 Found 1198 hits with Last Name = 'cook' and Initial = 'c'
Found 1198 hits with Last Name = 'cook' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM22
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(C\C=C\c2cn[nH]c2)C(=O)N(C\C=C\c2cn[nH]c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C31H34N6O3/c38-29-27(17-23-9-3-1-4-10-23)36(15-7-13-25-19-32-33-20-25)31(40)37(16-8-14-26-21-34-35-22-26)28(30(29)39)18-24-11-5-2-6-12-24/h1-14,19-22,27-30,38-39H,15-18H2,(H,32,33)(H,34,35)/b13-7+,14-8+/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards HIV-Protease enzyme. |
Bioorg Med Chem Lett 6: 797-802 (1996)
Article DOI: 10.1016/0960-894X(96)00108-4
BindingDB Entry DOI: 10.7270/Q2N879SZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM23

((4R,5S,6S,7R)-4,7-dibenzyl-1-(cyclopropylmethyl)-5...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(CC2CC2)C(=O)N(C\C=C\c2cn[nH]c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C29H34N4O3/c34-27-25(16-21-8-3-1-4-9-21)32(15-7-12-24-18-30-31-19-24)29(36)33(20-23-13-14-23)26(28(27)35)17-22-10-5-2-6-11-22/h1-12,18-19,23,25-28,34-35H,13-17,20H2,(H,30,31)/b12-7+/t25-,26-,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for binding affinity towards HIV-Protease enzyme. |
Bioorg Med Chem Lett 6: 797-802 (1996)
Article DOI: 10.1016/0960-894X(96)00108-4
BindingDB Entry DOI: 10.7270/Q2N879SZ |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50240434
(2-[1-(6-Amino-purin-9-yl)-2-oxo-ethoxy]-propenal |...)Show InChI InChI=1S/C10H9N5O3/c1-6(2-16)18-7(3-17)15-5-14-8-9(11)12-4-13-10(8)15/h2-5,7H,1H2,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of S-adenosyl homocysteine (SAH) hydrolase . |
Bioorg Med Chem Lett 6: 1381-1386 (1996)
Article DOI: 10.1016/0960-894X(96)00234-X
BindingDB Entry DOI: 10.7270/Q29P31NC |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50049735
(5-Methyl-6-oxo-8-trimethylsilanylethynyl-5,6-dihyd...)Show SMILES CN1Cc2c(ncn2-c2ccc(cc2C1=O)C#C[Si](C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C22H27N3O3Si/c1-22(2,3)28-21(27)19-18-13-24(4)20(26)16-12-15(10-11-29(5,6)7)8-9-17(16)25(18)14-23-19/h8-9,12,14H,13H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 488-93 (1997)
BindingDB Entry DOI: 10.7270/Q2PN945P |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50049735

(5-Methyl-6-oxo-8-trimethylsilanylethynyl-5,6-dihyd...)Show SMILES CN1Cc2c(ncn2-c2ccc(cc2C1=O)C#C[Si](C)(C)C)C(=O)OC(C)(C)C Show InChI InChI=1S/C22H27N3O3Si/c1-22(2,3)28-21(27)19-18-13-24(4)20(26)16-12-15(10-11-29(5,6)7)8-9-17(16)25(18)14-23-19/h8-9,12,14H,13H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.03 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 488-93 (1997)
BindingDB Entry DOI: 10.7270/Q2PN945P |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50029130

(Adenosine dialdehyde | CHEMBL165876)Show InChI InChI=1S/C10H11N5O4/c11-9-8-10(13-4-12-9)15(5-14-8)7(3-18)19-6(1-16)2-17/h1,3-7,17H,2H2,(H2,11,12,13)/t6-,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of S-adenosyl homocysteine (SAH) hydrolase . |
Bioorg Med Chem Lett 6: 1381-1386 (1996)
Article DOI: 10.1016/0960-894X(96)00234-X
BindingDB Entry DOI: 10.7270/Q29P31NC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50289122
((3aS,4R,8R,8aS)-4,8-Dibenzyl-2,2-dimethyl-5,7-di-p...)Show SMILES CC1(C)O[C@@H]2[C@@H](O1)[C@@H](Cc1ccccc1)N(CC#C)C(=O)N(CC#C)[C@@H]2Cc1ccccc1 Show InChI InChI=1S/C28H30N2O3/c1-5-17-29-23(19-21-13-9-7-10-14-21)25-26(33-28(3,4)32-25)24(30(18-6-2)27(29)31)20-22-15-11-8-12-16-22/h1-2,7-16,23-26H,17-20H2,3-4H3/t23-,24-,25+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to HIV-protease |
Bioorg Med Chem Lett 6: 797-802 (1996)
Article DOI: 10.1016/0960-894X(96)00108-4
BindingDB Entry DOI: 10.7270/Q2N879SZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50083882

(1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,...)Show InChI InChI=1S/C17H13N3O/c1-20-15-8-7-12(10-18)9-14(15)17(19-11-16(20)21)13-5-3-2-4-6-13/h2-9H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 283: 488-93 (1997)
BindingDB Entry DOI: 10.7270/Q2PN945P |
More data for this
Ligand-Target Pair | |
Adenosylhomocysteinase
(Homo sapiens (Human)) | BDBM50287214

(2-[1-(6-Amino-purin-9-yl)-2-hydroxy-ethoxy]-3-hydr...)Show SMILES Nc1ncnc2n(cnc12)[C@@H](CO)O[C@H](CO)C=O |r| Show InChI InChI=1S/C10H13N5O4/c11-9-8-10(13-4-12-9)15(5-14-8)7(3-18)19-6(1-16)2-17/h1,4-7,17-18H,2-3H2,(H2,11,12,13)/t6-,7+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for competitive inhibition against S-adenosyl homocysteine (SAH) hydrolase . |
Bioorg Med Chem Lett 6: 1381-1386 (1996)
Article DOI: 10.1016/0960-894X(96)00234-X
BindingDB Entry DOI: 10.7270/Q29P31NC |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... |
Bioorg Med Chem Lett 17: 5754-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.064
BindingDB Entry DOI: 10.7270/Q2Z03905 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337733
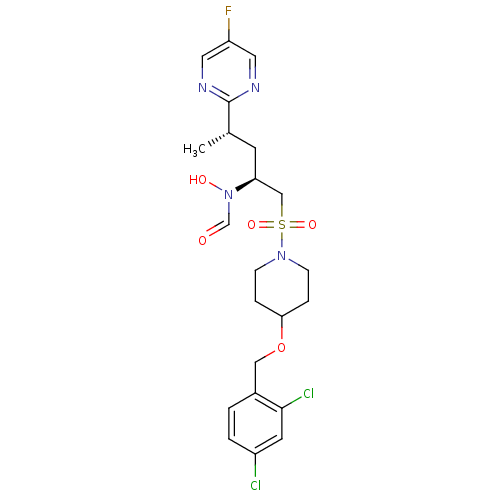
(CHEMBL1683444 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H27Cl2FN4O5S/c1-15(22-26-10-18(25)11-27-22)8-19(29(31)14-30)13-35(32,33)28-6-4-20(5-7-28)34-12-16-2-3-17(23)9-21(16)24/h2-3,9-11,14-15,19-20,31H,4-8,12-13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337721
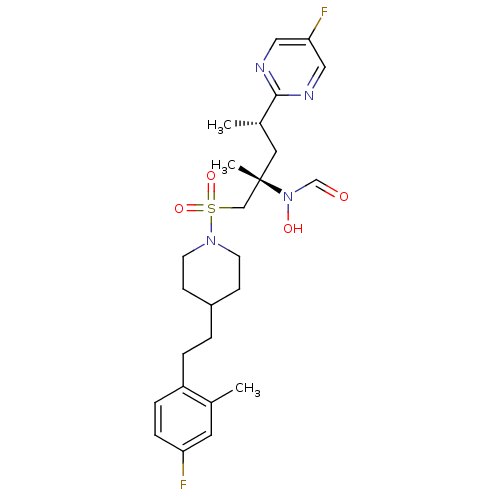
(CHEMBL1683454 | N-((2S,4S)-1-(4-(4-fluoro-2-methyl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(F)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34F2N4O4S/c1-18-12-22(26)7-6-21(18)5-4-20-8-10-30(11-9-20)36(34,35)16-25(3,31(33)17-32)13-19(2)24-28-14-23(27)15-29-24/h6-7,12,14-15,17,19-20,33H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265958
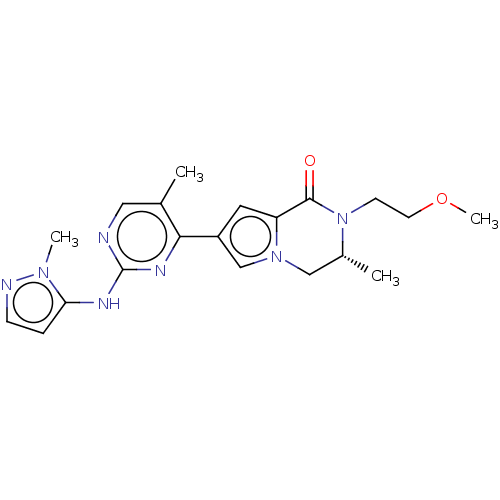
(CHEMBL4086507)Show SMILES COCCN1[C@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337722
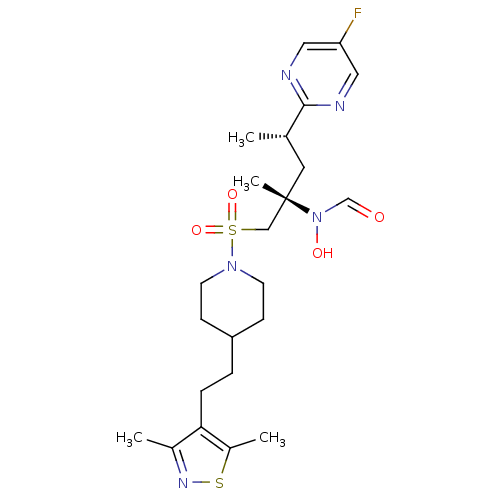
(CHEMBL1683460 | N-((2S,4S)-1-(4-(2-(3,5-dimethylis...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2c(C)nsc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H34FN5O4S2/c1-16(22-25-12-20(24)13-26-22)11-23(4,29(31)15-30)14-35(32,33)28-9-7-19(8-10-28)5-6-21-17(2)27-34-18(21)3/h12-13,15-16,19,31H,5-11,14H2,1-4H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959
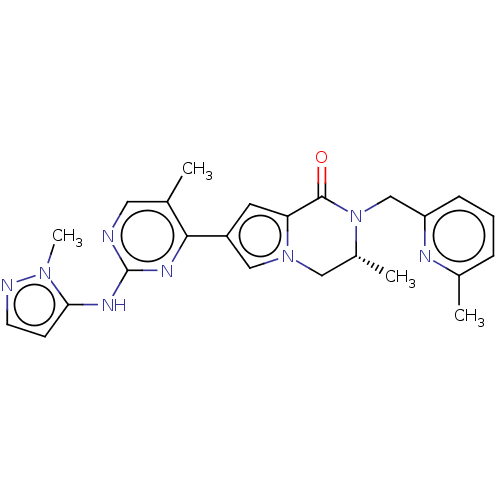
(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265960

(CHEMBL4098608)Show SMILES COCCN1[C@@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265961

(CHEMBL4090886)Show InChI InChI=1S/C19H23N7O2/c1-13-11-20-19(22-16-4-5-21-24(16)2)23-17(13)14-10-15-18(27)25(8-9-28-3)6-7-26(15)12-14/h4-5,10-12H,6-9H2,1-3H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265962
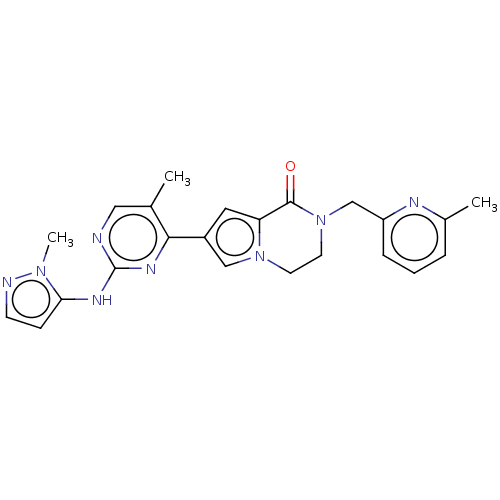
(CHEMBL4101072)Show SMILES Cc1cccc(CN2CCn3cc(cc3C2=O)-c2nc(Nc3ccnn3C)ncc2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-12-24-23(27-20-7-8-25-29(20)3)28-21(15)17-11-19-22(32)31(10-9-30(19)13-17)14-18-6-4-5-16(2)26-18/h4-8,11-13H,9-10,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265958

(CHEMBL4086507)Show SMILES COCCN1[C@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465
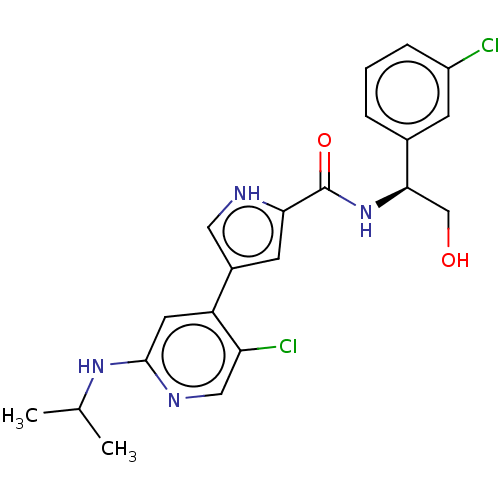
(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505988
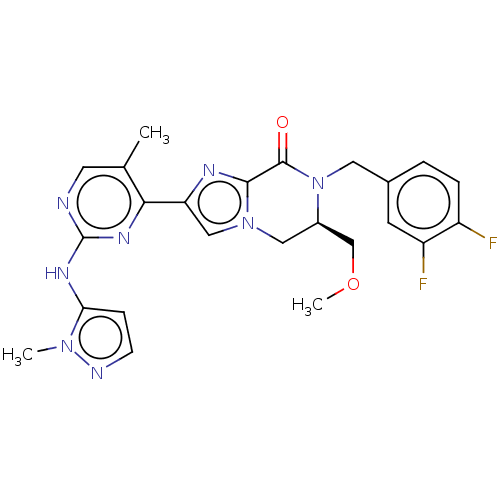
(CHEMBL4482864)Show SMILES COC[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H24F2N8O2/c1-14-9-27-24(30-20-6-7-28-32(20)2)31-21(14)19-12-33-11-16(13-36-3)34(23(35)22(33)29-19)10-15-4-5-17(25)18(26)8-15/h4-9,12,16H,10-11,13H2,1-3H3,(H,27,30,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505989

(CHEMBL4551714)Show SMILES COC[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C25H28N8O2/c1-16-11-26-25(29-22-8-9-27-31(22)3)30-23(16)18-10-21-24(34)33(13-19-7-5-6-17(2)28-19)20(15-35-4)14-32(21)12-18/h5-12,20H,13-15H2,1-4H3,(H,26,29,30)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265934

(CHEMBL4060050)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 |r| Show InChI InChI=1S/C19H23N7O2/c1-12-10-15-13(18(27)26(12)8-9-28-3)11-16(22-15)14-4-6-20-19(23-14)24-17-5-7-21-25(17)2/h4-7,11-12,22H,8-10H2,1-3H3,(H,20,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265962

(CHEMBL4101072)Show SMILES Cc1cccc(CN2CCn3cc(cc3C2=O)-c2nc(Nc3ccnn3C)ncc2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-12-24-23(27-20-7-8-25-29(20)3)28-21(15)17-11-19-22(32)31(10-9-30(19)13-17)14-18-6-4-5-16(2)26-18/h4-8,11-13H,9-10,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265971

(CHEMBL4087393)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265973

(CHEMBL4075638)Show SMILES C[C@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505991

(CHEMBL4470113)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3cccc(Cl)c3)CCn2c1 Show InChI InChI=1S/C23H22ClN7O/c1-15-12-25-23(27-20-6-7-26-29(20)2)28-21(15)17-11-19-22(32)31(9-8-30(19)14-17)13-16-4-3-5-18(24)10-16/h3-7,10-12,14H,8-9,13H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265959

(CHEMBL4071576)Show SMILES C[C@@H]1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C24H26N8O/c1-15-11-25-24(28-21-8-9-26-30(21)4)29-22(15)18-10-20-23(33)32(17(3)12-31(20)13-18)14-19-7-5-6-16(2)27-19/h5-11,13,17H,12,14H2,1-4H3,(H,25,28,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265970

(CHEMBL4097186)Show SMILES O=C1N(Cc2ccccc2)CCc2[nH]c(cc12)-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C23H25N5O2/c29-22-18-14-21(20-6-10-24-23(27-20)25-17-8-12-30-13-9-17)26-19(18)7-11-28(22)15-16-4-2-1-3-5-16/h1-6,10,14,17,26H,7-9,11-13,15H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265971

(CHEMBL4087393)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265968

(CHEMBL4065992)Show SMILES COCCN1[C@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265961

(CHEMBL4090886)Show InChI InChI=1S/C19H23N7O2/c1-13-11-20-19(22-16-4-5-21-24(16)2)23-17(13)14-10-15-18(27)25(8-9-28-3)6-7-26(15)12-14/h4-5,10-12H,6-9H2,1-3H3,(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265960

(CHEMBL4098608)Show SMILES COCCN1[C@@H](C)Cn2cc(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-13-10-21-20(23-17-5-6-22-25(17)3)24-18(13)15-9-16-19(28)27(7-8-29-4)14(2)11-26(16)12-15/h5-6,9-10,12,14H,7-8,11H2,1-4H3,(H,21,23,24)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265968

(CHEMBL4065992)Show SMILES COCCN1[C@H](C)Cc2[nH]c(cc2C1=O)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C20H25N7O2/c1-12-11-21-20(24-17-5-6-22-26(17)3)25-18(12)16-10-14-15(23-16)9-13(2)27(19(14)28)7-8-29-4/h5-6,10-11,13,23H,7-9H2,1-4H3,(H,21,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265938
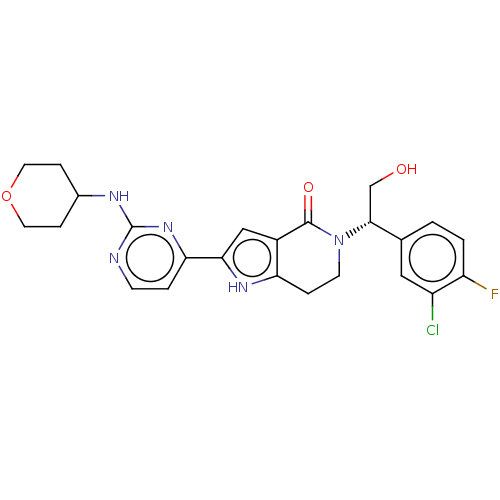
(CHEMBL4096522)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c25-17-11-14(1-2-18(17)26)22(13-32)31-8-4-19-16(23(31)33)12-21(29-19)20-3-7-27-24(30-20)28-15-5-9-34-10-6-15/h1-3,7,11-12,15,22,29,32H,4-6,8-10,13H2,(H,27,28,30)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265970

(CHEMBL4097186)Show SMILES O=C1N(Cc2ccccc2)CCc2[nH]c(cc12)-c1ccnc(NC2CCOCC2)n1 Show InChI InChI=1S/C23H25N5O2/c29-22-18-14-21(20-6-10-24-23(27-20)25-17-8-12-30-13-9-17)26-19(18)7-11-28(22)15-16-4-2-1-3-5-16/h1-6,10,14,17,26H,7-9,11-13,15H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505994

(CHEMBL4440109)Show SMILES C[C@H]1Cn2cc(nc2C(=O)N1Cc1ccc(F)c(F)c1)-c1nc(Nc2ccnn2C)ncc1C |r| Show InChI InChI=1S/C23H22F2N8O/c1-13-9-26-23(29-19-6-7-27-31(19)3)30-20(13)18-12-32-10-14(2)33(22(34)21(32)28-18)11-15-4-5-16(24)17(25)8-15/h4-9,12,14H,10-11H2,1-3H3,(H,26,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265934

(CHEMBL4060050)Show SMILES COCCN1[C@@H](C)Cc2[nH]c(cc2C1=O)-c1ccnc(Nc2ccnn2C)n1 |r| Show InChI InChI=1S/C19H23N7O2/c1-12-10-15-13(18(27)26(12)8-9-28-3)11-16(22-15)14-4-6-20-19(23-14)24-17-5-7-21-25(17)2/h4-7,11-12,22H,8-10H2,1-3H3,(H,20,23,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265939

(CHEMBL4078489)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C24H27N5O3/c30-15-22(16-4-2-1-3-5-16)29-11-7-19-18(23(29)31)14-21(27-19)20-6-10-25-24(28-20)26-17-8-12-32-13-9-17/h1-6,10,14,17,22,27,30H,7-9,11-13,15H2,(H,25,26,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50222089
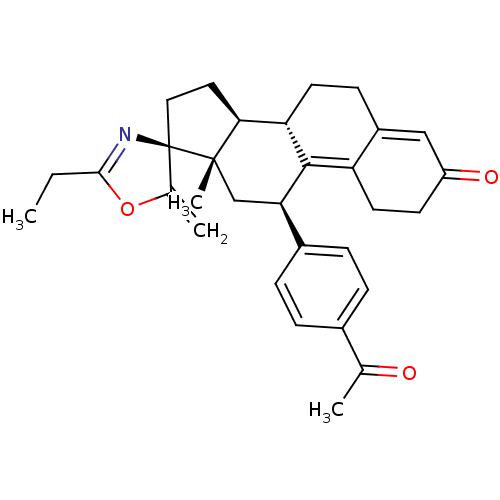
((3S,10'S,11'S,15'S,17'R)-17'-(4-acetylphenyl)-5-et...)Show SMILES CCC1=N[C@]2(CC[C@H]3[C@@H]4CCC5=CC(=O)CCC5=C4[C@H](C[C@]23C)c2ccc(cc2)C(C)=O)C(=C)O1 |c:18,t:2,11| Show InChI InChI=1S/C31H35NO3/c1-5-28-32-31(19(3)35-28)15-14-27-25-12-10-22-16-23(34)11-13-24(22)29(25)26(17-30(27,31)4)21-8-6-20(7-9-21)18(2)33/h6-9,16,25-27H,3,5,10-15,17H2,1-2,4H3/t25-,26+,27-,30-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor expressed in human T47D cells assessed as inhibition of promegestone-induced alkaline phosphatase activi... |
Bioorg Med Chem Lett 17: 5754-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.064
BindingDB Entry DOI: 10.7270/Q2Z03905 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505992

(CHEMBL4452853)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cn2CCN(Cc3cccc(Cl)c3)C(=O)c2n1 Show InChI InChI=1S/C22H21ClN8O/c1-14-11-24-22(27-18-6-7-25-29(18)2)28-19(14)17-13-30-8-9-31(21(32)20(30)26-17)12-15-4-3-5-16(23)10-15/h3-7,10-11,13H,8-9,12H2,1-2H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50505993

(CHEMBL4587118)Show SMILES Cc1cnc(Nc2ccnn2C)nc1-c1cc2C(=O)N(Cc3ccc(F)c(F)c3)CCn2c1 Show InChI InChI=1S/C23H21F2N7O/c1-14-11-26-23(28-20-5-6-27-30(20)2)29-21(14)16-10-19-22(33)32(8-7-31(19)13-16)12-15-3-4-17(24)18(25)9-15/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of MEK U911-activated ERK2 (unknown origin) using ERKtide as substrate preincubated for 20 mins followed by substrate addition in presence... |
J Med Chem 62: 11004-11018 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01295
BindingDB Entry DOI: 10.7270/Q24X5C3Z |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337723
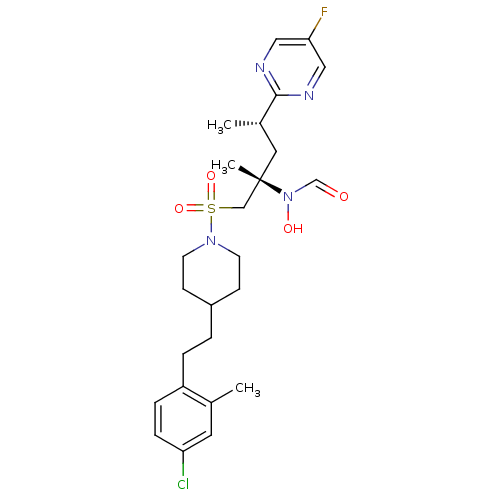
(CHEMBL1683450 | N-((2S,4S)-1-(4-(4-chloro-2-methyl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(Cl)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34ClFN4O4S/c1-18-12-22(26)7-6-21(18)5-4-20-8-10-30(11-9-20)36(34,35)16-25(3,31(33)17-32)13-19(2)24-28-14-23(27)15-29-24/h6-7,12,14-15,17,19-20,33H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50108227

((S)-2-[((R)-1-Benzyl-5-oxo-pyrrolidine-2-carbonyl)...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cccc2Cl)cc1)NC(=O)[C@H]1CCC(=O)N1Cc1ccccc1 Show InChI InChI=1S/C28H25Cl2N3O5/c29-20-7-4-8-21(30)25(20)27(36)31-19-11-9-17(10-12-19)15-22(28(37)38)32-26(35)23-13-14-24(34)33(23)16-18-5-2-1-3-6-18/h1-12,22-23H,13-16H2,(H,31,36)(H,32,35)(H,37,38)/t22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the VCAM-Very late antigen4 (VLA4) interaction in Ramos cell-based assay |
Bioorg Med Chem Lett 12: 137-40 (2001)
BindingDB Entry DOI: 10.7270/Q2QN6825 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265963
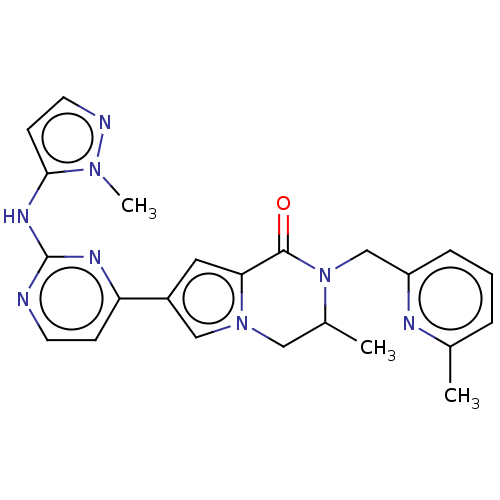
(CHEMBL4083098)Show SMILES CC1Cn2cc(cc2C(=O)N1Cc1cccc(C)n1)-c1ccnc(Nc2ccnn2C)n1 Show InChI InChI=1S/C23H24N8O/c1-15-5-4-6-18(26-15)14-31-16(2)12-30-13-17(11-20(30)22(31)32)19-7-9-24-23(27-19)28-21-8-10-25-29(21)3/h4-11,13,16H,12,14H2,1-3H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 60 uM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50265939

(CHEMBL4078489)Show SMILES OC[C@@H](N1CCc2[nH]c(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C24H27N5O3/c30-15-22(16-4-2-1-3-5-16)29-11-7-19-18(23(29)31)14-21(27-19)20-6-10-25-24(28-20)26-17-8-12-32-13-9-17/h1-6,10,14,17,22,27,30H,7-9,11-13,15H2,(H,25,26,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) in the presence of 1 mM ATP |
J Med Chem 60: 3438-3450 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00267
BindingDB Entry DOI: 10.7270/Q2FJ2K8J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data