

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, muscle form (Homo sapiens (Human)) | BDBM50330796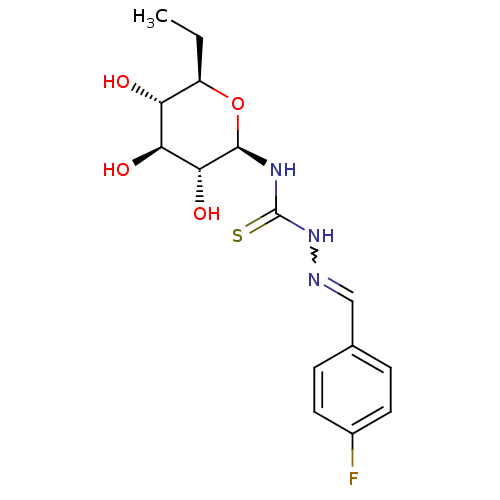 (4-Fluoro-benzaldehyde4-(beta-D-glucopyranosyl)thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Hellenic Research Foundation Curated by ChEMBL | Assay Description Inhibition of glycogen phosphorylase b | Bioorg Med Chem 18: 7911-22 (2010) Article DOI: 10.1016/j.bmc.2010.09.039 BindingDB Entry DOI: 10.7270/Q2DV1K3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50507492 (Loxo-195 | Selitrectinib | US10966985, Compound 33...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM374727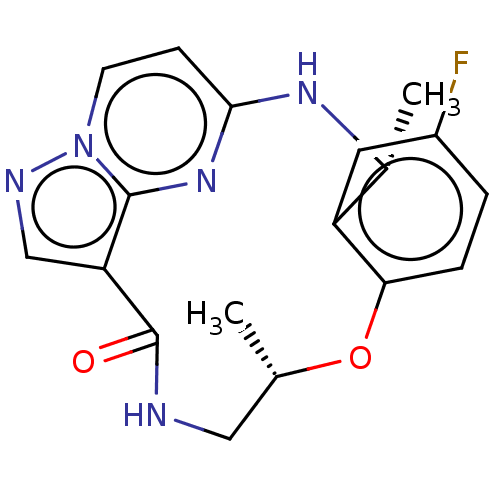 ((7S,13R)-11-fluoro-7,13-dimethyl-6,7,13,14- tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50579500 (CHEMBL4864729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKC (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50181441 (BCX-1812 | CHEBI:85202 | Peramivir) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Vietnam/1194/2004 (H5N1)) recombinant neuraminidase expressed in HEK293 cells pre-incubated for 10 mins before 4-M... | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50181441 (BCX-1812 | CHEBI:85202 | Peramivir) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) recombinant neuraminidase expressed in HEK293 cells pre-incubated for 10 mins before 4-MUNANA subs... | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464877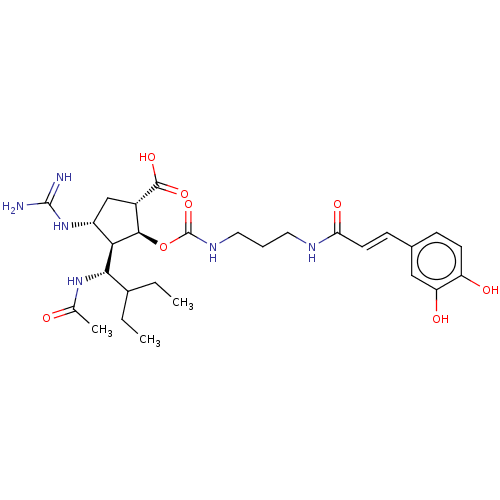 (CHEMBL4288789) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase pre-incubated for 10 mins before 4-MUNANA substrate addition by fluorometry | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50181441 (BCX-1812 | CHEBI:85202 | Peramivir) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase pre-incubated for 10 mins before 4-MUNANA substrate addition by fluorometry | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464875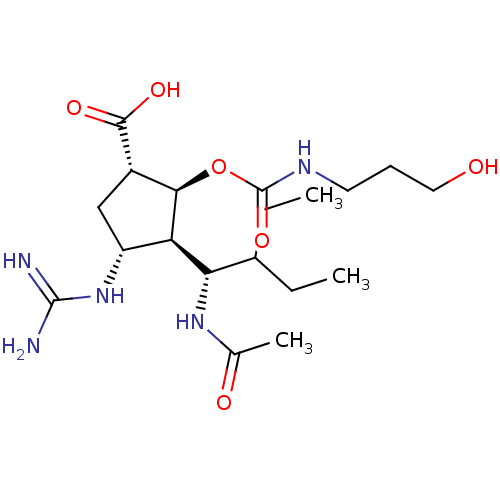 (CHEMBL4285430) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase pre-incubated for 10 mins before 4-MUNANA substrate addition by fluorometry | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464874 (CHEMBL4278125) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase pre-incubated for 10 mins before 4-MUNANA substrate addition by fluorometry | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464876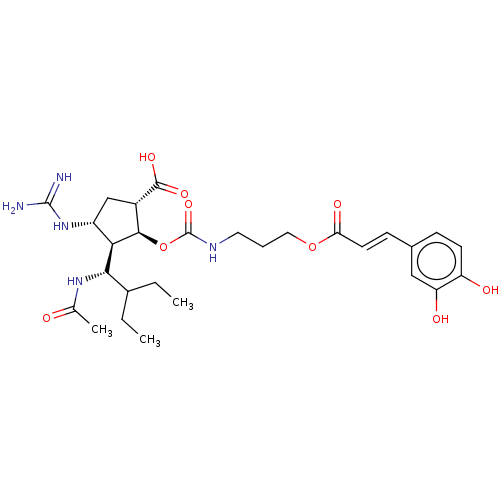 (CHEMBL4288995) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) recombinant neuraminidase expressed in HEK293 cells pre-incubated for 10 mins before 4-MUNANA subs... | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464872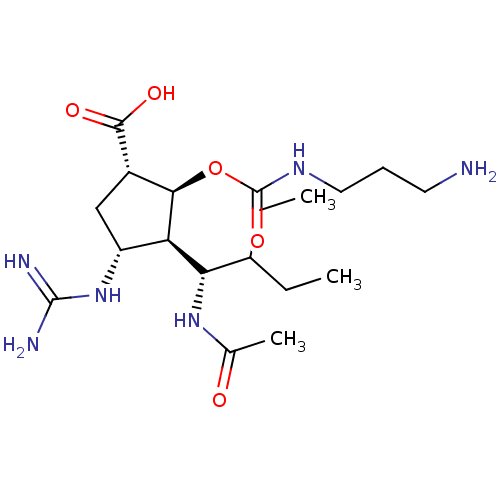 (CHEMBL4293261) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase pre-incubated for 10 mins before 4-MUNANA substrate addition by fluorometry | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464873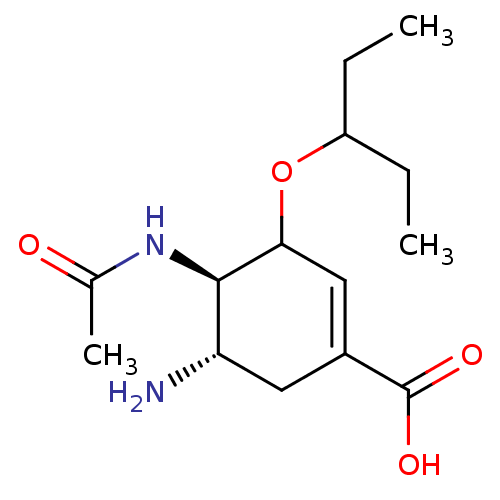 (CHEMBL4280263) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase pre-incubated for 10 mins before 4-MUNANA substrate addition by fluorometry | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) wild type neuraminidase using MUNANA as substrate preincubated for 10 mins followed by substrate a... | Eur J Med Chem 154: 314-323 (2018) Article DOI: 10.1016/j.ejmech.2018.05.030 BindingDB Entry DOI: 10.7270/Q2MC92JD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50573631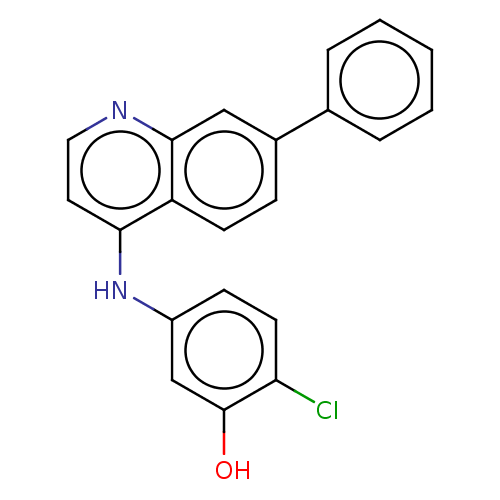 (CHEMBL4873767) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of VEGFR2 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00156 BindingDB Entry DOI: 10.7270/Q21C21PV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50028503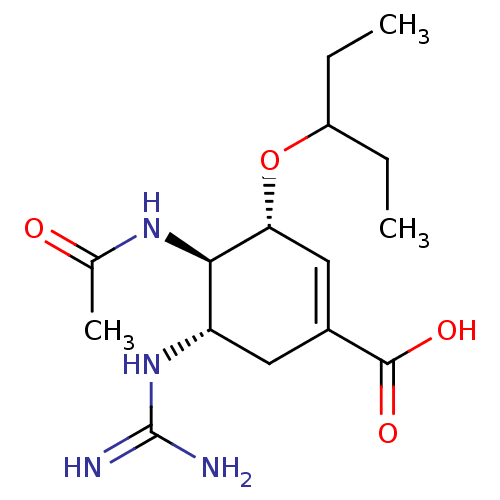 (CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) wild type neuraminidase using MUNANA as substrate preincubated for 10 mins followed by substrate a... | Eur J Med Chem 154: 314-323 (2018) Article DOI: 10.1016/j.ejmech.2018.05.030 BindingDB Entry DOI: 10.7270/Q2MC92JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50389234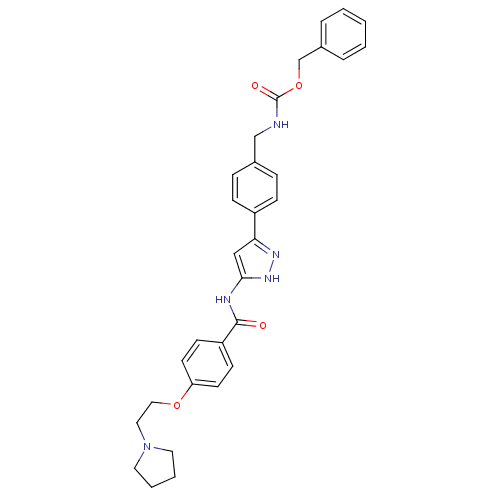 (CHEMBL2063324) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of FLT3 autophosphorylation in human MV4-11 cells after 2 hrs by Western blot analysis | Bioorg Med Chem Lett 22: 4654-9 (2012) Article DOI: 10.1016/j.bmcl.2012.05.116 BindingDB Entry DOI: 10.7270/Q2XS5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Perugia Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate after 5 mins by LC-MS analysis | J Med Chem 59: 3340-52 (2016) Article DOI: 10.1021/acs.jmedchem.6b00030 BindingDB Entry DOI: 10.7270/Q29K4D50 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) wild type neuraminidase using MUNANA as substrate preincubated for 10 mins followed by substrate a... | Eur J Med Chem 154: 314-323 (2018) Article DOI: 10.1016/j.ejmech.2018.05.030 BindingDB Entry DOI: 10.7270/Q2MC92JD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464876 (CHEMBL4288995) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase pre-incubated for 10 mins before 4-MUNANA substrate addition by fluorometry | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50431093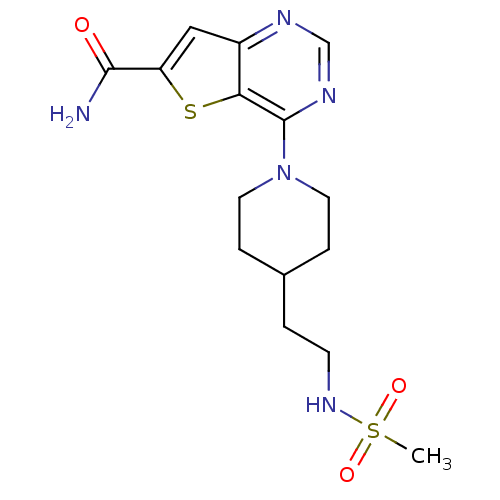 (CHEMBL2338810) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company Curated by ChEMBL | Assay Description Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... | J Med Chem 56: 3666-79 (2013) Article DOI: 10.1021/jm400204k BindingDB Entry DOI: 10.7270/Q2D50P9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50431121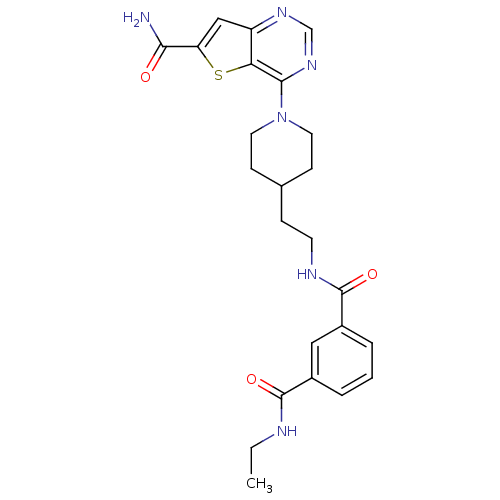 (CHEMBL2332037) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company Curated by ChEMBL | Assay Description Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... | J Med Chem 56: 3666-79 (2013) Article DOI: 10.1021/jm400204k BindingDB Entry DOI: 10.7270/Q2D50P9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507415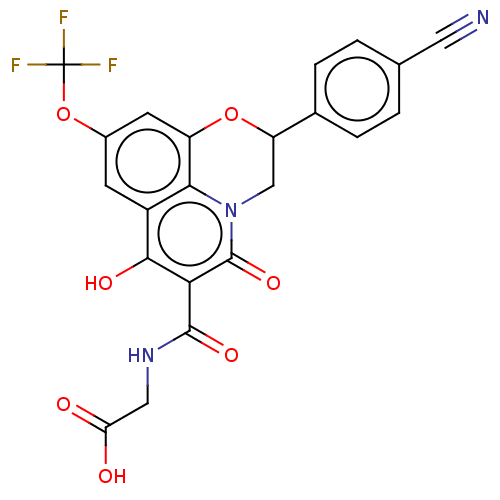 (CHEMBL4451025) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Wilson-Smith/1933 H1N1...) | BDBM50464877 (CHEMBL4288789) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) recombinant neuraminidase expressed in HEK293 cells pre-incubated for 10 mins before 4-MUNANA subs... | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50579500 (CHEMBL4864729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKB (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50431118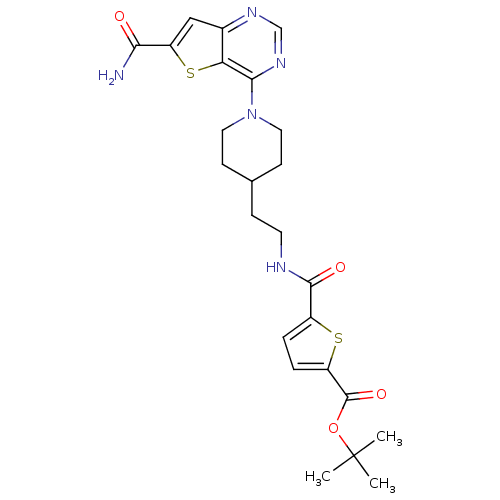 (CHEMBL2332041) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company Curated by ChEMBL | Assay Description Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... | J Med Chem 56: 3666-79 (2013) Article DOI: 10.1021/jm400204k BindingDB Entry DOI: 10.7270/Q2D50P9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50181441 (BCX-1812 | CHEBI:85202 | Peramivir) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Brisbane/10/2007(H3N2)) recombinant neuraminidase expressed in HEK293 cells pre-incubated for 10 mins before 4-MUN... | Eur J Med Chem 145: 224-234 (2018) Article DOI: 10.1016/j.ejmech.2017.12.072 BindingDB Entry DOI: 10.7270/Q22R3VBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase H275Y mutant using MUNANA as substrate preincubated for 10 mins followed by substrat... | Eur J Med Chem 154: 314-323 (2018) Article DOI: 10.1016/j.ejmech.2018.05.030 BindingDB Entry DOI: 10.7270/Q2MC92JD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50028503 (CHEMBL81717 | Guanidino-Oseltamivir Carboxylicacid) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/WSN/1933(H1N1)) neuraminidase H275Y mutant using MUNANA as substrate preincubated for 10 mins followed by substrat... | Eur J Med Chem 154: 314-323 (2018) Article DOI: 10.1016/j.ejmech.2018.05.030 BindingDB Entry DOI: 10.7270/Q2MC92JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50434621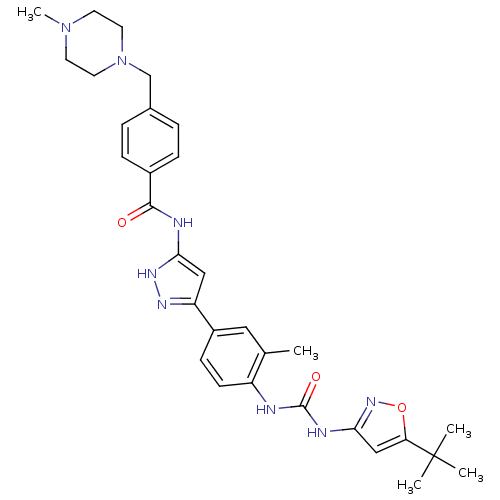 (CHEMBL2386796) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type GST tagged FLT3 kinase (567 to 993) (unknown origin) transfected in insect sf9 cells after 4 hrs by wallac counting analysis | Bioorg Med Chem 21: 2856-67 (2013) Article DOI: 10.1016/j.bmc.2013.03.083 BindingDB Entry DOI: 10.7270/Q22N53NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36462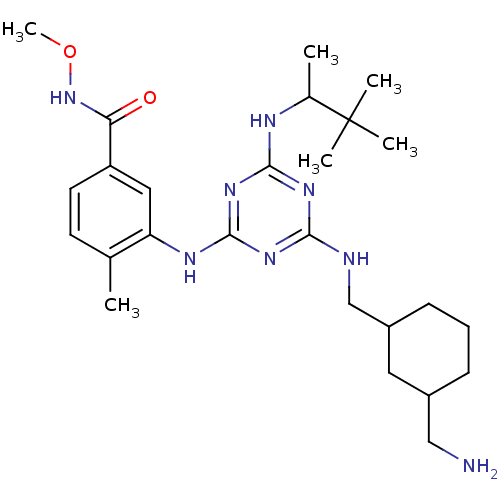 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36463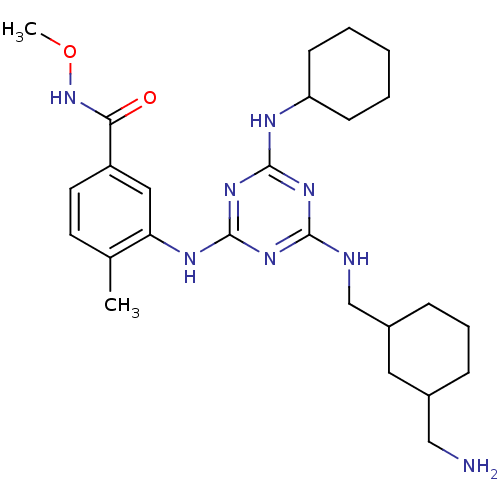 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50507415 (CHEMBL4451025) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD2 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50586463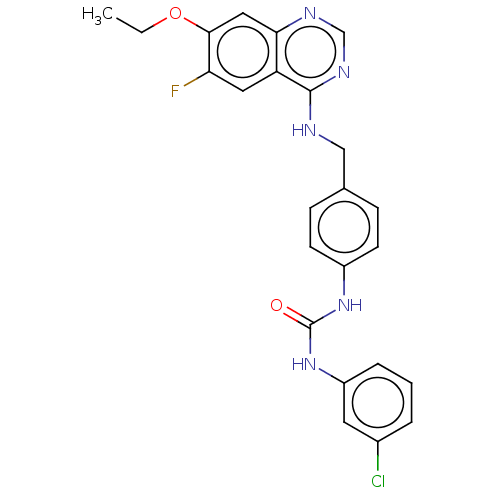 (CHEMBL5083023) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged AURA Ser123 to 401 residues) (unknown origin) expressed in Sf9 insect cells using tetra-LLRASLG peptide as substrate incubat... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507404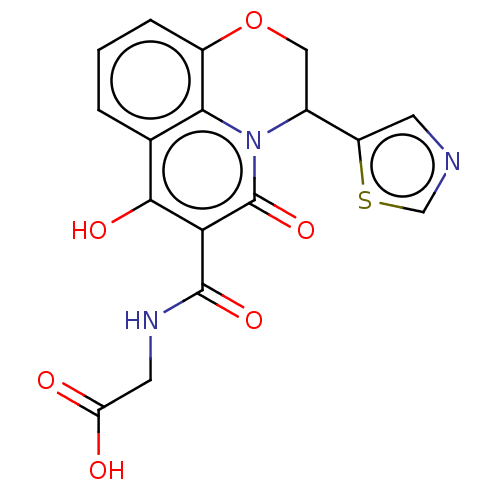 (CHEMBL4443606) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507419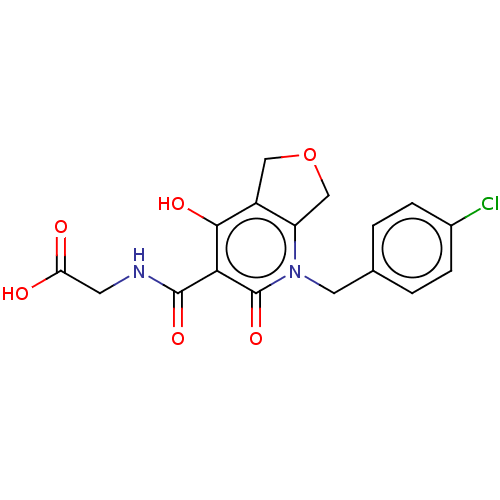 (CHEMBL4462855) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507404 (CHEMBL4443606) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507420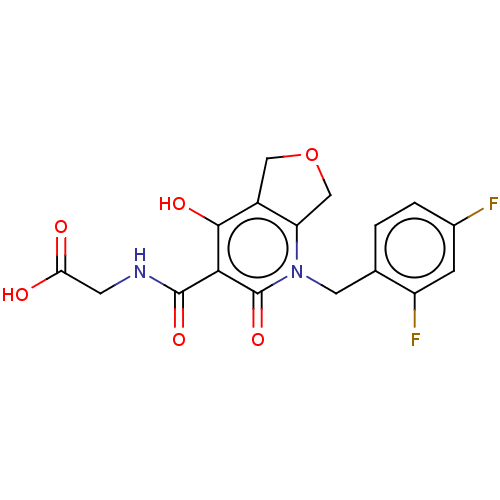 (CHEMBL4529447) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50507419 (CHEMBL4462855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD2 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507406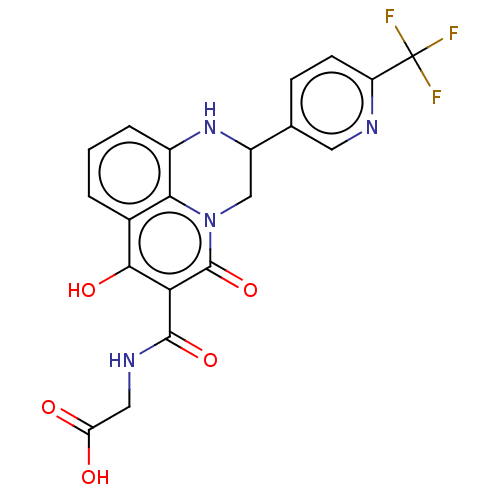 (CHEMBL4452218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507407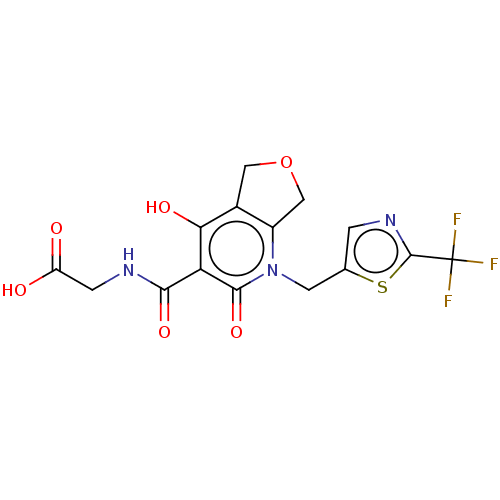 (CHEMBL4590677) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507408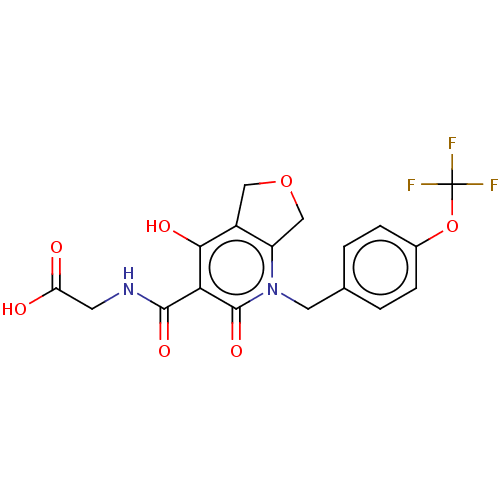 (CHEMBL4561977) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50507420 (CHEMBL4529447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD2 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50431097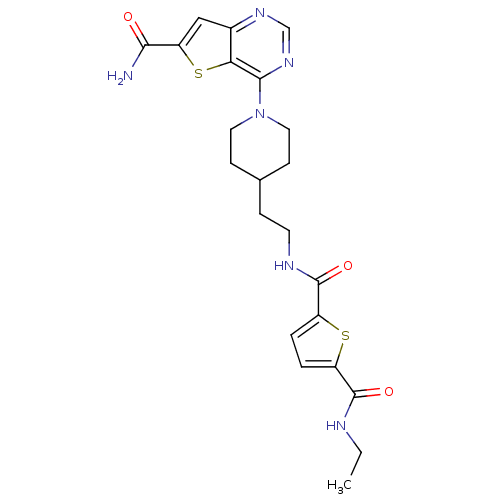 (CHEMBL2332039) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company Curated by ChEMBL | Assay Description Inhibition of His-tagged SIRT2 (1 to 389) (unknown origin)-mediated deacetylation of Ac-RHKKAcW-NH2 substrate incubated for 20 mins prior to substrat... | J Med Chem 56: 3666-79 (2013) Article DOI: 10.1021/jm400204k BindingDB Entry DOI: 10.7270/Q2D50P9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50399540 (FORETINIB | US10464902, Foretinib | US10882853, Co...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50507408 (CHEMBL4561977) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD2 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507421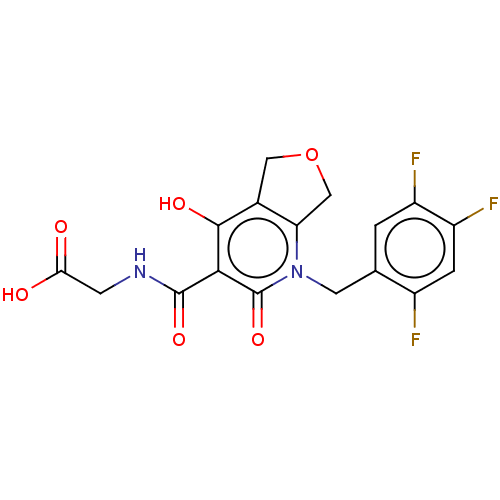 (CHEMBL4462741) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507409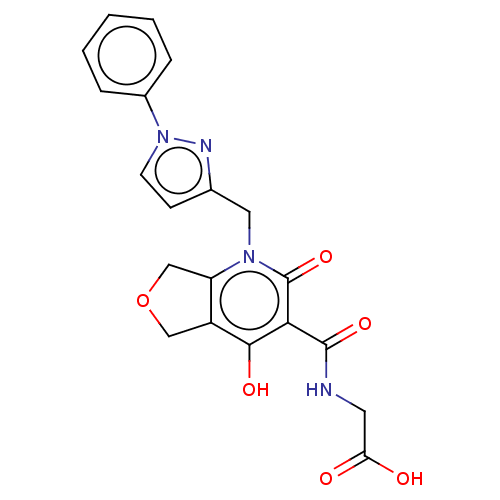 (CHEMBL4476576) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-3, mitochondrial (Homo sapiens (Human)) | BDBM50431118 (CHEMBL2332041) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sirtris a GSK Company Curated by ChEMBL | Assay Description Inhibition of human His-tagged SIRT3 (102 to 399) expressed in Escherichia coli BL21(DE3) assessed as inhibition of deacetylation of Ac-RHKKAcW-NH2 s... | J Med Chem 56: 3666-79 (2013) Article DOI: 10.1021/jm400204k BindingDB Entry DOI: 10.7270/Q2D50P9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM50507413 (CHEMBL4564341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human HIF-PHD1 expressed in baculovirus-infected Sf9 cells using biotin labelled DLDLEMLAPYIPMDDDFQL and 2-oxoglutarate as ... | ACS Med Chem Lett 9: 1193-1198 (2018) Article DOI: 10.1021/acsmedchemlett.8b00274 BindingDB Entry DOI: 10.7270/Q2MG7SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 970 total ) | Next | Last >> |