

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytosolic purine 5'-nucleotidase (Homo sapiens) | BDBM50500514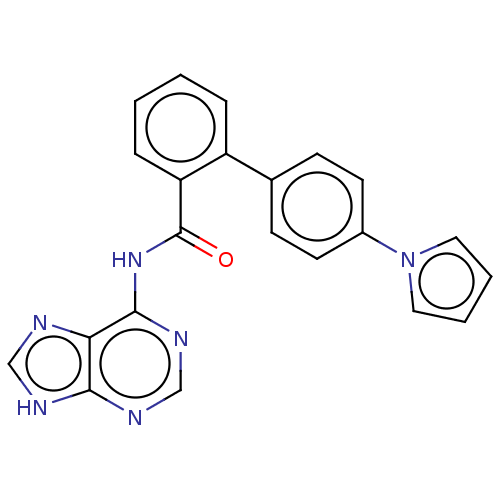 (CHEMBL3746819) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate h... | J Med Chem 58: 9680-96 (2015) Article DOI: 10.1021/acs.jmedchem.5b01616 BindingDB Entry DOI: 10.7270/Q2SF3062 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM68391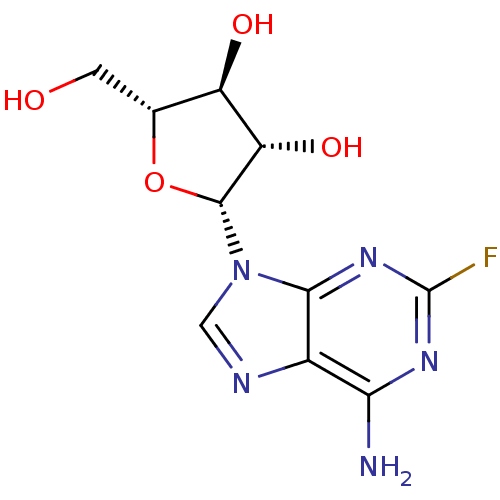 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive type of inhibition of human 5'-nucleotidase using IMP as substrate assessed as inhibitor constant for free enzyme by Lineweaver-Burk plot... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522285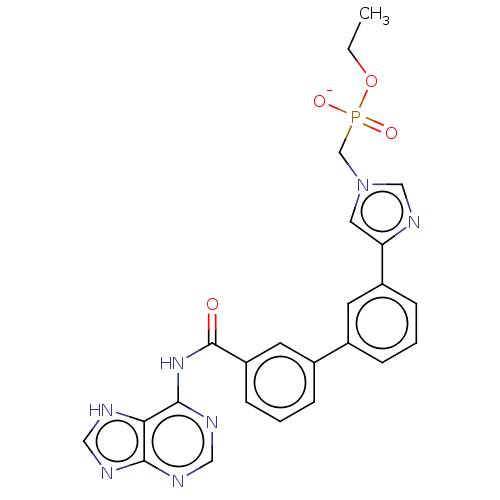 (CHEMBL4549109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant cN-II (unknown origin) assessed as inhibitor constant for free enzyme using varying level of IMP as substrate by... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522285 (CHEMBL4549109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot analys... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic purine 5'-nucleotidase (Homo sapiens) | BDBM50500511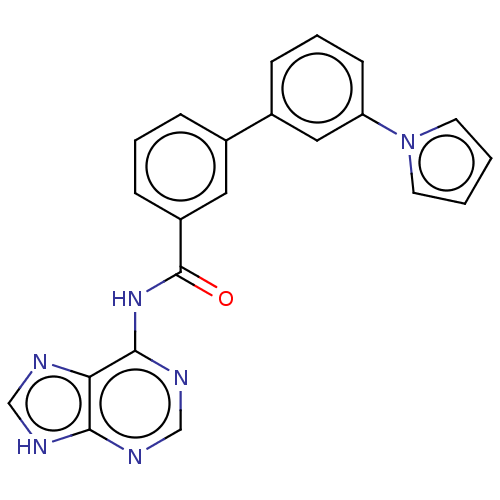 (CHEMBL3746624) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate h... | J Med Chem 58: 9680-96 (2015) Article DOI: 10.1021/acs.jmedchem.5b01616 BindingDB Entry DOI: 10.7270/Q2SF3062 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522288 (CHEMBL4518527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot analys... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50500515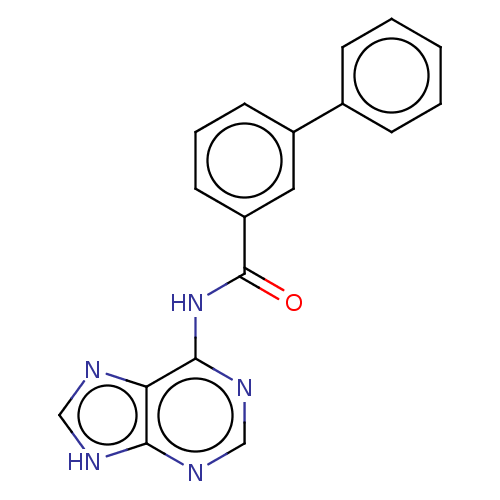 (CHEMBL3747390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 8.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot analys... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM68391 ((2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Non-competitive type of inhibition of human 5'-nucleotidase using IMP as substrate assessed as inhibitor constant for enzyme substrate complex by Lin... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic purine 5'-nucleotidase (Homo sapiens) | BDBM50500515 (CHEMBL3747390) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate hydro... | J Med Chem 58: 9680-96 (2015) Article DOI: 10.1021/acs.jmedchem.5b01616 BindingDB Entry DOI: 10.7270/Q2SF3062 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50500515 (CHEMBL3747390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of 5'-nucleotidase (unknown origin) using varying level of IMP as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522288 (CHEMBL4518527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant cN-II (unknown origin) assessed as inhibitor constant for free enzyme using varying level of IMP as substrate by... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522286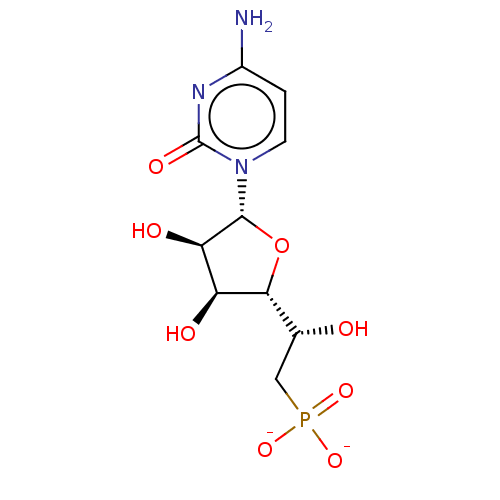 (CHEMBL3237671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of 5'-nucleotidase (unknown origin) using varying level of IMP as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522285 (CHEMBL4549109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.35E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot anal... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50500515 (CHEMBL3747390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.48E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant cN-II (unknown origin) assessed as inhibitor constant for free enzyme using varying level of IMP as substrate by... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic purine 5'-nucleotidase (Homo sapiens) | BDBM50500513 (CHEMBL3746419) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.65E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate h... | J Med Chem 58: 9680-96 (2015) Article DOI: 10.1021/acs.jmedchem.5b01616 BindingDB Entry DOI: 10.7270/Q2SF3062 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522288 (CHEMBL4518527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.65E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot anal... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522285 (CHEMBL4549109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Noncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot ana... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic purine 5'-nucleotidase (Homo sapiens) | BDBM50500510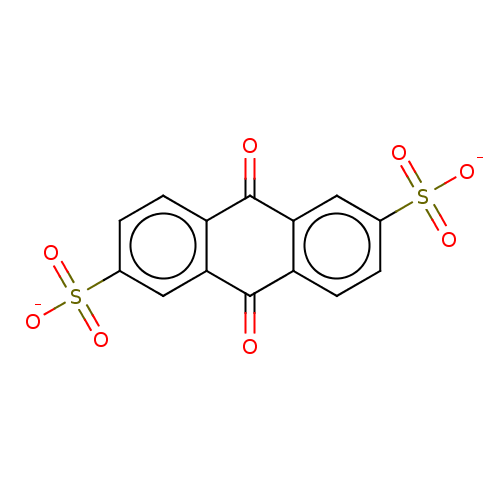 (CHEMBL3746211) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate hydrolysis by rap... | J Med Chem 58: 9680-96 (2015) Article DOI: 10.1021/acs.jmedchem.5b01616 BindingDB Entry DOI: 10.7270/Q2SF3062 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50500510 (CHEMBL3746211) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of 5'-nucleotidase (unknown origin) using varying level of IMP as substrate by Lineweaver-Burk plot analysis | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522287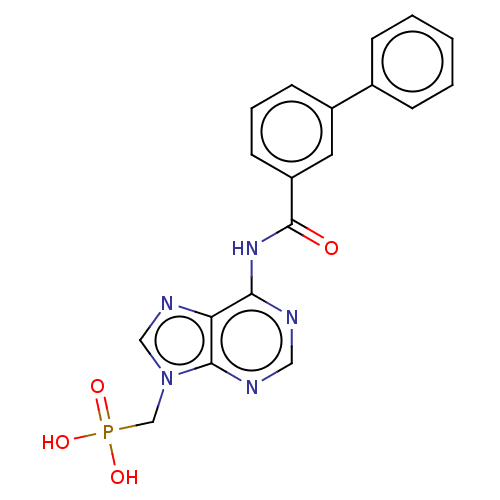 (CHEMBL4438573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot analys... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522287 (CHEMBL4438573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant cN-II (unknown origin) assessed as inhibitor constant for free enzyme using varying level of IMP as substrate by... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50500515 (CHEMBL3747390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.44E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot anal... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522288 (CHEMBL4518527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.52E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Noncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot ana... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosolic purine 5'-nucleotidase (Homo sapiens) | BDBM50500512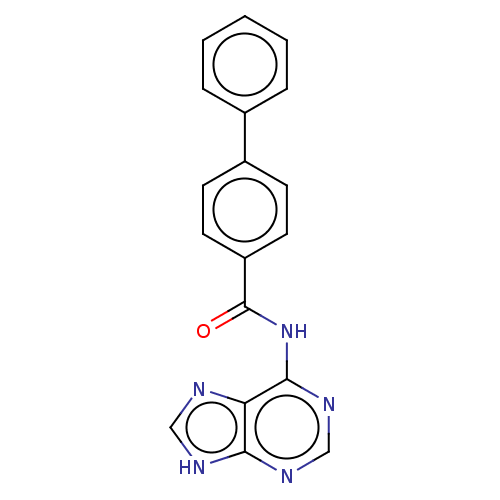 (CHEMBL3746515) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 2.78E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate hydro... | J Med Chem 58: 9680-96 (2015) Article DOI: 10.1021/acs.jmedchem.5b01616 BindingDB Entry DOI: 10.7270/Q2SF3062 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50500515 (CHEMBL3747390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.36E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Noncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot ana... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522287 (CHEMBL4438573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.98E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot anal... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50500515 (CHEMBL3747390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 6.27E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant cN-II (unknown origin) assessed as inhibitor constant for enzyme substrate complex using varying level of IMP as... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522287 (CHEMBL4438573) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.97E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Noncompetitive inhibition of recombinant cN-II (unknown origin) using varying level of IMP as substrate by HPLC method based Lineweaver-Burk plot ana... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50522288 (CHEMBL4518527) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.13E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant cN-II (unknown origin) assessed as inhibitor constant for enzyme substrate complex using varying level of IMP as... | Eur J Med Chem 168: 28-44 (2019) Article DOI: 10.1016/j.ejmech.2019.02.040 BindingDB Entry DOI: 10.7270/Q25D8W8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50193013 (Duvelisib | INK-1147 | INK-1197 | IPI-145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCdelta/PIK3R1 (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50193013 (Duvelisib | INK-1147 | INK-1197 | IPI-145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCgamma (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50403068 (CHEMBL2216870 | IDELALISIB | US9745321, CAL-101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCdelta/PIK3R1 (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50193013 (Duvelisib | INK-1147 | INK-1197 | IPI-145) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCbeta/PIK3R1 (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50470564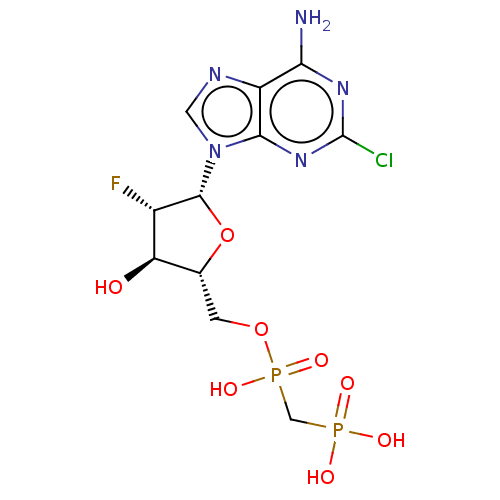 (CHEMBL4287314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of recombinant CD73 (27 to 549 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells assessed as reduction in conv... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50326928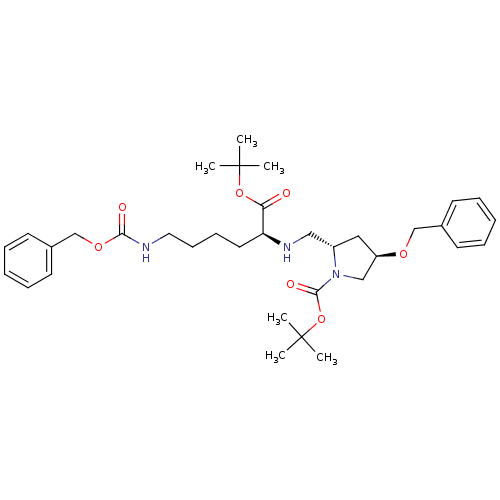 ((2S,4R)-tert-butyl 4-(benzyloxy)-2-(((S)-6-(benzyl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 5086 CNRS Curated by ChEMBL | Assay Description Inhibition of human MDR1 expressed in mouse NIH3T3 cells assessed as inhibition of drug efflux by flow cytometry | J Med Chem 53: 6720-9 (2010) Article DOI: 10.1021/jm100839w BindingDB Entry DOI: 10.7270/Q22N52GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50470564 (CHEMBL4287314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of CD73 in human NCI-H292 cells assessed as reduction in conversion of AMP to adenosine incubated for 30 mins by malachite green reagent b... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50403068 (CHEMBL2216870 | IDELALISIB | US9745321, CAL-101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCgamma (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50193013 (Duvelisib | INK-1147 | INK-1197 | IPI-145) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCalpha/PIK3R1 (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50403068 (CHEMBL2216870 | IDELALISIB | US9745321, CAL-101) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCbeta/PIK3R1 (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50326930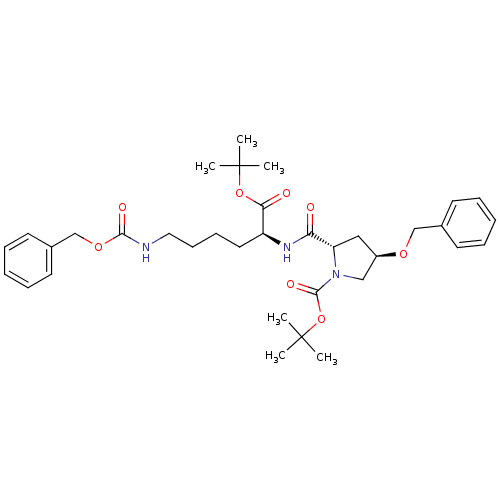 ((2S,4R)-tert-butyl 4-(benzyloxy)-2-((S)-6-(benzylo...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 5086 CNRS Curated by ChEMBL | Assay Description Inhibition of human MDR1 expressed in mouse NIH3T3 cells assessed as inhibition of drug efflux by flow cytometry | J Med Chem 53: 6720-9 (2010) Article DOI: 10.1021/jm100839w BindingDB Entry DOI: 10.7270/Q22N52GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50326931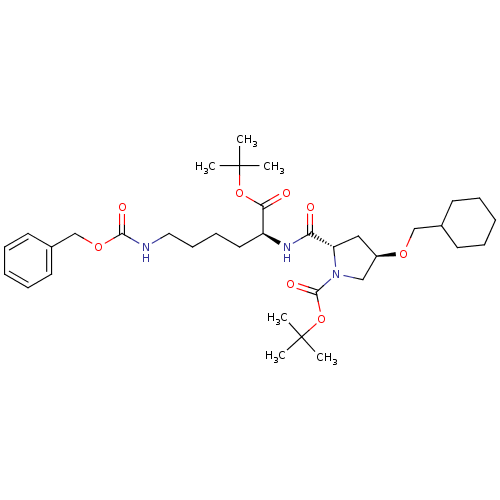 ((2S,4R)-tert-butyl 2-((S)-6-(benzyloxycarbonylamin...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 5086 CNRS Curated by ChEMBL | Assay Description Inhibition of human MDR1 expressed in mouse NIH3T3 cells assessed as inhibition of drug efflux by flow cytometry | J Med Chem 53: 6720-9 (2010) Article DOI: 10.1021/jm100839w BindingDB Entry DOI: 10.7270/Q22N52GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50326927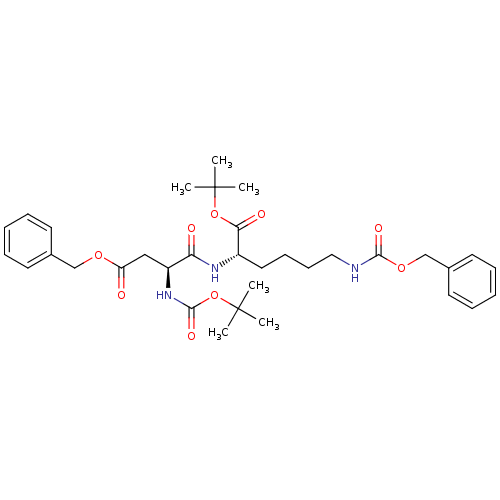 ((S)-tert-butyl 2-((S)-4-(benzyloxy)-2-(tert-butoxy...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 5086 CNRS Curated by ChEMBL | Assay Description Inhibition of human MDR1 expressed in mouse NIH3T3 cells assessed as inhibition of drug efflux by flow cytometry | J Med Chem 53: 6720-9 (2010) Article DOI: 10.1021/jm100839w BindingDB Entry DOI: 10.7270/Q22N52GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50326926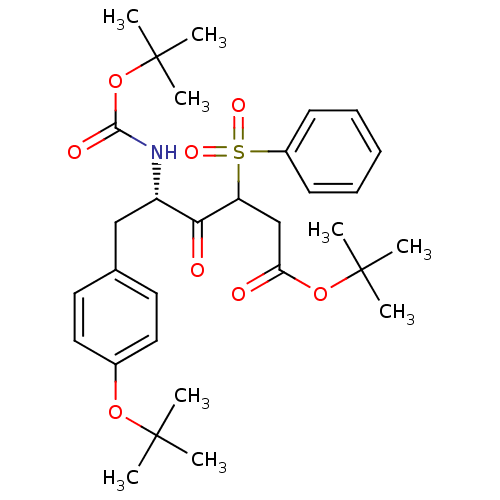 ((5S)-tert-butyl 5-(tert-butoxycarbonylamino)-6-(4-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 5086 CNRS Curated by ChEMBL | Assay Description Inhibition of human MDR1 expressed in mouse NIH3T3 cells assessed as inhibition of drug efflux by flow cytometry | J Med Chem 53: 6720-9 (2010) Article DOI: 10.1021/jm100839w BindingDB Entry DOI: 10.7270/Q22N52GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50326929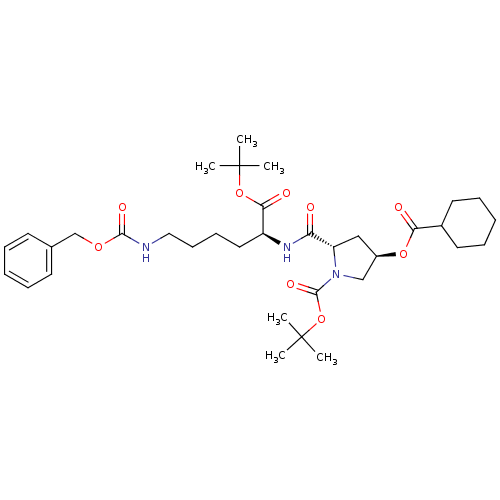 ((2S,4R)-tert-butyl 2-((S)-6-(benzyloxycarbonylamin...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 5086 CNRS Curated by ChEMBL | Assay Description Inhibition of human MDR1 expressed in mouse NIH3T3 cells assessed as inhibition of drug efflux by flow cytometry | J Med Chem 53: 6720-9 (2010) Article DOI: 10.1021/jm100839w BindingDB Entry DOI: 10.7270/Q22N52GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50403068 (CHEMBL2216870 | IDELALISIB | US9745321, CAL-101) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCalpha/PIK3R1 (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50470563 (CHEMBL4283386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of CD73 in human NCI-H292 cells assessed as reduction in conversion of AMP to adenosine incubated for 30 mins by malachite green reagent b... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50470565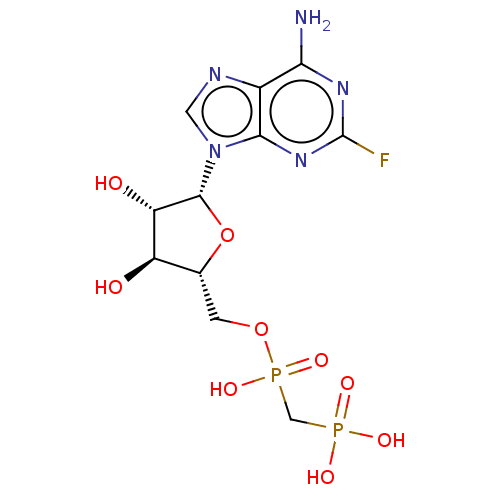 (CHEMBL4276670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of CD73 in human NCI-H292 cells assessed as reduction in conversion of AMP to adenosine incubated for 30 mins by malachite green reagent b... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50470564 (CHEMBL4287314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of CD73 in human MDA-MB-231 cells assessed as reduction in conversion of AMP to adenosine incubated for 30 mins by malachite green reagent... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM18136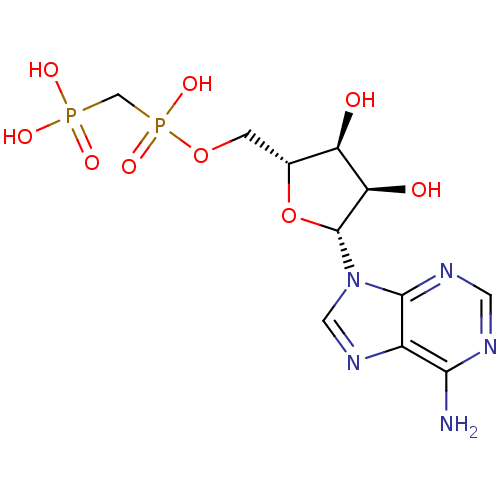 (ADP, alpha beta-me | AMPCPP | [({[(2R,3S,4R,5R)-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of recombinant CD73 (27 to 549 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells assessed as reduction in conv... | Eur J Med Chem 157: 1051-1055 (2018) Article DOI: 10.1016/j.ejmech.2018.08.035 BindingDB Entry DOI: 10.7270/Q2QJ7M16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50467362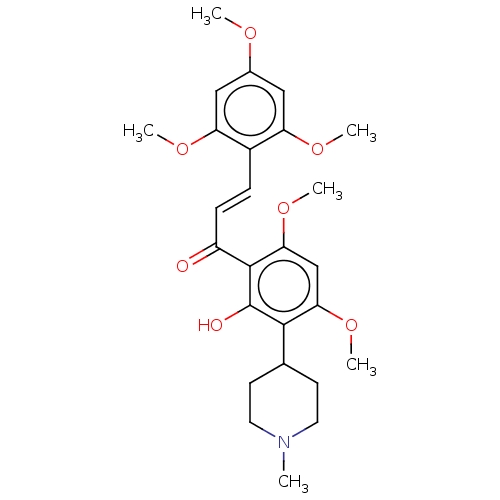 (CHEMBL4287949) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM 1052/CNRS 5286/University of Lyon Curated by ChEMBL | Assay Description Inhibition of PI3KCdelta/PIK3R1 (unknown origin) | Eur J Med Chem 158: 405-413 (2018) Article DOI: 10.1016/j.ejmech.2018.09.033 BindingDB Entry DOI: 10.7270/Q2KS6V81 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |