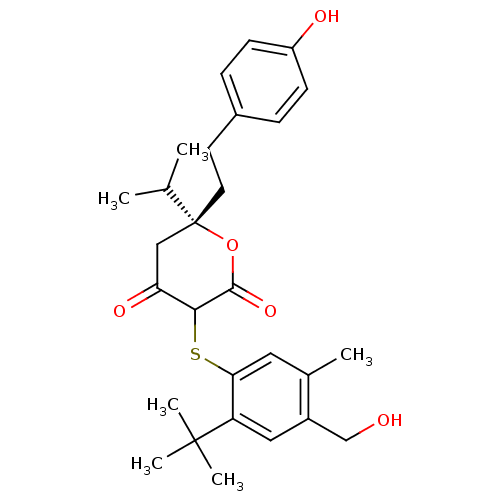 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2209 ((6R)-3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006848 (CHEMBL88091 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50045292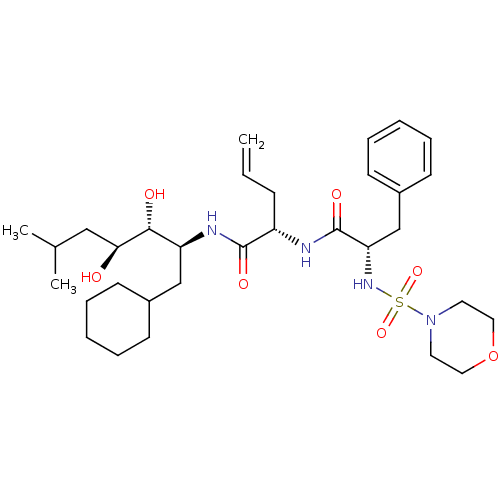 ((S)-2-[(S)-2-(Morpholine-4-sulfonylamino)-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006850 (CHEMBL313063 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006858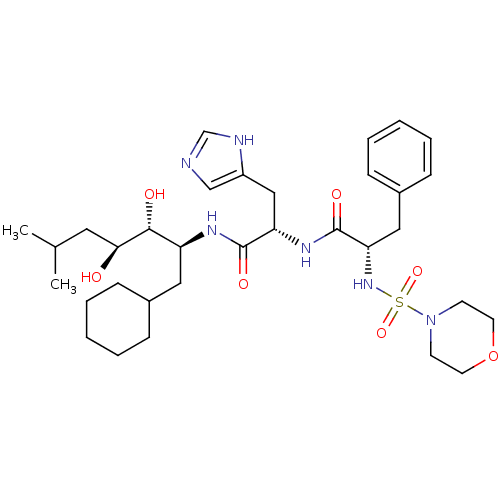 (CHEMBL315380 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006854 (CHEMBL88069 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006853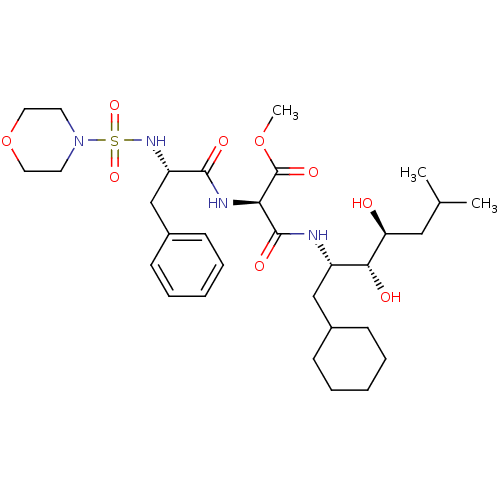 (CHEMBL315211 | N-(1-Cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006845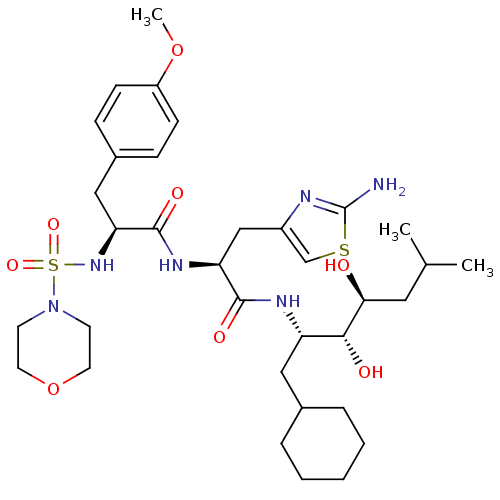 (CHEMBL316208 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844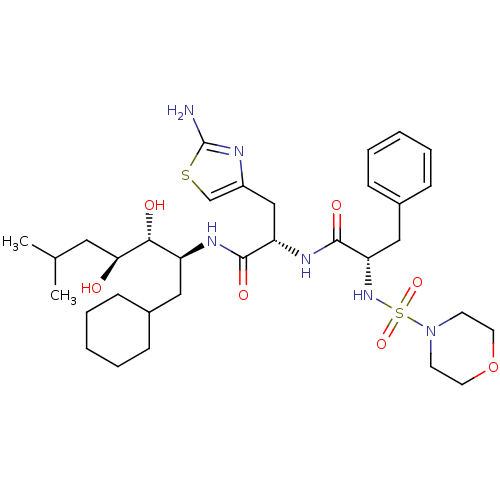 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of plasma renin activity in monkey | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50283014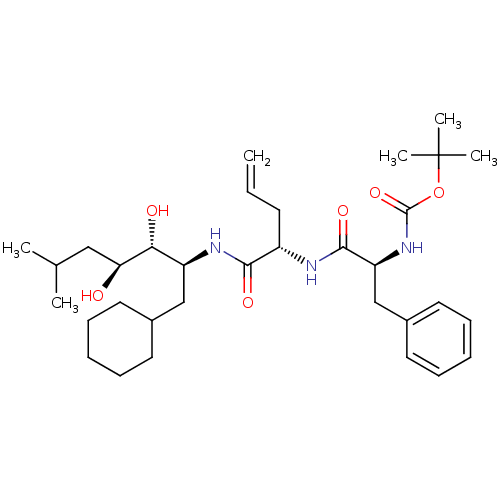 (CHEMBL291139 | {(S)-1-[(S)-1-((1S,2R,3S)-1-Cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against monkey plasma renin | Bioorg Med Chem Lett 4: 2029-2034 (1994) Article DOI: 10.1016/S0960-894X(01)80557-6 BindingDB Entry DOI: 10.7270/Q2RB74JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006851 (CHEMBL263531 | N-[1-(1-Cyclohexylmethyl-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006856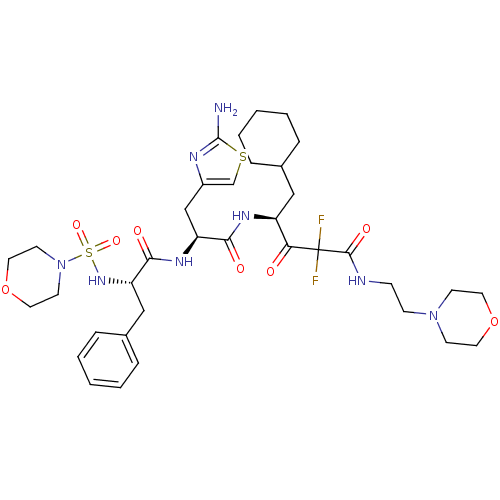 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006846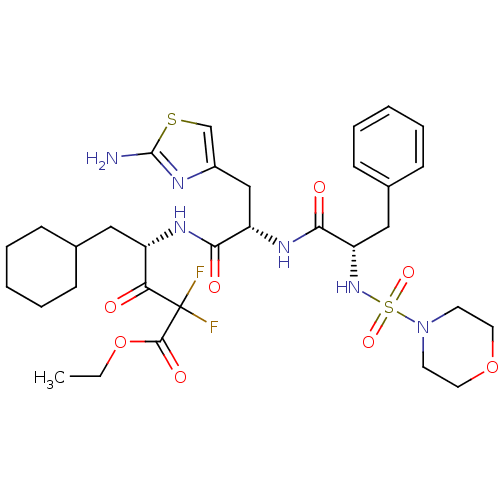 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of plasma renin activity in human | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006843 (CHEMBL314066 | N-[(2-Amino-thiazol-4-yl)-(1-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.581 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006849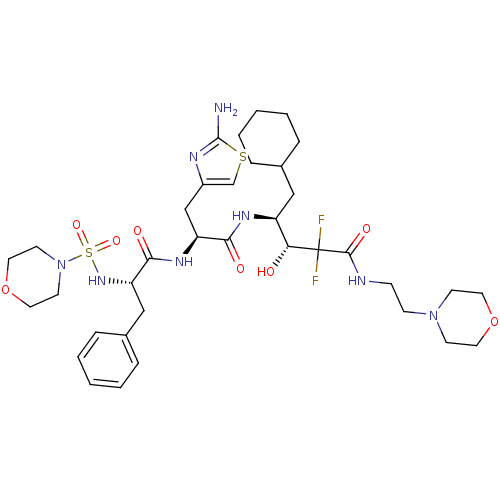 (4-{3-(2-Amino-thiazol-4-yl)-2-[2-(morpholine-4-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006202 (3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17941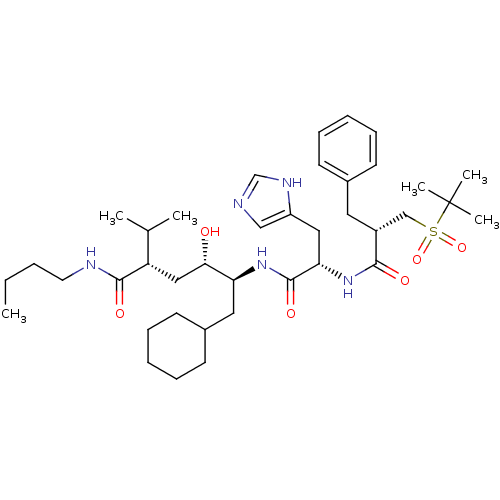 ((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006860 (CHEMBL85672 | {1-[2-(2-Amino-thiazol-4-yl)-1-(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2179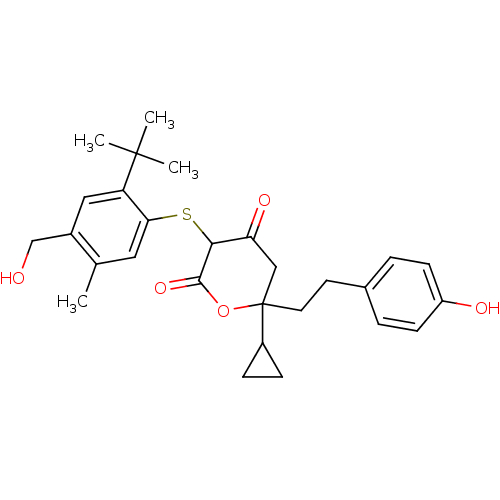 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50231035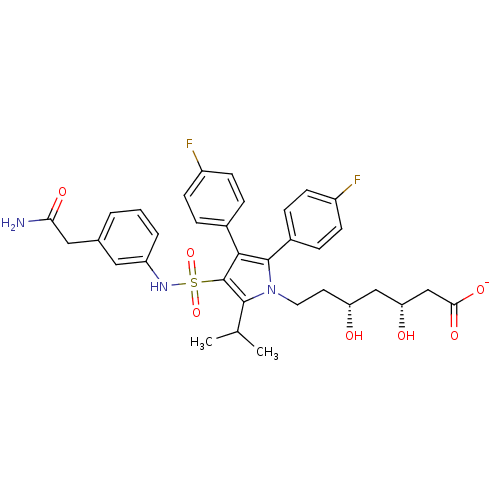 (CHEMBL401950 | sodium; (3R,5R)-7-[3-(3-carbamoylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat microsomal HMGCoA reductase | Bioorg Med Chem Lett 18: 1151-6 (2008) Article DOI: 10.1016/j.bmcl.2007.11.124 BindingDB Entry DOI: 10.7270/Q2TQ6191 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2198 ((3-(2-tert-Butyl-5-methyl-phenylsulfanyl)-4-hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2526 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | J Med Chem 40: 3707-11 (1997) Article DOI: 10.1021/jm970522y BindingDB Entry DOI: 10.7270/Q2ZC812F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50231037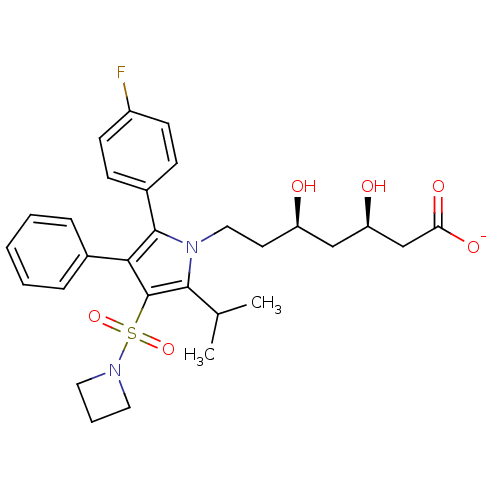 (CHEMBL253054 | sodium (3R,5R)-7-(3-(azetidin-1-yls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat microsomal HMGCoA reductase | Bioorg Med Chem Lett 18: 1151-6 (2008) Article DOI: 10.1016/j.bmcl.2007.11.124 BindingDB Entry DOI: 10.7270/Q2TQ6191 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50231039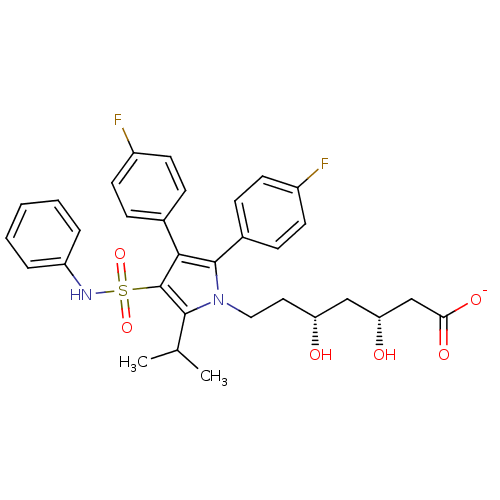 (CHEMBL252657 | sodium; (3R,5R)-7-[2,3-bis-(4-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat microsomal HMGCoA reductase | Bioorg Med Chem Lett 18: 1151-6 (2008) Article DOI: 10.1016/j.bmcl.2007.11.124 BindingDB Entry DOI: 10.7270/Q2TQ6191 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006844 ((S)-N-[(S)-2-(2-Amino-thiazol-4-yl)-1-((1S,2R,3S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of plasma renin activity in monkey | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2180 (3-(2- tert -Butyl-4-hydroxymethyl -5-methyl-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2200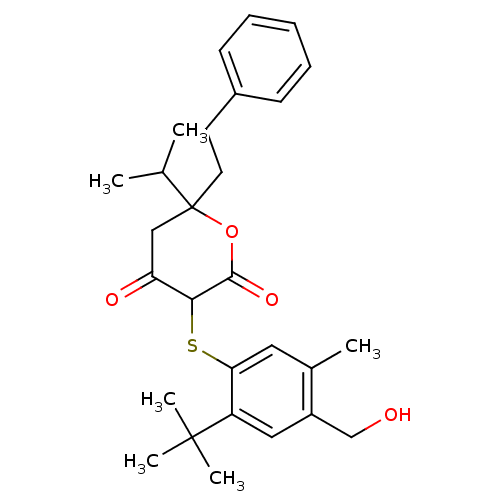 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006864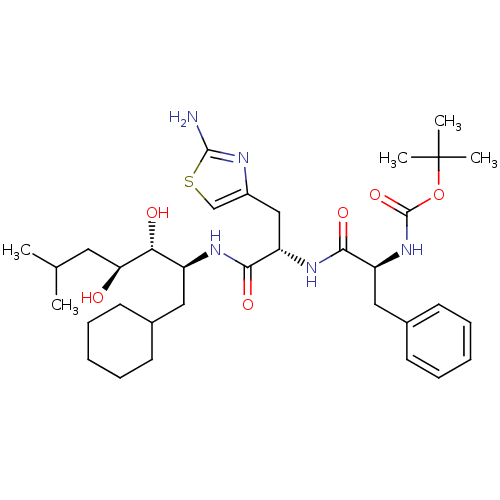 (CHEMBL420417 | {1-[2-(2-Amino-thiazol-4-yl)-1-(1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2532 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2523 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | J Med Chem 40: 3707-11 (1997) Article DOI: 10.1021/jm970522y BindingDB Entry DOI: 10.7270/Q2ZC812F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50231028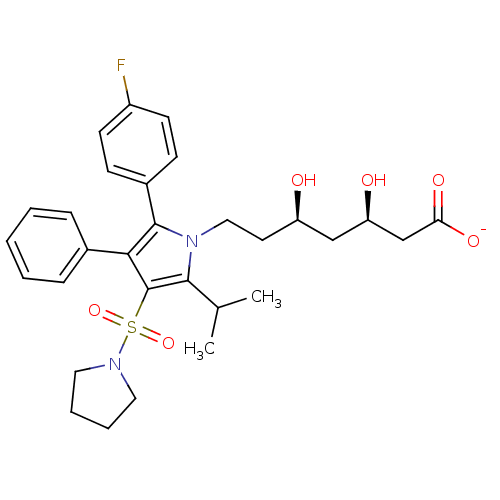 (CHEMBL429487 | sodium (3R,5R)-7-(2-(4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat microsomal HMGCoA reductase | Bioorg Med Chem Lett 18: 1151-6 (2008) Article DOI: 10.1016/j.bmcl.2007.11.124 BindingDB Entry DOI: 10.7270/Q2TQ6191 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2174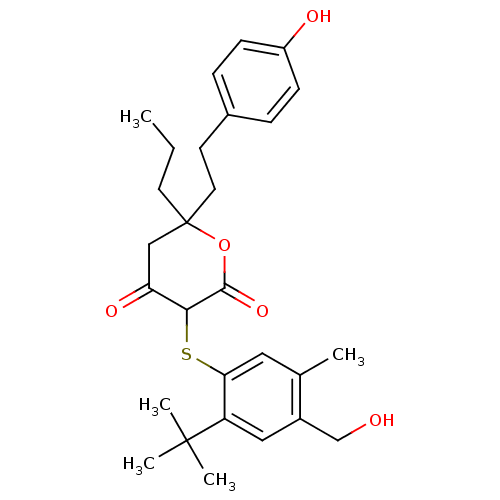 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2203 ((3-(2-tert-Butyl-4-(2-hydroxy-ethoxy)-5-methyl-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50231019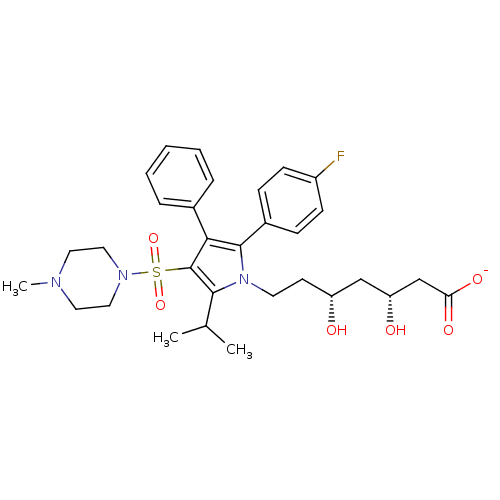 (CHEMBL402622 | sodium (3R,5R)-7-(2-(4-fluorophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat microsomal HMGCoA reductase | Bioorg Med Chem Lett 18: 1151-6 (2008) Article DOI: 10.1016/j.bmcl.2007.11.124 BindingDB Entry DOI: 10.7270/Q2TQ6191 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2536 (6-Alkyl-6-phenethyldihydropyrone 13y | 6-[2-(4-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | J Med Chem 40: 3707-11 (1997) Article DOI: 10.1021/jm970522y BindingDB Entry DOI: 10.7270/Q2ZC812F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006859 (2-((R)-(S)-Morpholine-4-sulfonyl)-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Division of Warner-Lambert Co. Curated by ChEMBL | Assay Description In vitro inhibition of monkey renin. | J Med Chem 35: 2562-72 (1992) BindingDB Entry DOI: 10.7270/Q2ZK5H8V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2536 (6-Alkyl-6-phenethyldihydropyrone 13y | 6-[2-(4-ami...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 188 total ) | Next | Last >> |