

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280415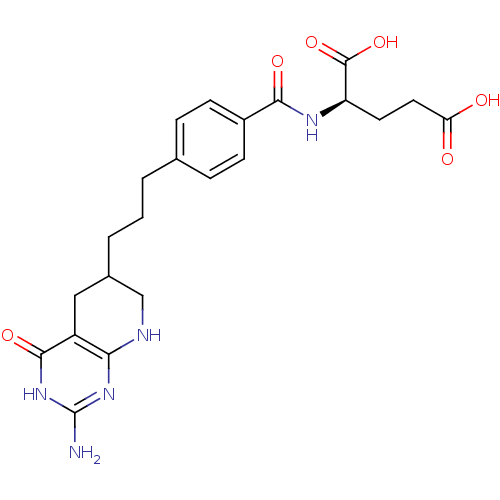 ((R)-2-{4-[3-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.0000190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM22590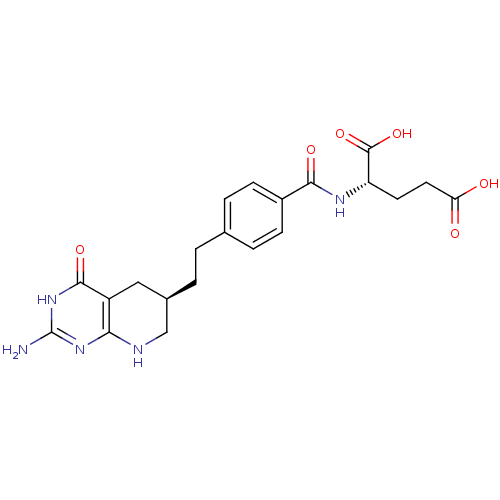 ((2S)-2-[(4-{2-[(6R)-2-amino-4-oxo-1H,4H,5H,6H,7H,8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 0.000120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50280414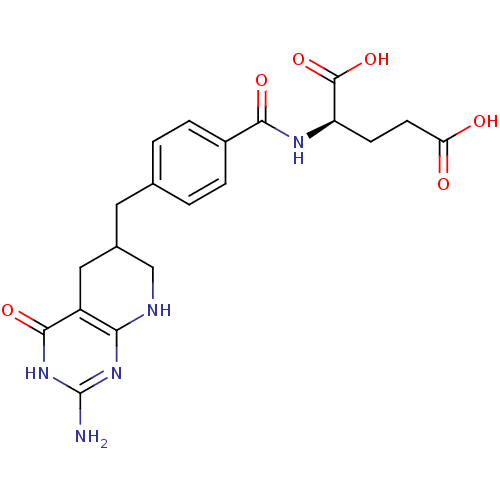 ((R)-2-[4-(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against glycinamide ribonucleotide formyltransferase (GARFT) from L1210 murine leukemic cells | Bioorg Med Chem Lett 2: 339-342 (1992) Article DOI: 10.1016/S0960-894X(01)80214-6 BindingDB Entry DOI: 10.7270/Q2RF5TXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM85097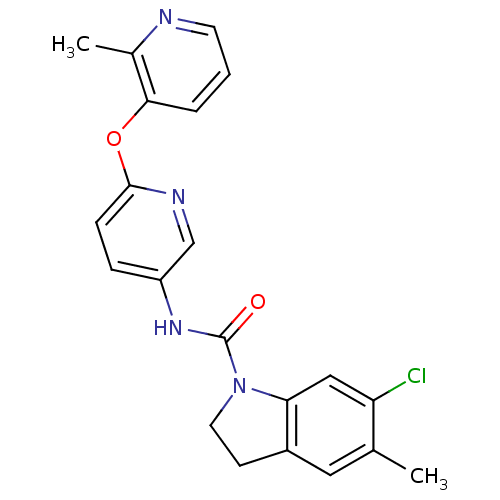 (CAS_181632-25-7 | CHEMBL14563 | SB 242084) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583877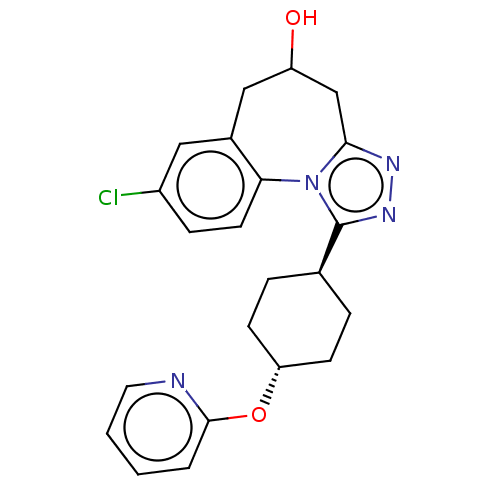 (CHEMBL5070261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583878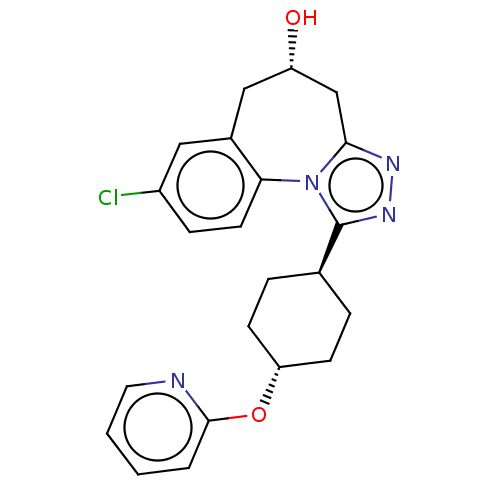 (CHEMBL5074709) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583879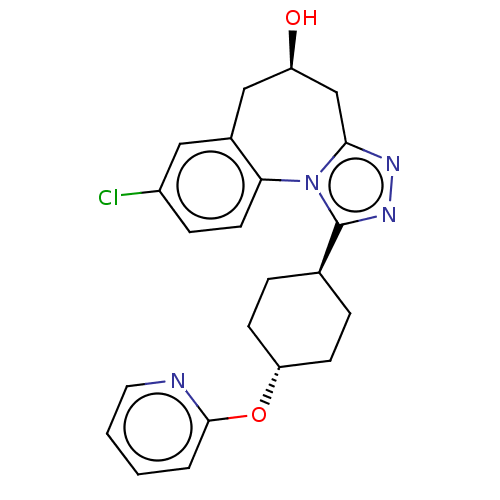 (CHEMBL5070075) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583881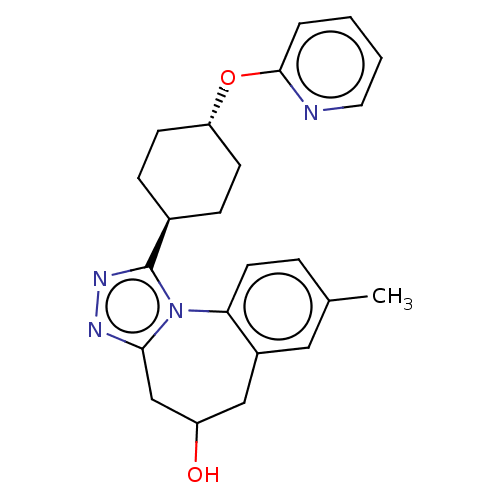 (CHEMBL5081323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140071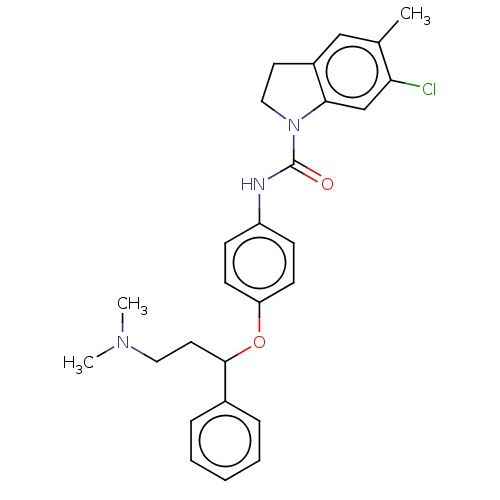 (CHEMBL3752465) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50546439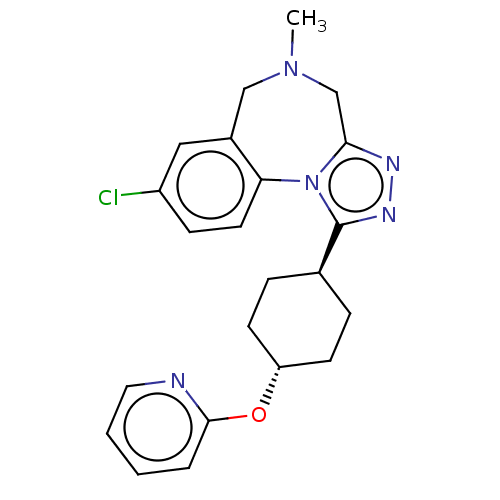 (Balovaptan | RG-7314 | RO-5285119 | RO5285119 | Rg...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50546436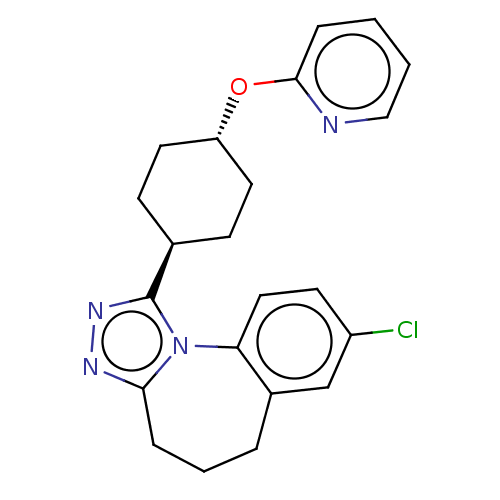 (CHEMBL4799793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V2 receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50140065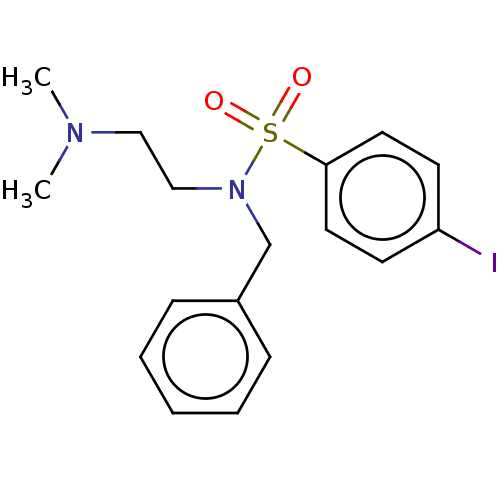 (CHEMBL3753894) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Binding affinity to 5HT2A (unknown origin) | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50200120 (CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC | Bioorg Med Chem Lett 22: 2536-43 (2012) Article DOI: 10.1016/j.bmcl.2012.01.140 BindingDB Entry DOI: 10.7270/Q2JS9T9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50013050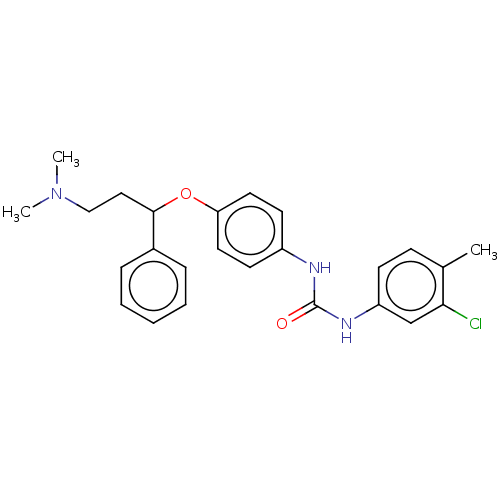 (CHEMBL3261693) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50140065 (CHEMBL3753894) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A in Wistar rat frontal cortex membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50354894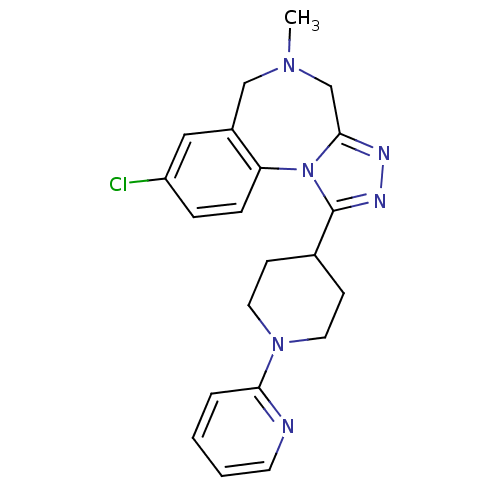 (CHEMBL1837037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277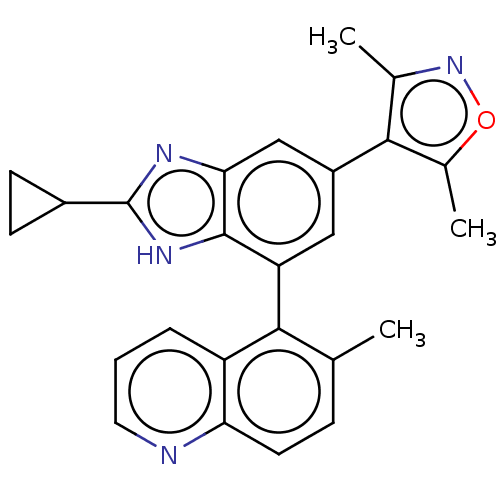 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain2 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140069 (CHEMBL3753329) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077217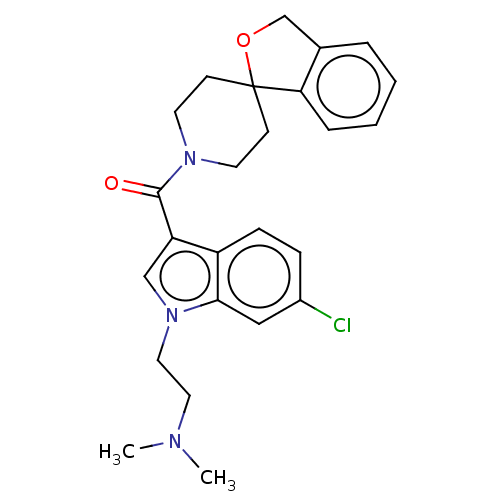 (CHEMBL3416885) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140066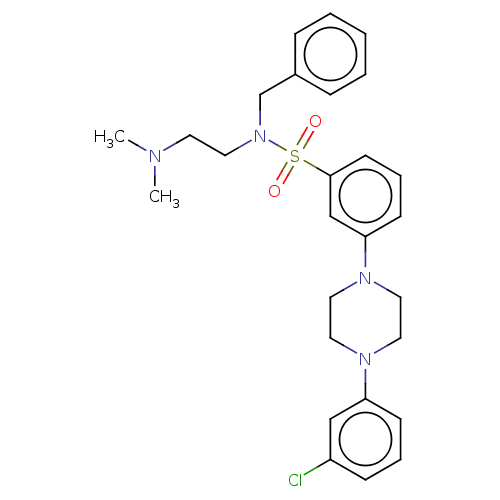 (CHEMBL3754784) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583883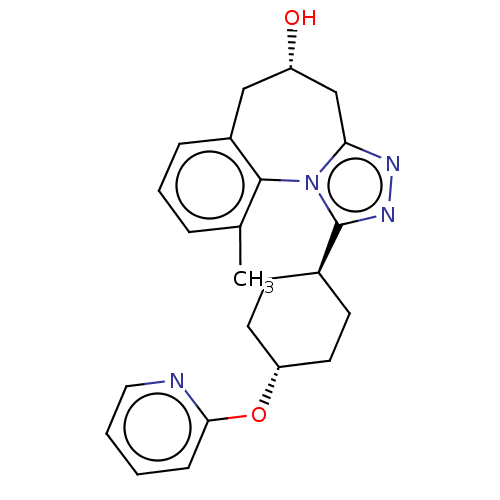 (CHEMBL5078950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583883 (CHEMBL5078950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of Cy5-linked JQ1 probe binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after 60 mins by HTR... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50140065 (CHEMBL3753894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Binding affinity to 5HT2C (unknown origin) | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140067 (CHEMBL3753681) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583880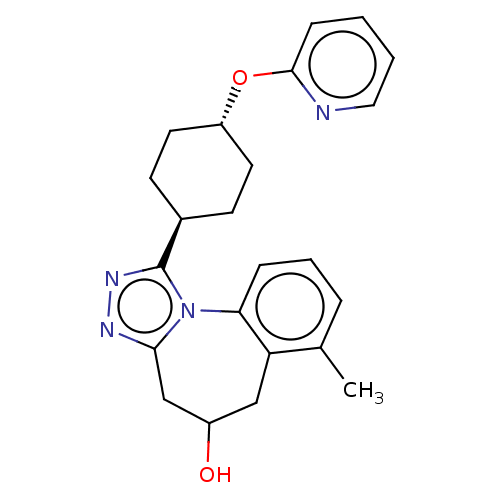 (CHEMBL5088219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50013069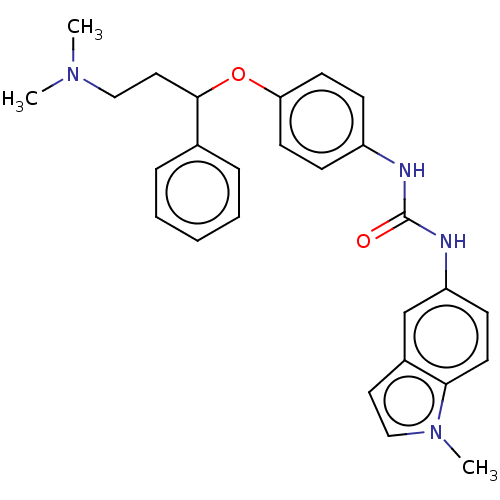 (CHEMBL3261686) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50140067 (CHEMBL3753681) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A in Wistar rat frontal cortex membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50013102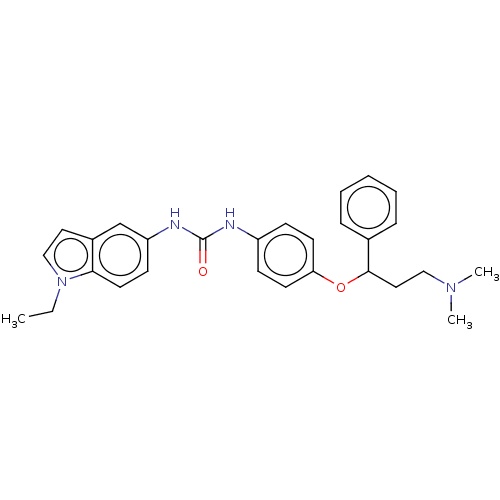 (CHEMBL3261690) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50013052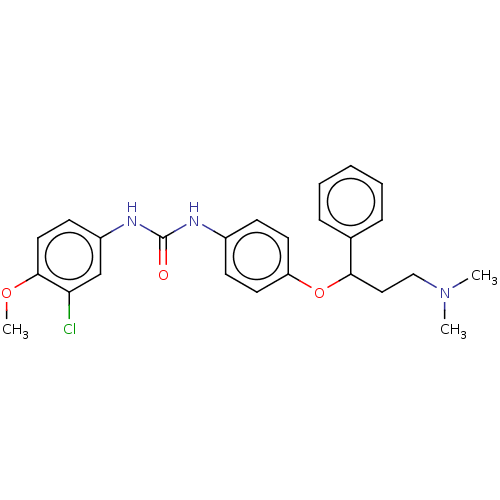 (CHEMBL3261695) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583887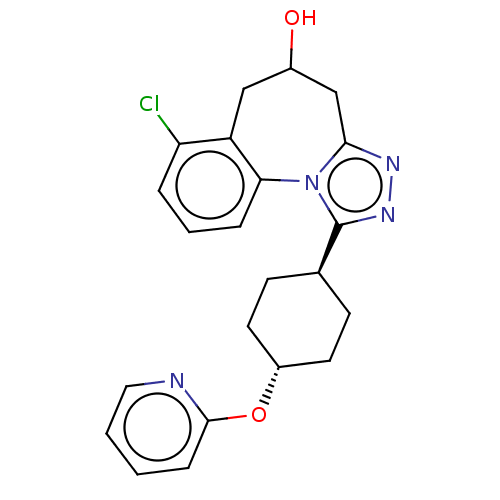 (CHEMBL5074961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140065 (CHEMBL3753894) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140064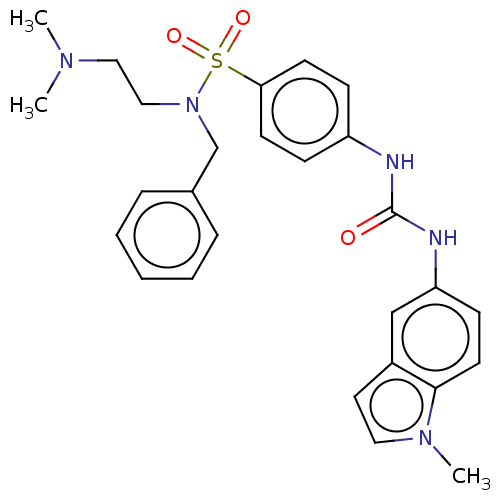 (CHEMBL3752844) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140070 (CHEMBL3754137) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM249277 (US10017501, Compound 1020-18 | US9458145, 1020-18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of tetra-acetylated Histone H4 peptide binding to recombinant human N-terminal TEV-cleavable hexa-histidine tagged BRD4 bromodomain1 after... | Bioorg Med Chem 27: 457-469 (2019) Article DOI: 10.1016/j.bmc.2018.11.020 BindingDB Entry DOI: 10.7270/Q2HD7ZZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50013069 (CHEMBL3261686) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50140065 (CHEMBL3753894) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50478376 (BB-78485 | CHEMBL261713) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of Escherichia coli LpxC | Bioorg Med Chem Lett 22: 2536-43 (2012) Article DOI: 10.1016/j.bmcl.2012.01.140 BindingDB Entry DOI: 10.7270/Q2JS9T9W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50255538 ((4-(4-(3-chlorobenzyloxy)-3-methylbenzoyl)piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from human ERG channel | Bioorg Med Chem Lett 19: 665-9 (2009) Article DOI: 10.1016/j.bmcl.2008.12.054 BindingDB Entry DOI: 10.7270/Q28C9W3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50140064 (CHEMBL3752844) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50140063 (CHEMBL3753516) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5HT2C in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50001915 (1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Binding affinity to 5HT2C (unknown origin) | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50140065 (CHEMBL3753894) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Binding affinity to SERT (unknown origin) | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50140067 (CHEMBL3753681) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Mus musculus) | BDBM50003896 ((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University Curated by ChEMBL | Assay Description Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase | J Med Chem 35: 4450-4 (1992) BindingDB Entry DOI: 10.7270/Q2RR1ZV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583885 (CHEMBL5079921) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583882 (CHEMBL5085869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50140073 (CHEMBL3752059) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem Lett 26: 914-20 (2016) Article DOI: 10.1016/j.bmcl.2015.12.071 BindingDB Entry DOI: 10.7270/Q2VX0JC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3998 total ) | Next | Last >> |