 Found 1080 hits with Last Name = 'harris' and Initial = 'cm'
Found 1080 hits with Last Name = 'harris' and Initial = 'cm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506950
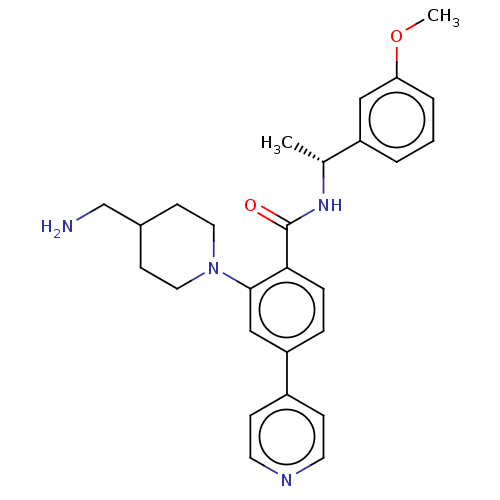
(CHEMBL4434688)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1N1CCC(CN)CC1)-c1ccncc1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(22-4-3-5-24(16-22)33-2)30-27(32)25-7-6-23(21-8-12-29-13-9-21)17-26(25)31-14-10-20(18-28)11-15-31/h3-9,12-13,16-17,19-20H,10-11,14-15,18,28H2,1-2H3,(H,30,32)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506948
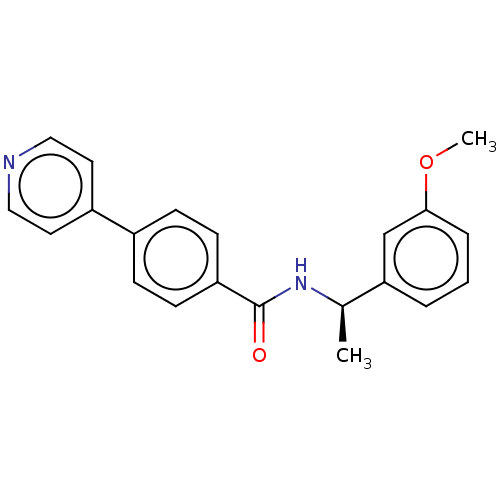
(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
cGMP-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50506948

(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of PKG1A (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499639

(CHEMBL3739440)Show InChI InChI=1S/C20H31NO3/c1-2-3-4-5-6-7-8-13-24-19-11-9-17(10-12-19)14-21-15-18(16-21)20(22)23/h9-12,18H,2-8,13-16H2,1H3,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499678
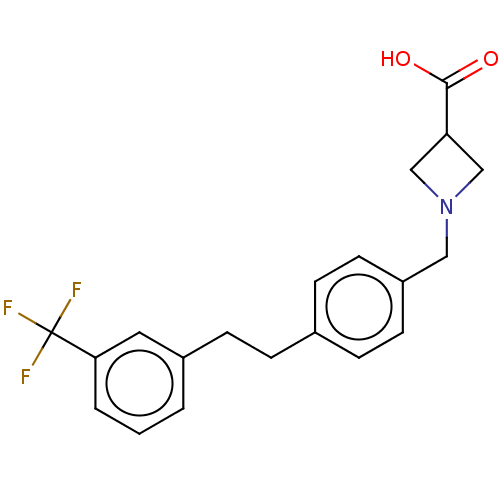
(CHEMBL1973936)Show SMILES OC(=O)C1CN(Cc2ccc(CCc3cccc(c3)C(F)(F)F)cc2)C1 Show InChI InChI=1S/C20H20F3NO2/c21-20(22,23)18-3-1-2-15(10-18)7-4-14-5-8-16(9-6-14)11-24-12-17(13-24)19(25)26/h1-3,5-6,8-10,17H,4,7,11-13H2,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50499644
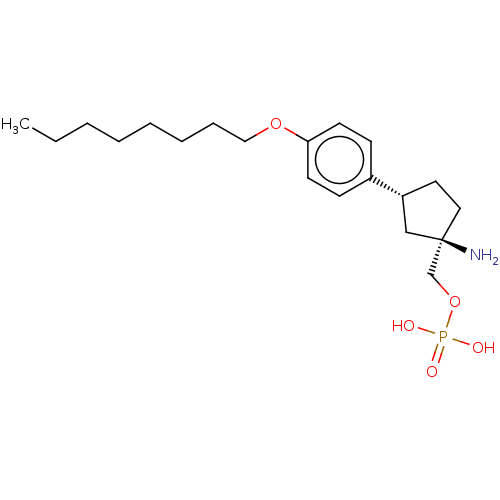
(CHEMBL3741414)Show SMILES CCCCCCCCOc1ccc(cc1)[C@@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C20H34NO5P/c1-2-3-4-5-6-7-14-25-19-10-8-17(9-11-19)18-12-13-20(21,15-18)16-26-27(22,23)24/h8-11,18H,2-7,12-16,21H2,1H3,(H2,22,23,24)/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P1 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499650
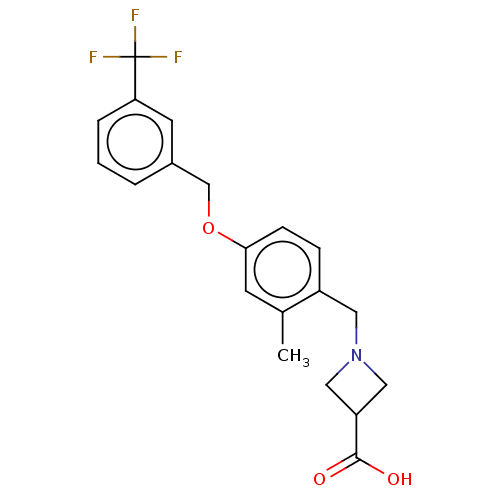
(CHEMBL1966501)Show SMILES Cc1cc(OCc2cccc(c2)C(F)(F)F)ccc1CN1CC(C1)C(O)=O Show InChI InChI=1S/C20H20F3NO3/c1-13-7-18(6-5-15(13)9-24-10-16(11-24)19(25)26)27-12-14-3-2-4-17(8-14)20(21,22)23/h2-8,16H,9-12H2,1H3,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499687
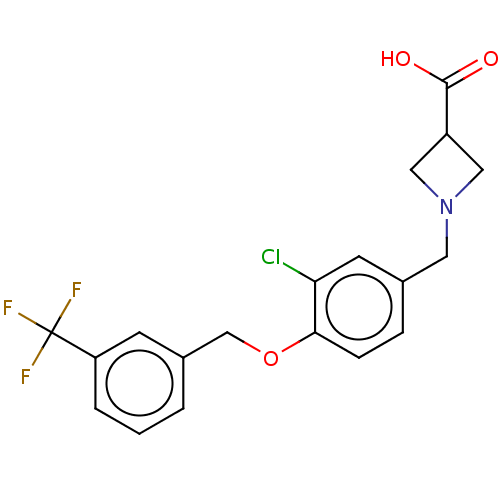
(CHEMBL3741092)Show SMILES OC(=O)C1CN(Cc2ccc(OCc3cccc(c3)C(F)(F)F)c(Cl)c2)C1 Show InChI InChI=1S/C19H17ClF3NO3/c20-16-7-12(8-24-9-14(10-24)18(25)26)4-5-17(16)27-11-13-2-1-3-15(6-13)19(21,22)23/h1-7,14H,8-11H2,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506946

(CHEMBL4472858)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1F |r| Show InChI InChI=1S/C21H19FN2O2/c1-14(17-4-3-5-18(12-17)26-2)24-21(25)16-8-6-15(7-9-16)19-10-11-23-13-20(19)22/h3-14H,1-2H3,(H,24,25)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50506948

(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499644

(CHEMBL3741414)Show SMILES CCCCCCCCOc1ccc(cc1)[C@@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C20H34NO5P/c1-2-3-4-5-6-7-14-25-19-10-8-17(9-11-19)18-12-13-20(21,15-18)16-26-27(22,23)24/h8-11,18H,2-7,12-16,21H2,1H3,(H2,22,23,24)/t18-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499651

(CHEMBL1990223)Show SMILES Cc1cc(OCc2ccc(Cl)c(Cl)c2)ccc1CN1CC(C1)C(O)=O Show InChI InChI=1S/C19H19Cl2NO3/c1-12-6-16(25-11-13-2-5-17(20)18(21)7-13)4-3-14(12)8-22-9-15(10-22)19(23)24/h2-7,15H,8-11H2,1H3,(H,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506948

(CHEMBL4448806)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C21H20N2O2/c1-15(19-4-3-5-20(14-19)25-2)23-21(24)18-8-6-16(7-9-18)17-10-12-22-13-11-17/h3-15H,1-2H3,(H,23,24)/t15-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499648
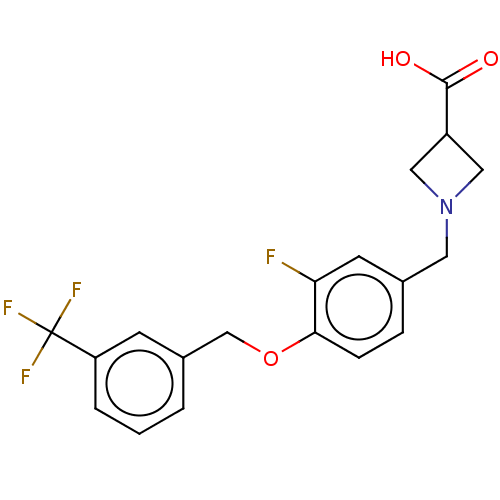
(CHEMBL3741572)Show SMILES OC(=O)C1CN(Cc2ccc(OCc3cccc(c3)C(F)(F)F)c(F)c2)C1 Show InChI InChI=1S/C19H17F4NO3/c20-16-7-12(8-24-9-14(10-24)18(25)26)4-5-17(16)27-11-13-2-1-3-15(6-13)19(21,22)23/h1-7,14H,8-11H2,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499670

(CHEMBL1998478)Show SMILES OC(=O)C1CN(Cc2ccc(OCc3cccc(c3)C(F)(F)F)cc2F)C1 Show InChI InChI=1S/C19H17F4NO3/c20-17-7-16(5-4-13(17)8-24-9-14(10-24)18(25)26)27-11-12-2-1-3-15(6-12)19(21,22)23/h1-7,14H,8-11H2,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499683
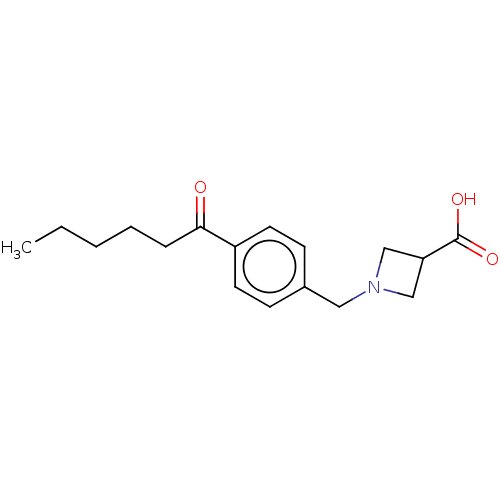
(CHEMBL3740009)Show InChI InChI=1S/C17H23NO3/c1-2-3-4-5-16(19)14-8-6-13(7-9-14)10-18-11-15(12-18)17(20)21/h6-9,15H,2-5,10-12H2,1H3,(H,20,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor (unknown origin) expressed in CHO cell membranes after 30 mins by GTPgammaS binding based MFB method |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50210272
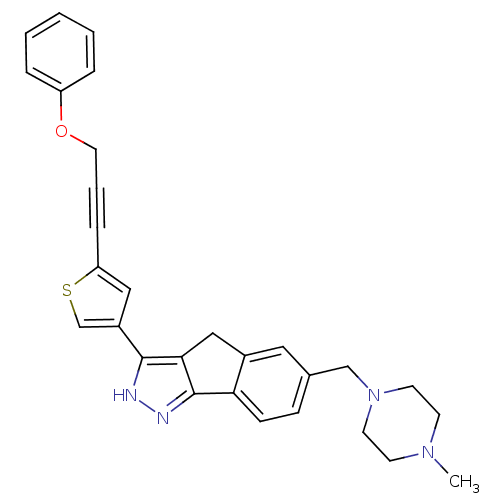
(6-((4-methylpiperazin-1-yl)methyl)-3-(5-(3-phenoxy...)Show SMILES CN1CCN(Cc2ccc-3c(Cc4c-3n[nH]c4-c3csc(c3)C#CCOc3ccccc3)c2)CC1 Show InChI InChI=1S/C29H28N4OS/c1-32-11-13-33(14-12-32)19-21-9-10-26-22(16-21)18-27-28(30-31-29(26)27)23-17-25(35-20-23)8-5-15-34-24-6-3-2-4-7-24/h2-4,6-7,9-10,16-17,20H,11-15,18-19H2,1H3,(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Flt1 |
J Med Chem 50: 2011-29 (2007)
Article DOI: 10.1021/jm061223o
BindingDB Entry DOI: 10.7270/Q2TB16K0 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50499668

(CHEMBL3741743)Show InChI InChI=1S/C19H21NO2/c21-19(22)18-13-20(14-18)12-17-10-8-16(9-11-17)7-6-15-4-2-1-3-5-15/h1-5,8-11,18H,6-7,12-14H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P1 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50210309
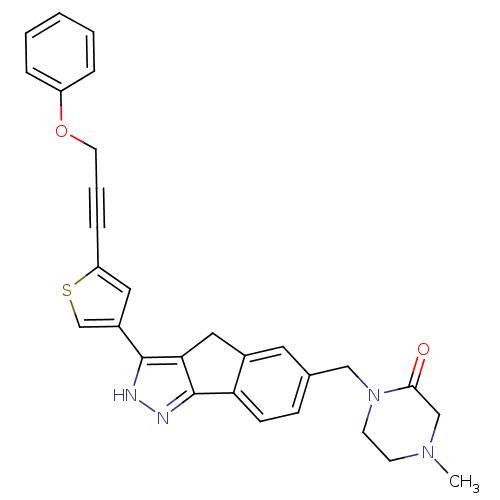
(4-methyl-1-((3-(5-(3-phenoxyprop-1-ynyl)thiophen-3...)Show SMILES CN1CCN(Cc2ccc-3c(Cc4c-3n[nH]c4-c3csc(c3)C#CCOc3ccccc3)c2)C(=O)C1 Show InChI InChI=1S/C29H26N4O2S/c1-32-11-12-33(27(34)18-32)17-20-9-10-25-21(14-20)16-26-28(30-31-29(25)26)22-15-24(36-19-22)8-5-13-35-23-6-3-2-4-7-23/h2-4,6-7,9-10,14-15,19H,11-13,16-18H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced phosphorylation of human KDR expressed in mouse NIH3T3 cell line by Western blot |
J Med Chem 50: 2011-29 (2007)
Article DOI: 10.1021/jm061223o
BindingDB Entry DOI: 10.7270/Q2TB16K0 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50499654

(CHEMBL3739878)Show InChI InChI=1S/C19H29NO3/c1-2-3-4-5-6-7-12-23-18-10-8-16(9-11-18)13-20-14-17(15-20)19(21)22/h8-11,17H,2-7,12-15H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P1 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50207507
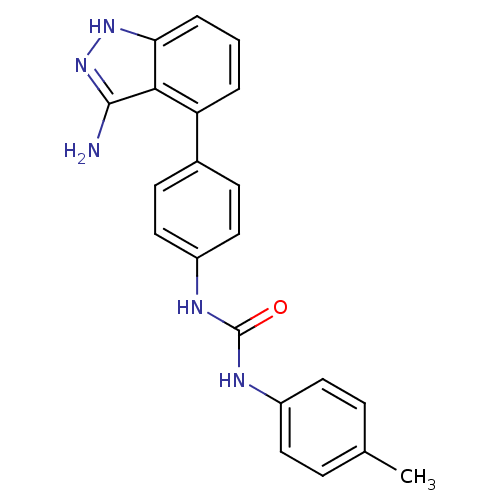
(1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-p-tolylure...)Show SMILES Cc1ccc(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)cc1 Show InChI InChI=1S/C21H19N5O/c1-13-5-9-15(10-6-13)23-21(27)24-16-11-7-14(8-12-16)17-3-2-4-18-19(17)20(22)26-25-18/h2-12H,1H3,(H3,22,25,26)(H2,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499682
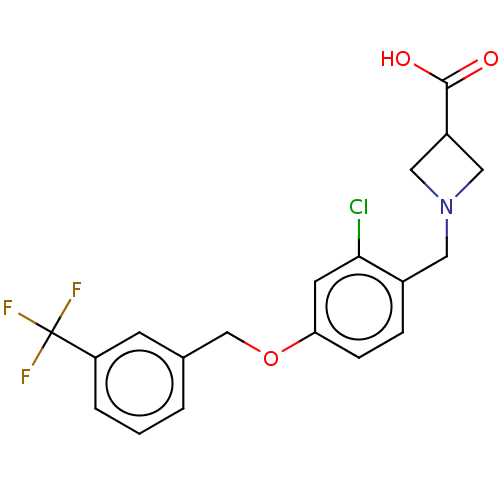
(CHEMBL1986265)Show SMILES OC(=O)C1CN(Cc2ccc(OCc3cccc(c3)C(F)(F)F)cc2Cl)C1 Show InChI InChI=1S/C19H17ClF3NO3/c20-17-7-16(5-4-13(17)8-24-9-14(10-24)18(25)26)27-11-12-2-1-3-15(6-12)19(21,22)23/h1-7,14H,8-11H2,(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50506950

(CHEMBL4434688)Show SMILES COc1cccc(c1)[C@@H](C)NC(=O)c1ccc(cc1N1CCC(CN)CC1)-c1ccncc1 |r| Show InChI InChI=1S/C27H32N4O2/c1-19(22-4-3-5-24(16-22)33-2)30-27(32)25-7-6-23(21-8-12-29-13-9-21)17-26(25)31-14-10-20(18-28)11-15-31/h3-9,12-13,16-17,19-20H,10-11,14-15,18,28H2,1-2H3,(H,30,32)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged catalytic ROCK1 expressed in baculovirus system using STK S2 peptide substrate and and 33P-ATP after 60 mins by HTRF a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50210271
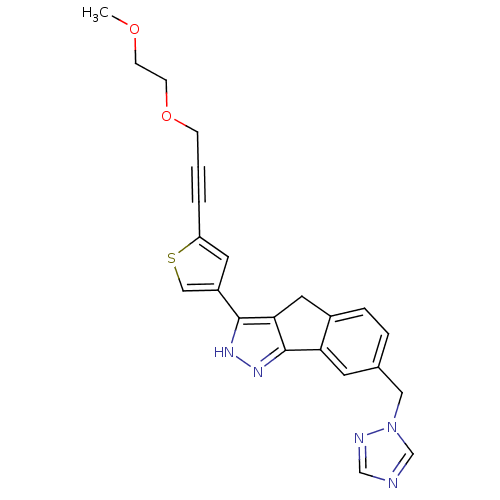
(7-((1H-1,2,4-triazol-1-yl)methyl)-3-(5-(3-(2-metho...)Show SMILES COCCOCC#Cc1cc(cs1)-c1[nH]nc-2c1Cc1ccc(Cn3cncn3)cc-21 Show InChI InChI=1S/C23H21N5O2S/c1-29-7-8-30-6-2-3-19-10-18(13-31-19)22-21-11-17-5-4-16(12-28-15-24-14-25-28)9-20(17)23(21)27-26-22/h4-5,9-10,13-15H,6-8,11-12H2,1H3,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Flt1 |
J Med Chem 50: 2011-29 (2007)
Article DOI: 10.1021/jm061223o
BindingDB Entry DOI: 10.7270/Q2TB16K0 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506954
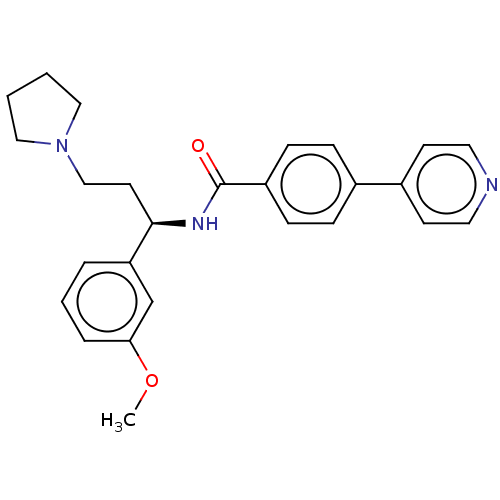
(CHEMBL4583341)Show SMILES COc1cccc(c1)[C@@H](CCN1CCCC1)NC(=O)c1ccc(cc1)-c1ccncc1 |r| Show InChI InChI=1S/C26H29N3O2/c1-31-24-6-4-5-23(19-24)25(13-18-29-16-2-3-17-29)28-26(30)22-9-7-20(8-10-22)21-11-14-27-15-12-21/h4-12,14-15,19,25H,2-3,13,16-18H2,1H3,(H,28,30)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499663
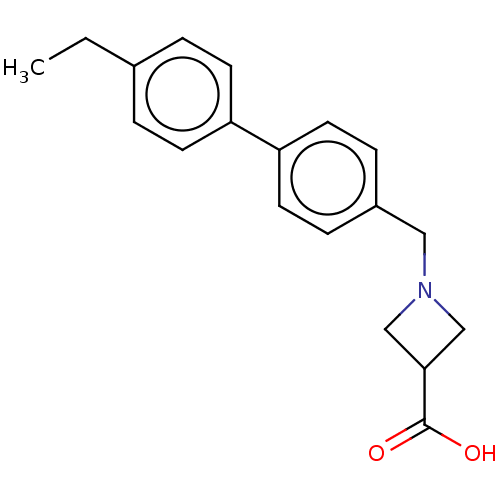
(CHEMBL3740359)Show InChI InChI=1S/C19H21NO2/c1-2-14-3-7-16(8-4-14)17-9-5-15(6-10-17)11-20-12-18(13-20)19(21)22/h3-10,18H,2,11-13H2,1H3,(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50499644

(CHEMBL3741414)Show SMILES CCCCCCCCOc1ccc(cc1)[C@@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C20H34NO5P/c1-2-3-4-5-6-7-14-25-19-10-8-17(9-11-19)18-12-13-20(21,15-18)16-26-27(22,23)24/h8-11,18H,2-7,12-16,21H2,1H3,(H2,22,23,24)/t18-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P3 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50210300
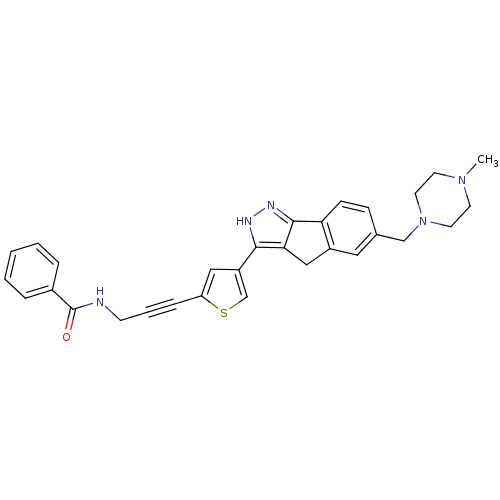
(CHEMBL225311 | N-(3-(4-(6-((4-methylpiperazin-1-yl...)Show SMILES CN1CCN(Cc2ccc-3c(Cc4c-3n[nH]c4-c3csc(c3)C#CCNC(=O)c3ccccc3)c2)CC1 Show InChI InChI=1S/C30H29N5OS/c1-34-12-14-35(15-13-34)19-21-9-10-26-23(16-21)18-27-28(32-33-29(26)27)24-17-25(37-20-24)8-5-11-31-30(36)22-6-3-2-4-7-22/h2-4,6-7,9-10,16-17,20H,11-15,18-19H2,1H3,(H,31,36)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced phosphorylation of human KDR expressed in mouse NIH3T3 cell line by Western blot |
J Med Chem 50: 2011-29 (2007)
Article DOI: 10.1021/jm061223o
BindingDB Entry DOI: 10.7270/Q2TB16K0 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499645
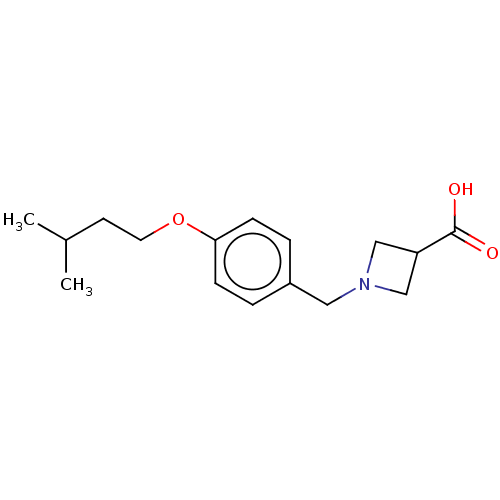
(CHEMBL3740090)Show InChI InChI=1S/C16H23NO3/c1-12(2)7-8-20-15-5-3-13(4-6-15)9-17-10-14(11-17)16(18)19/h3-6,12,14H,7-11H2,1-2H3,(H,18,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM21079
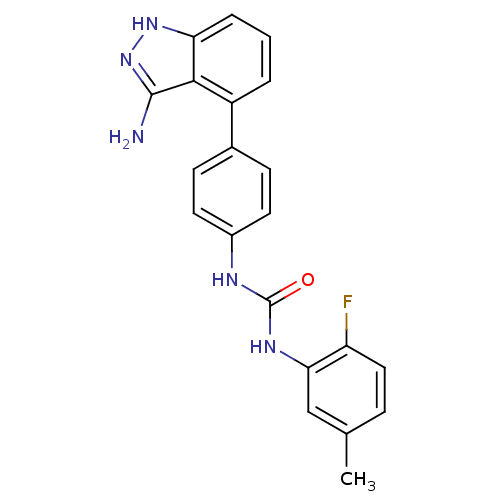
(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CSF1R by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50207509
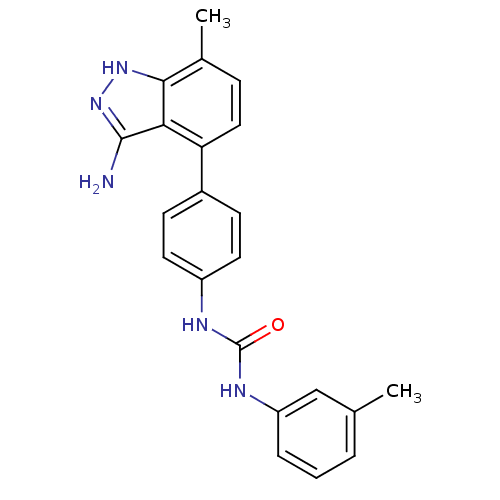
(1-(4-(3-amino-7-methyl-1H-indazol-4-yl)phenyl)-3-m...)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2ccc(C)c3[nH]nc(N)c23)c1 Show InChI InChI=1S/C22H21N5O/c1-13-4-3-5-17(12-13)25-22(28)24-16-9-7-15(8-10-16)18-11-6-14(2)20-19(18)21(23)27-26-20/h3-12H,1-2H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced human KDR phosphorylation in mouse 3T3 cells by ELISA |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50207484
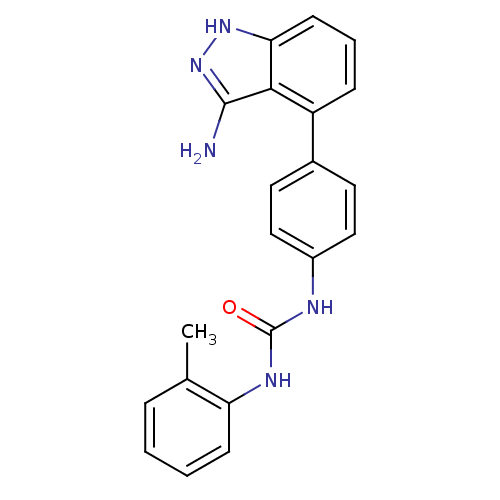
(1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-o-tolylure...)Show SMILES Cc1ccccc1NC(=O)Nc1ccc(cc1)-c1cccc2[nH]nc(N)c12 Show InChI InChI=1S/C21H19N5O/c1-13-5-2-3-7-17(13)24-21(27)23-15-11-9-14(10-12-15)16-6-4-8-18-19(16)20(22)26-25-18/h2-12H,1H3,(H3,22,25,26)(H2,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50210271

(7-((1H-1,2,4-triazol-1-yl)methyl)-3-(5-(3-(2-metho...)Show SMILES COCCOCC#Cc1cc(cs1)-c1[nH]nc-2c1Cc1ccc(Cn3cncn3)cc-21 Show InChI InChI=1S/C23H21N5O2S/c1-29-7-8-30-6-2-3-19-10-18(13-31-19)22-21-11-17-5-4-16(12-28-15-24-14-25-28)9-20(17)23(21)27-26-22/h4-5,9-10,13-15H,6-8,11-12H2,1H3,(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
J Med Chem 50: 2011-29 (2007)
Article DOI: 10.1021/jm061223o
BindingDB Entry DOI: 10.7270/Q2TB16K0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50210272

(6-((4-methylpiperazin-1-yl)methyl)-3-(5-(3-phenoxy...)Show SMILES CN1CCN(Cc2ccc-3c(Cc4c-3n[nH]c4-c3csc(c3)C#CCOc3ccccc3)c2)CC1 Show InChI InChI=1S/C29H28N4OS/c1-32-11-13-33(14-12-32)19-21-9-10-26-22(16-21)18-27-28(30-31-29(26)27)23-17-25(35-20-23)8-5-15-34-24-6-3-2-4-7-24/h2-4,6-7,9-10,16-17,20H,11-15,18-19H2,1H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Flt4 |
J Med Chem 50: 2011-29 (2007)
Article DOI: 10.1021/jm061223o
BindingDB Entry DOI: 10.7270/Q2TB16K0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50207473
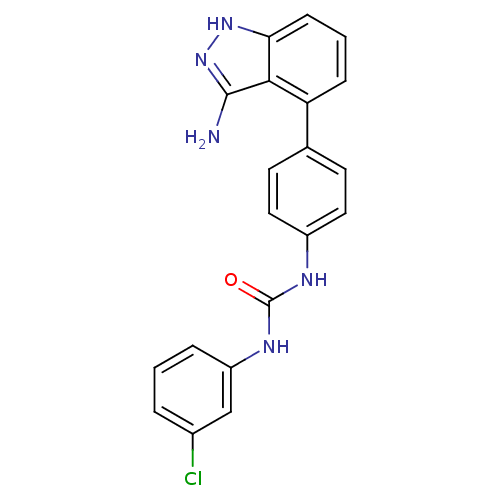
(1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-(3-chlorop...)Show SMILES Nc1n[nH]c2cccc(-c3ccc(NC(=O)Nc4cccc(Cl)c4)cc3)c12 Show InChI InChI=1S/C20H16ClN5O/c21-13-3-1-4-15(11-13)24-20(27)23-14-9-7-12(8-10-14)16-5-2-6-17-18(16)19(22)26-25-17/h1-11H,(H3,22,25,26)(H2,23,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLT1 by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 51: 1231-41 (2008)
Article DOI: 10.1021/jm701096v
BindingDB Entry DOI: 10.7270/Q2HX19Z7 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 51: 1231-41 (2008)
Article DOI: 10.1021/jm701096v
BindingDB Entry DOI: 10.7270/Q2HX19Z7 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50207499
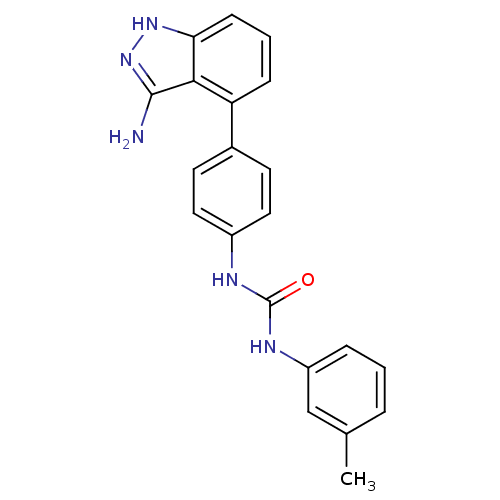
(1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-m-tolylure...)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H19N5O/c1-13-4-2-5-16(12-13)24-21(27)23-15-10-8-14(9-11-15)17-6-3-7-18-19(17)20(22)26-25-18/h2-12H,1H3,(H3,22,25,26)(H2,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KDR by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50506933
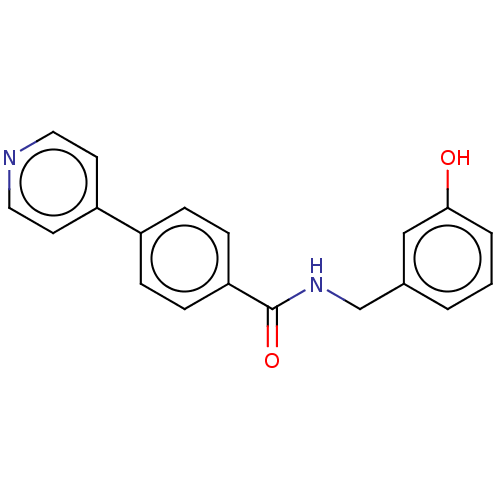
(CHEMBL4536833)Show InChI InChI=1S/C19H16N2O2/c22-18-3-1-2-14(12-18)13-21-19(23)17-6-4-15(5-7-17)16-8-10-20-11-9-16/h1-12,22H,13H2,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... |
J Med Chem 61: 11074-11100 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01098
BindingDB Entry DOI: 10.7270/Q2RB77XJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499658

(CHEMBL1971132)Show SMILES OC(=O)C1CN(Cc2ccc(OCc3cccc(c3)C(F)(F)F)cc2)C1 Show InChI InChI=1S/C19H18F3NO3/c20-19(21,22)16-3-1-2-14(8-16)12-26-17-6-4-13(5-7-17)9-23-10-15(11-23)18(24)25/h1-8,15H,9-12H2,(H,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50499685

(CHEMBL1968499)Show InChI InChI=1S/C18H17Cl2NO3/c19-15-4-3-13(17(20)7-15)11-24-16-5-1-12(2-6-16)8-21-9-14(10-21)18(22)23/h1-7,14H,8-11H2,(H,22,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from S1P5 receptor (unknown origin) expressed in HEK cell membranes after 45 to 60 mins by scintillation counting based RLB ... |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of VEGF-induced human KDR phosphorylation in mouse 3T3 cells by ELISA |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM21090
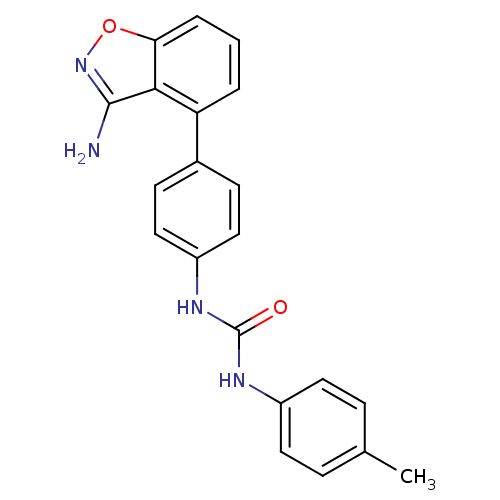
(3-[4-(3-amino-1,2-benzoxazol-4-yl)phenyl]-1-(4-met...)Show SMILES Cc1ccc(NC(=O)Nc2ccc(cc2)-c2cccc3onc(N)c23)cc1 Show InChI InChI=1S/C21H18N4O2/c1-13-5-9-15(10-6-13)23-21(26)24-16-11-7-14(8-12-16)17-3-2-4-18-19(17)20(22)25-27-18/h2-12H,1H3,(H2,22,25)(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 51: 1231-41 (2008)
Article DOI: 10.1021/jm701096v
BindingDB Entry DOI: 10.7270/Q2HX19Z7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM21085
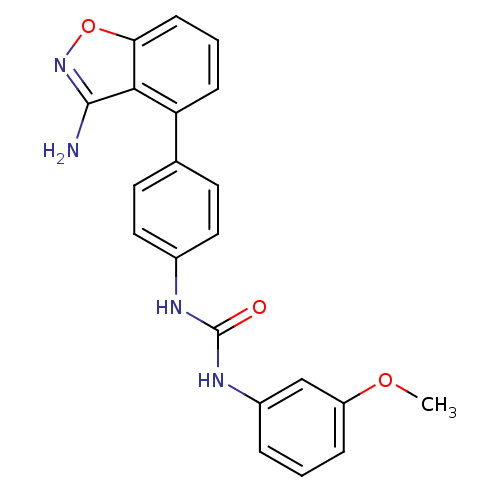
(3-[4-(3-amino-1,2-benzoxazol-4-yl)phenyl]-1-(3-met...)Show SMILES COc1cccc(NC(=O)Nc2ccc(cc2)-c2cccc3onc(N)c23)c1 Show InChI InChI=1S/C21H18N4O3/c1-27-16-5-2-4-15(12-16)24-21(26)23-14-10-8-13(9-11-14)17-6-3-7-18-19(17)20(22)25-28-18/h2-12H,1H3,(H2,22,25)(H2,23,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 51: 1231-41 (2008)
Article DOI: 10.1021/jm701096v
BindingDB Entry DOI: 10.7270/Q2HX19Z7 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50207474
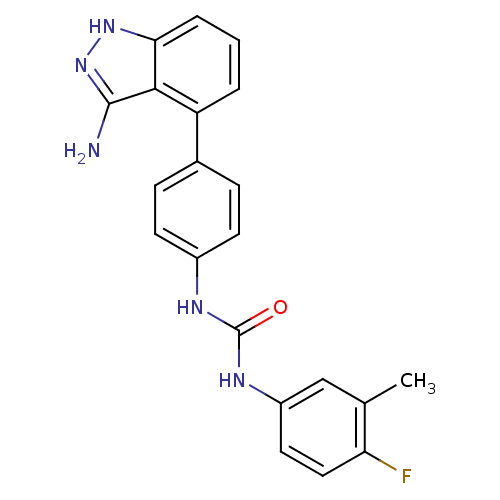
(1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-(4-fluoro-...)Show SMILES Cc1cc(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)ccc1F Show InChI InChI=1S/C21H18FN5O/c1-12-11-15(9-10-17(12)22)25-21(28)24-14-7-5-13(6-8-14)16-3-2-4-18-19(16)20(23)27-26-18/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KDR by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KDR by HTRF assay |
J Med Chem 50: 1584-97 (2007)
Article DOI: 10.1021/jm061280h
BindingDB Entry DOI: 10.7270/Q2DJ5F95 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50210271

(7-((1H-1,2,4-triazol-1-yl)methyl)-3-(5-(3-(2-metho...)Show SMILES COCCOCC#Cc1cc(cs1)-c1[nH]nc-2c1Cc1ccc(Cn3cncn3)cc-21 Show InChI InChI=1S/C23H21N5O2S/c1-29-7-8-30-6-2-3-19-10-18(13-31-19)22-21-11-17-5-4-16(12-28-15-24-14-25-28)9-20(17)23(21)27-26-22/h4-5,9-10,13-15H,6-8,11-12H2,1H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Flt4 |
J Med Chem 50: 2011-29 (2007)
Article DOI: 10.1021/jm061223o
BindingDB Entry DOI: 10.7270/Q2TB16K0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM21079

(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 51: 1231-41 (2008)
Article DOI: 10.1021/jm701096v
BindingDB Entry DOI: 10.7270/Q2HX19Z7 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM21093
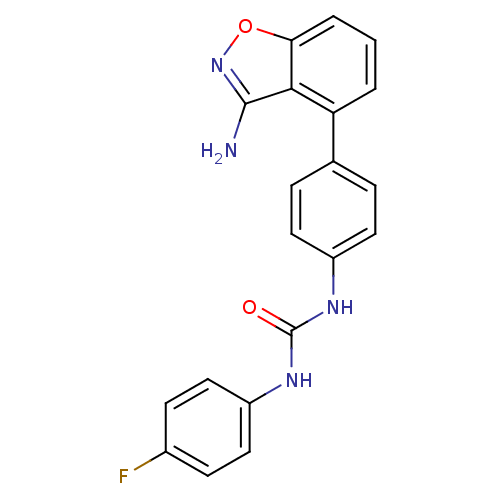
(3-[4-(3-amino-1,2-benzoxazol-4-yl)phenyl]-1-(4-flu...)Show SMILES Nc1noc2cccc(-c3ccc(NC(=O)Nc4ccc(F)cc4)cc3)c12 Show InChI InChI=1S/C20H15FN4O2/c21-13-6-10-15(11-7-13)24-20(26)23-14-8-4-12(5-9-14)16-2-1-3-17-18(16)19(22)25-27-17/h1-11H,(H2,22,25)(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
J Med Chem 51: 1231-41 (2008)
Article DOI: 10.1021/jm701096v
BindingDB Entry DOI: 10.7270/Q2HX19Z7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data