 Found 167 hits with Last Name = 'rodrigues' and Initial = 'cr'
Found 167 hits with Last Name = 'rodrigues' and Initial = 'cr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kallikrein-1
(Homo sapiens (Human)) | BDBM50205620
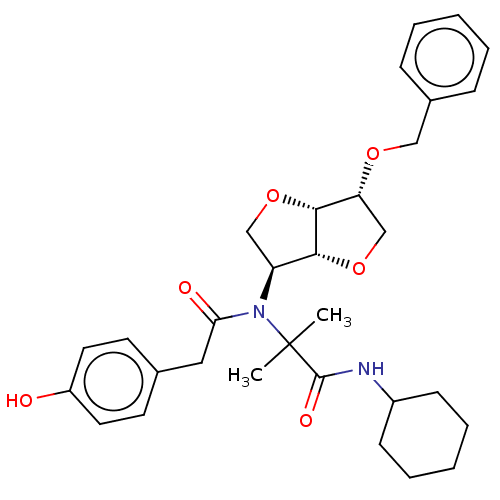
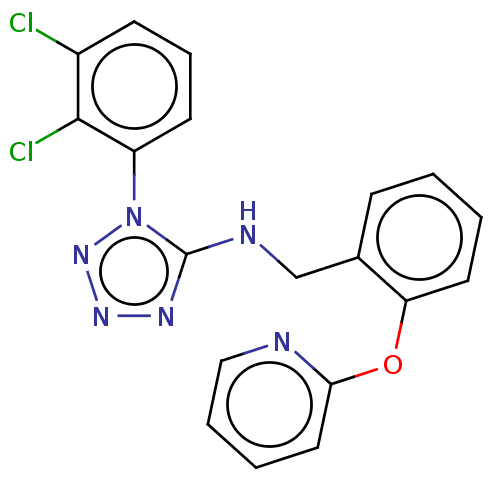
(CHEMBL3943212)Show SMILES [H][C@]12OC[C@H](N(C(=O)Cc3ccc(O)cc3)C(C)(C)C(=O)NC3CCCCC3)[C@@]1([H])OC[C@H]2OCc1ccccc1 |r| Show InChI InChI=1S/C31H40N2O6/c1-31(2,30(36)32-23-11-7-4-8-12-23)33(27(35)17-21-13-15-24(34)16-14-21)25-19-38-29-26(20-39-28(25)29)37-18-22-9-5-3-6-10-22/h3,5-6,9-10,13-16,23,25-26,28-29,34H,4,7-8,11-12,17-20H2,1-2H3,(H,32,36)/t25-,26+,28+,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal Fluminense
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human KLK1 expressed in baculovirus/insect cell expression system using Abz-KLRSSKQ-EDDnp peptide as substrate ... |
Bioorg Med Chem Lett 27: 314-318 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.051
BindingDB Entry DOI: 10.7270/Q2WW7KNV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032162
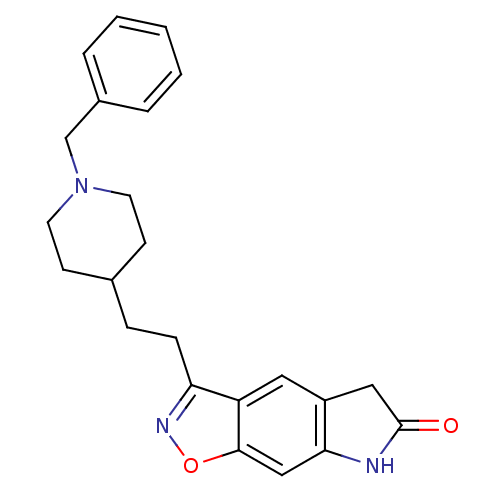
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...)Show SMILES O=C1Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-19-20(25-28-22(19)14-21(18)24-23)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032161
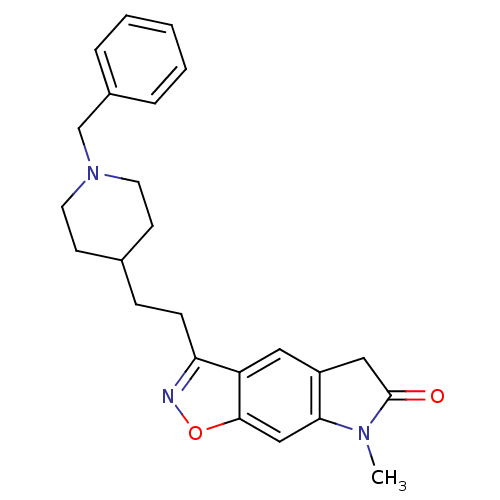
(3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-7-methyl-5,7...)Show SMILES CN1C(=O)Cc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc12 Show InChI InChI=1S/C24H27N3O2/c1-26-22-15-23-20(13-19(22)14-24(26)28)21(25-29-23)8-7-17-9-11-27(12-10-17)16-18-5-3-2-4-6-18/h2-6,13,15,17H,7-12,14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417809
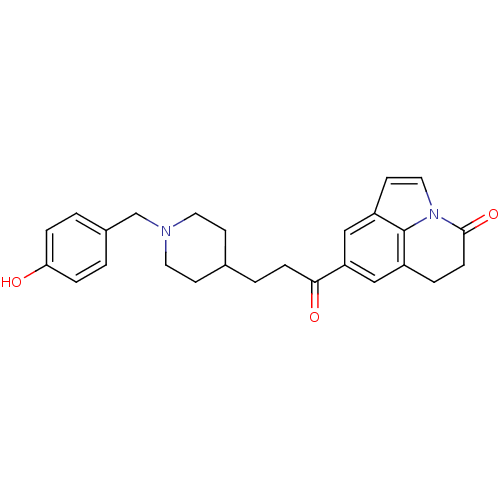
(CHEMBL1651249)Show SMILES Oc1ccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)cc1 Show InChI InChI=1S/C26H28N2O3/c29-23-5-1-19(2-6-23)17-27-12-9-18(10-13-27)3-7-24(30)22-15-20-4-8-25(31)28-14-11-21(16-22)26(20)28/h1-2,5-6,11,14-16,18,29H,3-4,7-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032164
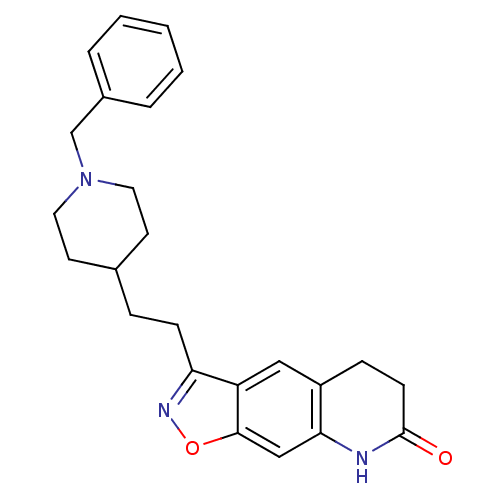
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...)Show SMILES O=C1CCc2cc3c(CCC4CCN(Cc5ccccc5)CC4)noc3cc2N1 Show InChI InChI=1S/C24H27N3O2/c28-24-9-7-19-14-20-21(26-29-23(20)15-22(19)25-24)8-6-17-10-12-27(13-11-17)16-18-4-2-1-3-5-18/h1-5,14-15,17H,6-13,16H2,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.575 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417798
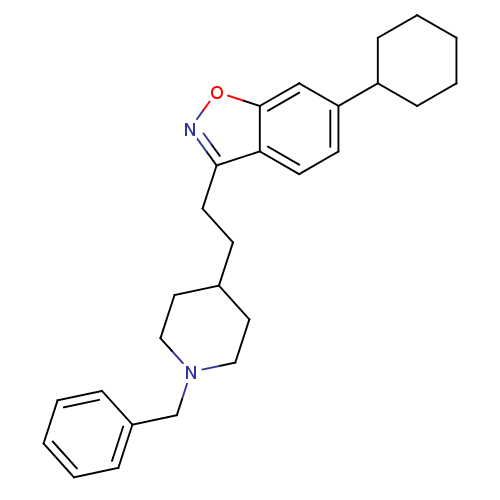
(CHEMBL1651247)Show SMILES C(Cc1noc2cc(ccc12)C1CCCCC1)C1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C27H34N2O/c1-3-7-22(8-4-1)20-29-17-15-21(16-18-29)11-14-26-25-13-12-24(19-27(25)30-28-26)23-9-5-2-6-10-23/h1,3-4,7-8,12-13,19,21,23H,2,5-6,9-11,14-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032163
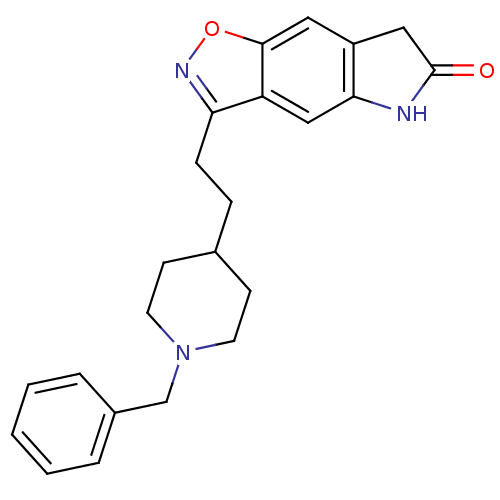
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...)Show SMILES O=C1Cc2cc3onc(CCC4CCN(Cc5ccccc5)CC4)c3cc2N1 Show InChI InChI=1S/C23H25N3O2/c27-23-13-18-12-22-19(14-21(18)24-23)20(25-28-22)7-6-16-8-10-26(11-9-16)15-17-4-2-1-3-5-17/h1-5,12,14,16H,6-11,13,15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417791
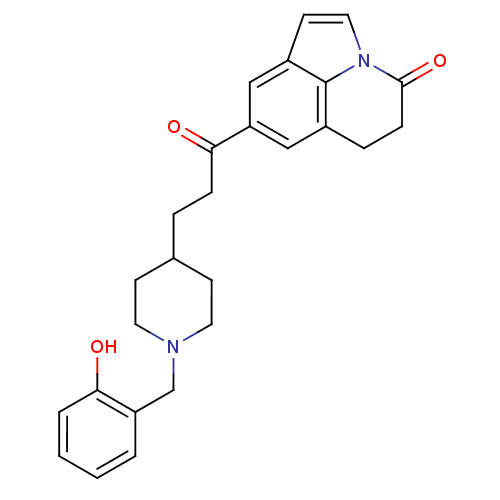
(CHEMBL1651139)Show SMILES Oc1ccccc1CN1CCC(CCC(=O)c2cc3CCC(=O)n4ccc(c2)c34)CC1 Show InChI InChI=1S/C26H28N2O3/c29-23-4-2-1-3-21(23)17-27-12-9-18(10-13-27)5-7-24(30)22-15-19-6-8-25(31)28-14-11-20(16-22)26(19)28/h1-4,11,14-16,18,29H,5-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417787
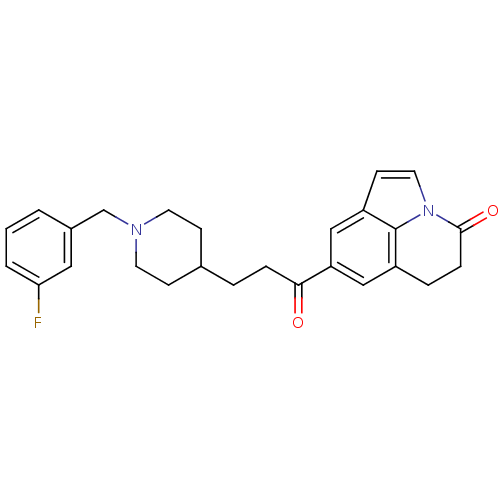
(CHEMBL1651132)Show SMILES Fc1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H27FN2O2/c27-23-3-1-2-19(14-23)17-28-11-8-18(9-12-28)4-6-24(30)22-15-20-5-7-25(31)29-13-10-21(16-22)26(20)29/h1-3,10,13-16,18H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417786
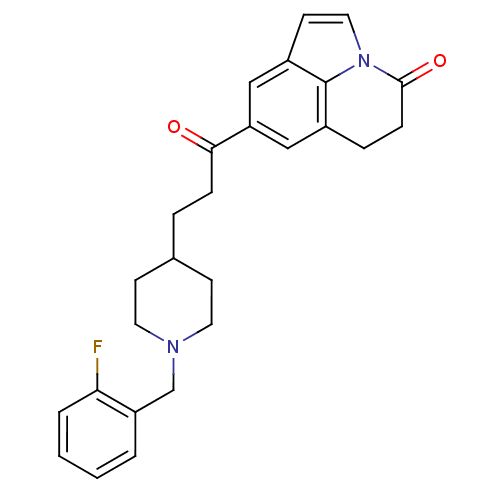
(CHEMBL1651131)Show SMILES Fc1ccccc1CN1CCC(CCC(=O)c2cc3CCC(=O)n4ccc(c2)c34)CC1 Show InChI InChI=1S/C26H27FN2O2/c27-23-4-2-1-3-21(23)17-28-12-9-18(10-13-28)5-7-24(30)22-15-19-6-8-25(31)29-14-11-20(16-22)26(19)29/h1-4,11,14-16,18H,5-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032165
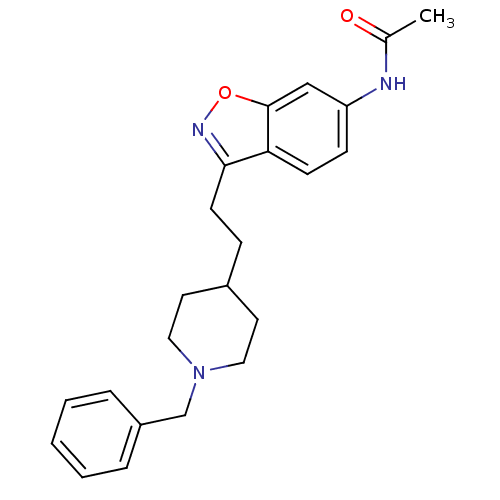
(CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...)Show SMILES CC(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C23H27N3O2/c1-17(27)24-20-8-9-21-22(25-28-23(21)15-20)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417793
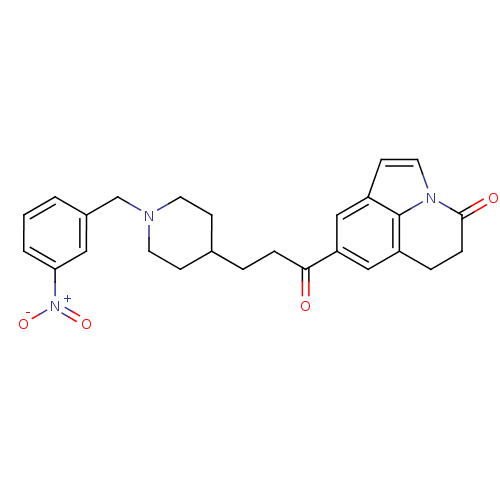
(CHEMBL1651243)Show SMILES [O-][N+](=O)c1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H27N3O4/c30-24(22-15-20-5-7-25(31)28-13-10-21(16-22)26(20)28)6-4-18-8-11-27(12-9-18)17-19-2-1-3-23(14-19)29(32)33/h1-3,10,13-16,18H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50032160
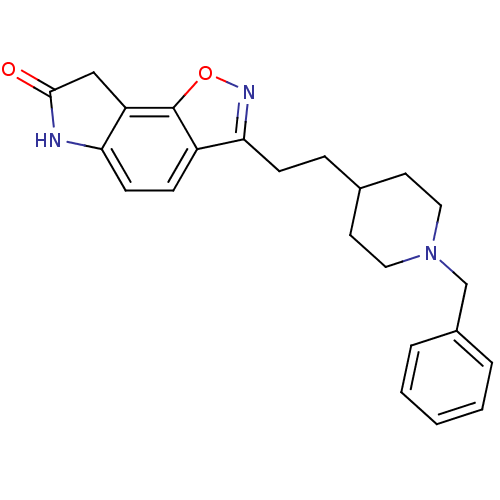
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...)Show SMILES O=C1Cc2c(N1)ccc1c(CCC3CCN(Cc4ccccc4)CC3)noc21 Show InChI InChI=1S/C23H25N3O2/c27-22-14-19-20(24-22)9-7-18-21(25-28-23(18)19)8-6-16-10-12-26(13-11-16)15-17-4-2-1-3-5-17/h1-5,7,9,16H,6,8,10-15H2,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417785
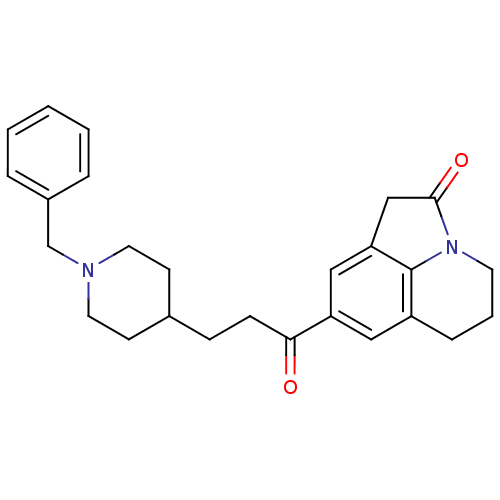
(CHEMBL1651129)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1cc2CC(=O)N3CCCc(c1)c23 Show InChI InChI=1S/C26H30N2O2/c29-24(22-15-21-7-4-12-28-25(30)17-23(16-22)26(21)28)9-8-19-10-13-27(14-11-19)18-20-5-2-1-3-6-20/h1-3,5-6,15-16,19H,4,7-14,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034001
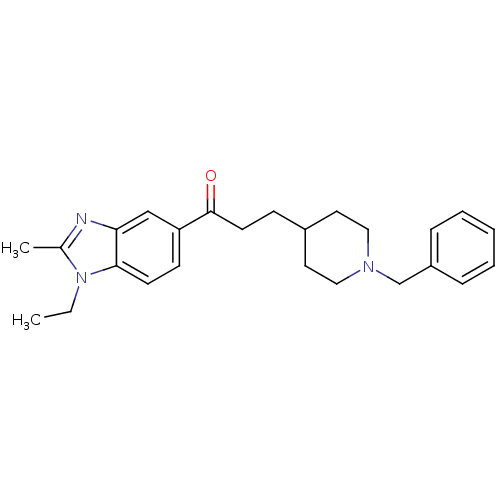
(3-(1-Benzyl-piperidin-4-yl)-1-(1-ethyl-2-methyl-1H...)Show SMILES CCn1c(C)nc2cc(ccc12)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C25H31N3O/c1-3-28-19(2)26-23-17-22(10-11-24(23)28)25(29)12-9-20-13-15-27(16-14-20)18-21-7-5-4-6-8-21/h4-8,10-11,17,20H,3,9,12-16,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417799
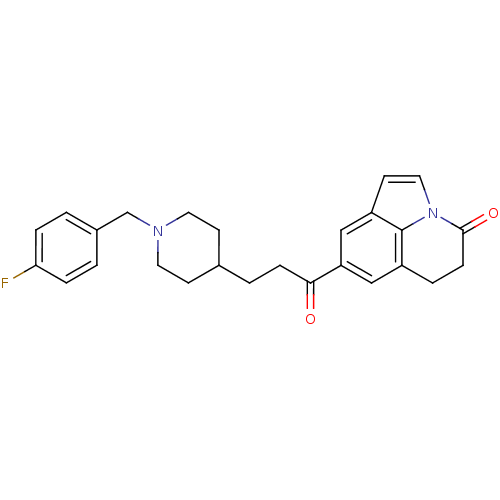
(CHEMBL1651248)Show SMILES Fc1ccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)cc1 Show InChI InChI=1S/C26H27FN2O2/c27-23-5-1-19(2-6-23)17-28-12-9-18(10-13-28)3-7-24(30)22-15-20-4-8-25(31)29-14-11-21(16-22)26(20)29/h1-2,5-6,11,14-16,18H,3-4,7-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417788
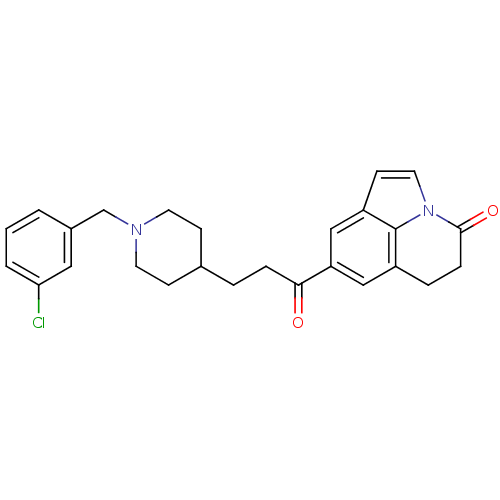
(CHEMBL1651134)Show SMILES Clc1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H27ClN2O2/c27-23-3-1-2-19(14-23)17-28-11-8-18(9-12-28)4-6-24(30)22-15-20-5-7-25(31)29-13-10-21(16-22)26(20)29/h1-3,10,13-16,18H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417803
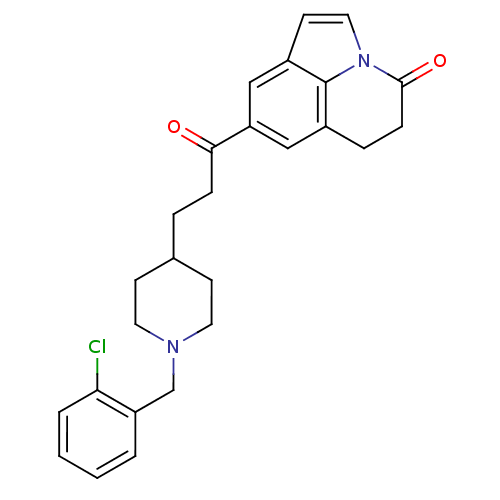
(CHEMBL1651133)Show SMILES Clc1ccccc1CN1CCC(CCC(=O)c2cc3CCC(=O)n4ccc(c2)c34)CC1 Show InChI InChI=1S/C26H27ClN2O2/c27-23-4-2-1-3-21(23)17-28-12-9-18(10-13-28)5-7-24(30)22-15-19-6-8-25(31)29-14-11-20(16-22)26(19)29/h1-4,11,14-16,18H,5-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50277559
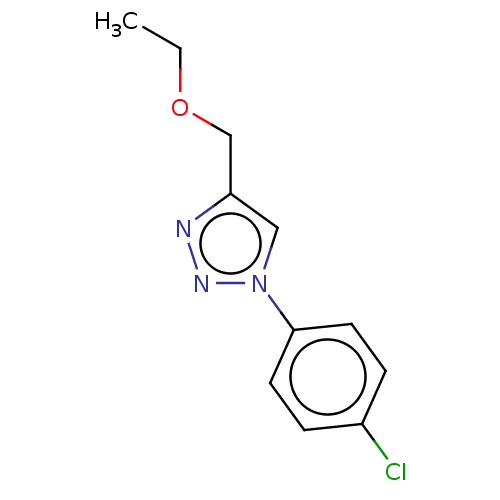
(CHEMBL4162056)Show InChI InChI=1S/C11H12ClN3O/c1-2-16-8-10-7-15(14-13-10)11-5-3-9(12)4-6-11/h3-7H,2,8H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of ATP-induced ethidium iodide uptake preincubated for 10... |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039729
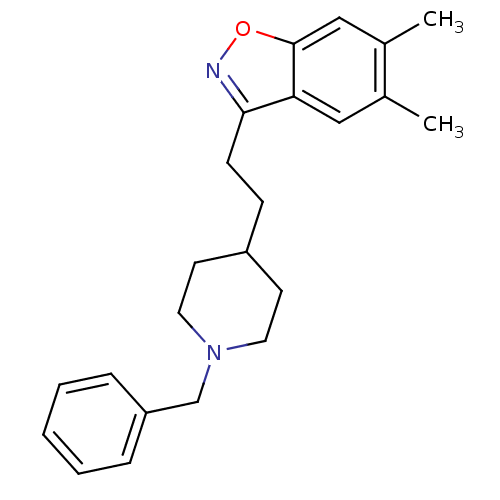
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dimethylbe...)Show InChI InChI=1S/C23H28N2O/c1-17-14-21-22(24-26-23(21)15-18(17)2)9-8-19-10-12-25(13-11-19)16-20-6-4-3-5-7-20/h3-7,14-15,19H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50277551
(A-839977 | CHEMBL1628691)Show InChI InChI=1S/C19H14Cl2N6O/c20-14-7-5-8-15(18(14)21)27-19(24-25-26-27)23-12-13-6-1-2-9-16(13)28-17-10-3-4-11-22-17/h1-11H,12H2,(H,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
Tested for inhibitory potency against rat liver microsomal squalene synthase |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034002

(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-benzothiaz...)Show InChI InChI=1S/C23H26N2OS/c1-17-24-21-9-8-20(15-23(21)27-17)22(26)10-7-18-11-13-25(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039713
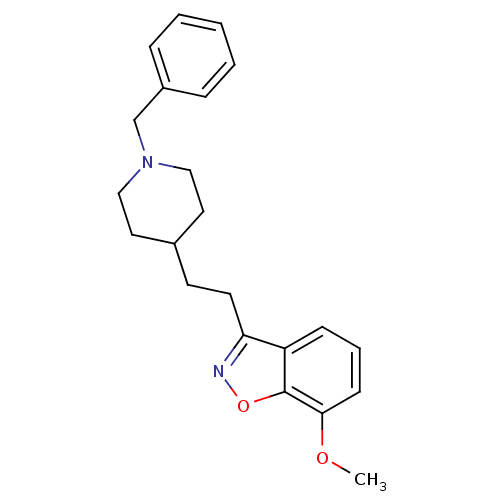
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-21-9-5-8-19-20(23-26-22(19)21)11-10-17-12-14-24(15-13-17)16-18-6-3-2-4-7-18/h2-9,17H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039712
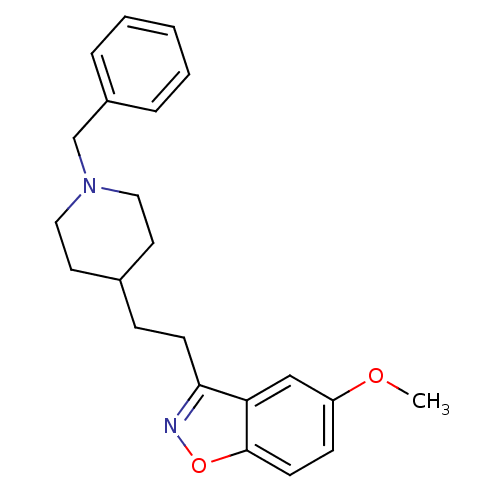
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-10-22-20(15-19)21(23-26-22)9-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8,10,15,17H,7,9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039728
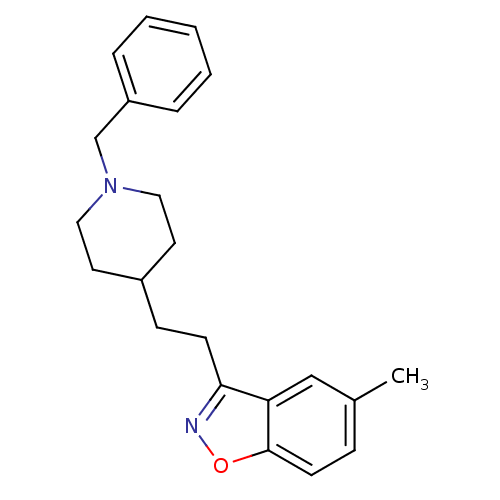
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methylbenzo[...)Show InChI InChI=1S/C22H26N2O/c1-17-7-10-22-20(15-17)21(23-25-22)9-8-18-11-13-24(14-12-18)16-19-5-3-2-4-6-19/h2-7,10,15,18H,8-9,11-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039717
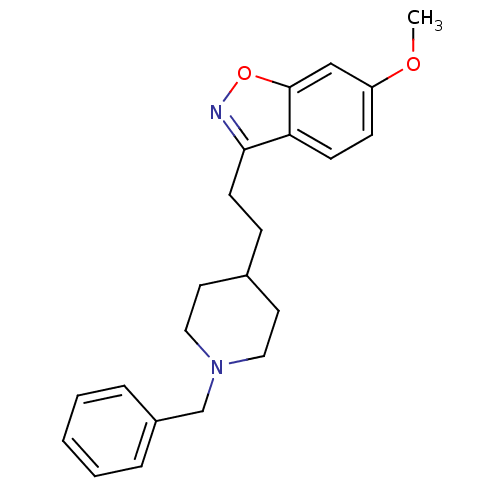
(3-(2-(1-benzylpiperidin-4-yl)ethyl)-6-methoxybenzo...)Show InChI InChI=1S/C22H26N2O2/c1-25-19-8-9-20-21(23-26-22(20)15-19)10-7-17-11-13-24(14-12-17)16-18-5-3-2-4-6-18/h2-6,8-9,15,17H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039723
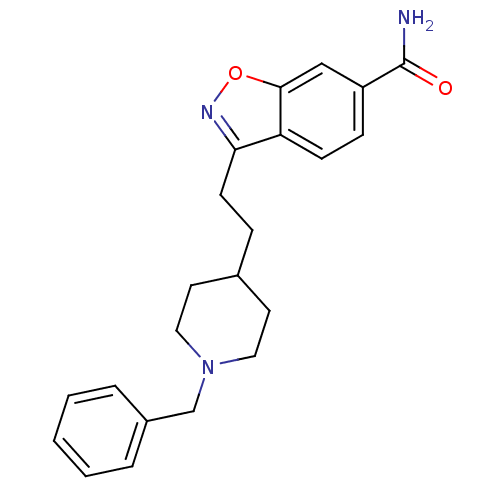
(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C22H25N3O2/c23-22(26)18-7-8-19-20(24-27-21(19)14-18)9-6-16-10-12-25(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417792

(CHEMBL1651140)Show SMILES Oc1cccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)c1 Show InChI InChI=1S/C26H28N2O3/c29-23-3-1-2-19(14-23)17-27-11-8-18(9-12-27)4-6-24(30)22-15-20-5-7-25(31)28-13-10-21(16-22)26(20)28/h1-3,10,13-16,18,29H,4-9,11-12,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039727
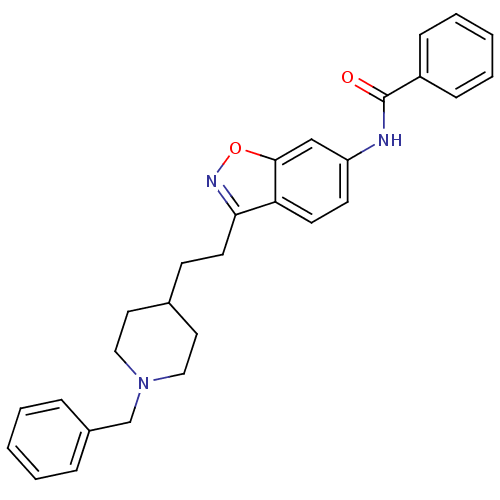
(CHEMBL93123 | N-(3-(2-(1-benzylpiperidin-4-yl)ethy...)Show SMILES O=C(Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1)c1ccccc1 Show InChI InChI=1S/C28H29N3O2/c32-28(23-9-5-2-6-10-23)29-24-12-13-25-26(30-33-27(25)19-24)14-11-21-15-17-31(18-16-21)20-22-7-3-1-4-8-22/h1-10,12-13,19,21H,11,14-18,20H2,(H,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417802

(CHEMBL1651130)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1cc2CCN3c2c(CCC3=O)c1 Show InChI InChI=1S/C26H30N2O2/c29-24(23-16-21-7-9-25(30)28-15-12-22(17-23)26(21)28)8-6-19-10-13-27(14-11-19)18-20-4-2-1-3-5-20/h1-5,16-17,19H,6-15,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034003
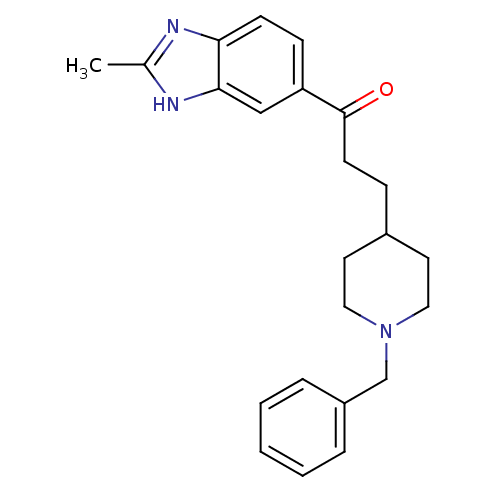
(3-(1-Benzyl-piperidin-4-yl)-1-(2-methyl-1H-benzoim...)Show SMILES Cc1nc2ccc(cc2[nH]1)C(=O)CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C23H27N3O/c1-17-24-21-9-8-20(15-22(21)25-17)23(27)10-7-18-11-13-26(14-12-18)16-19-5-3-2-4-6-19/h2-6,8-9,15,18H,7,10-14,16H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417808
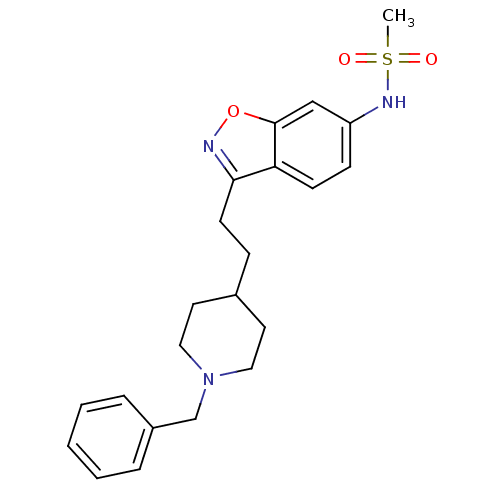
(CHEMBL1651246)Show SMILES CS(=O)(=O)Nc1ccc2c(CCC3CCN(Cc4ccccc4)CC3)noc2c1 Show InChI InChI=1S/C22H27N3O3S/c1-29(26,27)24-19-8-9-20-21(23-28-22(20)15-19)10-7-17-11-13-25(14-12-17)16-18-5-3-2-4-6-18/h2-6,8-9,15,17,24H,7,10-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417794

(CHEMBL1651244)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1cc2CCCN3C(=O)CCc(c1)c23 Show InChI InChI=1S/C27H32N2O2/c30-25(10-8-20-12-15-28(16-13-20)19-21-5-2-1-3-6-21)24-17-22-7-4-14-29-26(31)11-9-23(18-24)27(22)29/h1-3,5-6,17-18,20H,4,7-16,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039719

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H25N3O/c22-18-7-8-19-20(23-25-21(19)14-18)9-6-16-10-12-24(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16H,6,9-13,15,22H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Rattus norvegicus (Rat)) | BDBM50277551

(A-839977 | CHEMBL1628691)Show InChI InChI=1S/C19H14Cl2N6O/c20-14-7-5-8-15(18(14)21)27-19(24-25-26-27)23-12-13-6-1-2-9-16(13)28-17-10-3-4-11-22-17/h1-11H,12H2,(H,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
Tested for inhibitory potency against rat liver microsomal squalene synthase |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50277551

(A-839977 | CHEMBL1628691)Show InChI InChI=1S/C19H14Cl2N6O/c20-14-7-5-8-15(18(14)21)27-19(24-25-26-27)23-12-13-6-1-2-9-16(13)28-17-10-3-4-11-22-17/h1-11H,12H2,(H,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced platelet aggregation in human whole blood |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Mus musculus) | BDBM50277551

(A-839977 | CHEMBL1628691)Show InChI InChI=1S/C19H14Cl2N6O/c20-14-7-5-8-15(18(14)21)27-19(24-25-26-27)23-12-13-6-1-2-9-16(13)28-17-10-3-4-11-22-17/h1-11H,12H2,(H,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced platelet aggregation in human whole blood |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50464474
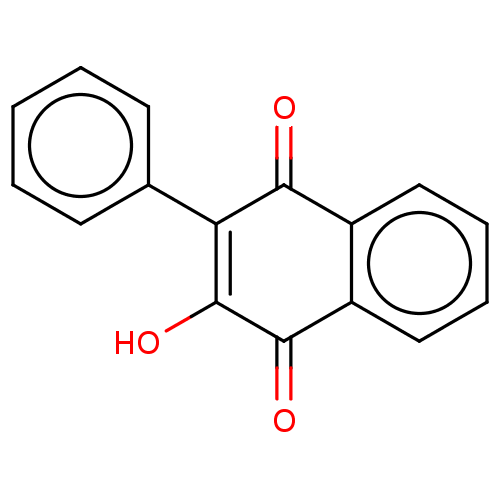
(CHEMBL4281588)Show InChI InChI=1S/C16H10O3/c17-14-11-8-4-5-9-12(11)15(18)16(19)13(14)10-6-2-1-3-7-10/h1-9,19H | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Oswaldo Cruz
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced ethidium iodide uptake preincubated for 10 mins fo... |
Eur J Med Chem 143: 1361-1372 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.033
BindingDB Entry DOI: 10.7270/Q2KS6V7K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417783

(CHEMBL1651126)Show InChI InChI=1S/C25H32N2O/c28-25(23-10-9-22-7-4-14-26-18-24(22)17-23)11-8-20-12-15-27(16-13-20)19-21-5-2-1-3-6-21/h1-3,5-6,9-10,17,20,26H,4,7-8,11-16,18-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Mus musculus) | BDBM50464474

(CHEMBL4281588)Show InChI InChI=1S/C16H10O3/c17-14-11-8-4-5-9-12(11)15(18)16(19)13(14)10-6-2-1-3-7-10/h1-9,19H | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Oswaldo Cruz
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R in Swiss Webster mouse peritoneal macrophages assessed as inhibition of ATP-induced propidium iodide uptake preincubated... |
Eur J Med Chem 143: 1361-1372 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.033
BindingDB Entry DOI: 10.7270/Q2KS6V7K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417801

(CHEMBL1651127)Show InChI InChI=1S/C25H32N2O/c28-25(24-8-7-22-10-14-26-15-11-23(22)18-24)9-6-20-12-16-27(17-13-20)19-21-4-2-1-3-5-21/h1-5,7-8,18,20,26H,6,9-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50039731

(3-(2-(1-benzylpiperidin-4-yl)ethyl)benzo[d]isoxazo...)Show InChI InChI=1S/C21H24N2O2/c24-18-7-8-19-20(22-25-21(19)14-18)9-6-16-10-12-23(13-11-16)15-17-4-2-1-3-5-17/h1-5,7-8,14,16,24H,6,9-13,15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50034000
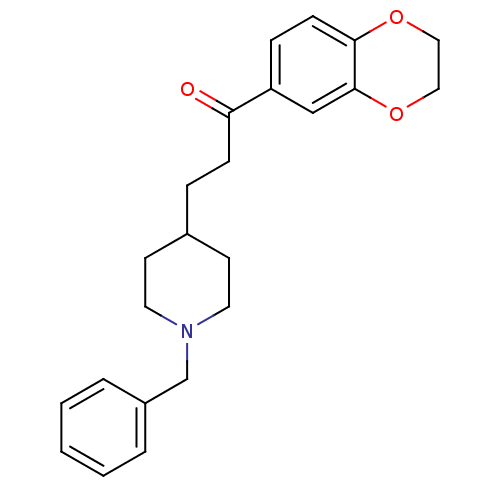
(3-(1-Benzyl-piperidin-4-yl)-1-(2,3-dihydro-benzo[1...)Show InChI InChI=1S/C23H27NO3/c25-21(20-7-9-22-23(16-20)27-15-14-26-22)8-6-18-10-12-24(13-11-18)17-19-4-2-1-3-5-19/h1-5,7,9,16,18H,6,8,10-15,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417796

(CHEMBL1651125)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1ccc2NC(Nc2c1)c1ccccc1 Show InChI InChI=1S/C28H31N3O/c32-27(14-11-21-15-17-31(18-16-21)20-22-7-3-1-4-8-22)24-12-13-25-26(19-24)30-28(29-25)23-9-5-2-6-10-23/h1-10,12-13,19,21,28-30H,11,14-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50277551

(A-839977 | CHEMBL1628691)Show InChI InChI=1S/C19H14Cl2N6O/c20-14-7-5-8-15(18(14)21)27-19(24-25-26-27)23-12-13-6-1-2-9-16(13)28-17-10-3-4-11-22-17/h1-11H,12H2,(H,23,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
In vitro thromboxane A2 receptor antagonism through inhibition of U-46619 induced contraction of rat isolated thoracic aortic strip |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417784
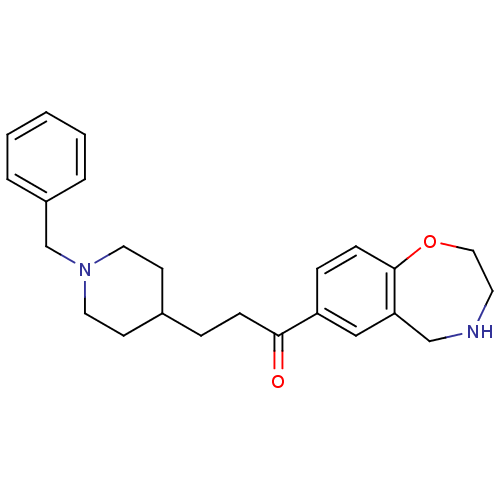
(CHEMBL1651128)Show InChI InChI=1S/C24H30N2O2/c27-23(21-7-9-24-22(16-21)17-25-12-15-28-24)8-6-19-10-13-26(14-11-19)18-20-4-2-1-3-5-20/h1-5,7,9,16,19,25H,6,8,10-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Mus musculus) | BDBM50277559

(CHEMBL4162056)Show InChI InChI=1S/C11H12ClN3O/c1-2-16-8-10-7-15(14-13-10)11-5-3-9(12)4-6-11/h3-7H,2,8H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in Swiss mouse peritoneal macrophages assessed as inhibition of BzATP-induced current at holding potential of -6... |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50417800
(CHEMBL1651250)Show SMILES [O-][N+](=O)c1ccc(CN2CCC(CCC(=O)c3cc4CCC(=O)n5ccc(c3)c45)CC2)cc1 Show InChI InChI=1S/C26H27N3O4/c30-24(22-15-20-4-8-25(31)28-14-11-21(16-22)26(20)28)7-3-18-9-12-27(13-10-18)17-19-1-5-23(6-2-19)29(32)33/h1-2,5-6,11,14-16,18H,3-4,7-10,12-13,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio de Janeiro (UFRJ)
Curated by ChEMBL
| Assay Description
Inhibition of human acetylcholinesterase |
Eur J Med Chem 46: 39-51 (2010)
Article DOI: 10.1016/j.ejmech.2010.10.009
BindingDB Entry DOI: 10.7270/Q2SX6FG0 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Mus musculus) | BDBM50234965
(A-740003 | CHEMBL255787 | N-(1-(2-cyano-3-(quinoli...)Show SMILES COc1ccc(CC(=O)NC(N=C(NC#N)Nc2cccc3ncccc23)C(C)(C)C)cc1OC |w:11.10| Show InChI InChI=1S/C26H30N6O3/c1-26(2,3)24(31-23(33)15-17-11-12-21(34-4)22(14-17)35-5)32-25(29-16-27)30-20-10-6-9-19-18(20)8-7-13-28-19/h6-14,24H,15H2,1-5H3,(H,31,33)(H2,29,30,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor in Swiss mouse peritoneal macrophages assessed as inhibition of BzATP-induced current at holding potential of -6... |
Eur J Med Chem 139: 698-717 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.034
BindingDB Entry DOI: 10.7270/Q2TX3HWC |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Mus musculus) | BDBM50464477
(CHEMBL4292211)Show InChI InChI=1S/C10H5IO3/c11-7-8(12)5-3-1-2-4-6(5)9(13)10(7)14/h1-4,14H | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Oswaldo Cruz
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R in Swiss Webster mouse peritoneal macrophages assessed as inhibition of ATP-induced propidium iodide uptake preincubated... |
Eur J Med Chem 143: 1361-1372 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.033
BindingDB Entry DOI: 10.7270/Q2KS6V7K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data