

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50530824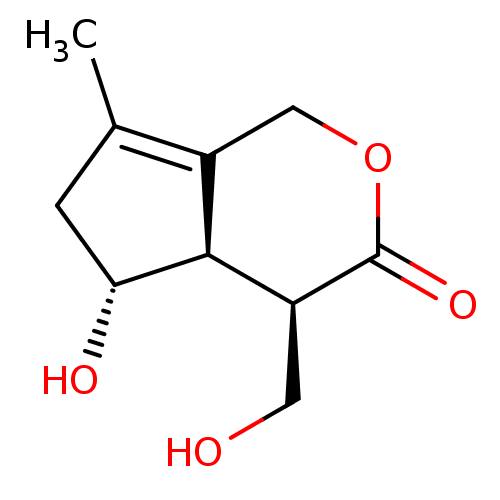 (CHEMBL4518867) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal GST-tagged recombinant human PTP1B catalytic domain (1 to 321 residues) expressed in Escherichia coli assessed a... | J Nat Prod 82: 2916-2924 (2019) Article DOI: 10.1021/acs.jnatprod.9b00770 BindingDB Entry DOI: 10.7270/Q2W66Q89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50530824 (CHEMBL4518867) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal GST-tagged recombinant human PTP1B catalytic domain (1 to 321 residues) expressed in Escherichia coli assessed a... | J Nat Prod 82: 2916-2924 (2019) Article DOI: 10.1021/acs.jnatprod.9b00770 BindingDB Entry DOI: 10.7270/Q2W66Q89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50530825 (CHEMBL4582106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal GST-tagged recombinant human PTP1B catalytic domain (1 to 321 residues) expressed in Escherichia coli assessed a... | J Nat Prod 82: 2916-2924 (2019) Article DOI: 10.1021/acs.jnatprod.9b00770 BindingDB Entry DOI: 10.7270/Q2W66Q89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50530825 (CHEMBL4582106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal GST-tagged recombinant human PTP1B catalytic domain (1 to 321 residues) expressed in Escherichia coli assessed a... | J Nat Prod 82: 2916-2924 (2019) Article DOI: 10.1021/acs.jnatprod.9b00770 BindingDB Entry DOI: 10.7270/Q2W66Q89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50020713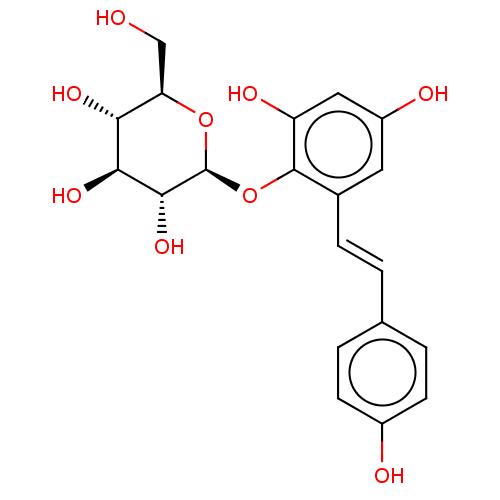 (CHEMBL460860) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 240 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50020713 (CHEMBL460860) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 120 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 120 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50020713 (CHEMBL460860) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 60 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50031467 (5-HYDROXY-2-(HYDROXYMETHYL)-4H-PYRAN-4-ONE | 5-Hyd...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse Melan-a cells assessed as decrease in L-DOPA Vmax at 120 uM by Lineweaver-Burk plot | J Nat Prod 77: 1270-4 (2014) Article DOI: 10.1021/np4008798 BindingDB Entry DOI: 10.7270/Q2MK6FFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D820Y single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623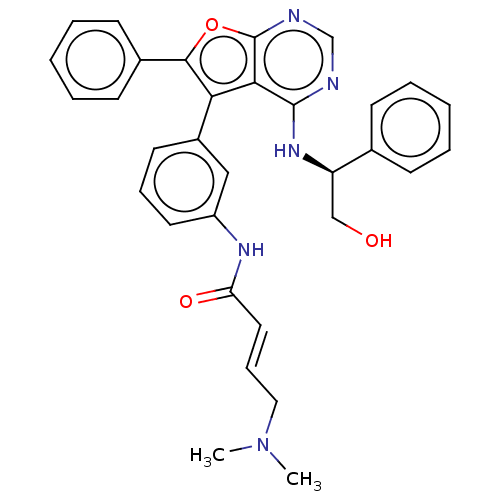 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50507492 (Loxo-195 | Selitrectinib | US10966985, Compound 33...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247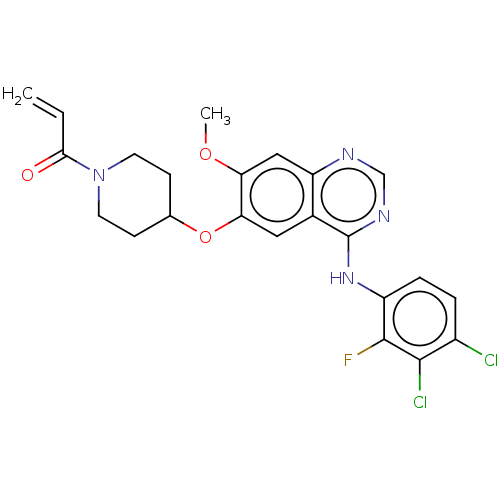 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 phosphorylation at 0.1 to 1000 nM after 1 hr by... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 D835Y mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 D835Y phosphorylation at 0.1 to 10... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50519725 (CHEMBL4513768) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type FLT3 ITD mutant (unknown origin) expressed in HEK293T cells assessed as decrease in FLT3 ITD phosphorylation at 0.1 to 1000 n... | J Med Chem 62: 11135-11150 (2019) Article DOI: 10.1021/acs.jmedchem.9b01229 BindingDB Entry DOI: 10.7270/Q2BV7M1W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM374727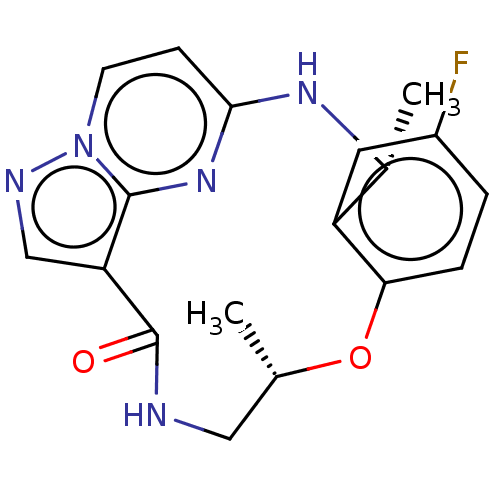 ((7S,13R)-11-fluoro-7,13-dimethyl-6,7,13,14- tetrah...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wildtype human TRKA using poly (Glu,Tyr) 4:1 as substrate in presence of [gamma-33P]ATP by hotspot kinase assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 Y823D single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530623 (CHEMBL4521381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618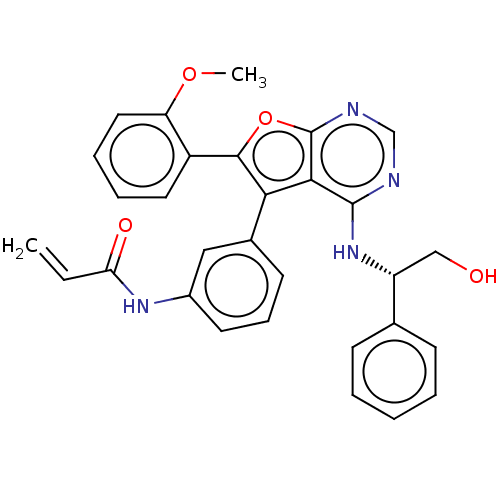 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 11/17 V560G/N822K double mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot k... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50579500 (CHEMBL4864729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TRKC (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113673 BindingDB Entry DOI: 10.7270/Q2Z323JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50468247 (HM 781-36B | HM-781-36B | NOV-120101 | Poziotinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.218 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50241089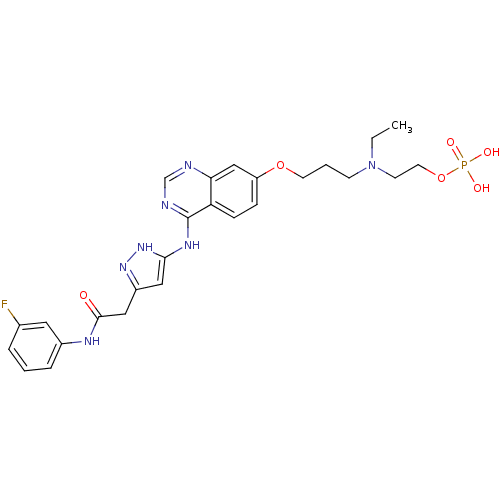 (2-(ethyl(3-(4-(5-(2-(3-fluorophenylamino)-2-oxoeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant GST-tagged N-terminal truncated human Aurora A (123 to 401 residues) expressed in Sf9 insect cell using tetra-LRRASLG pepti... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01806 BindingDB Entry DOI: 10.7270/Q2Q2444S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50579499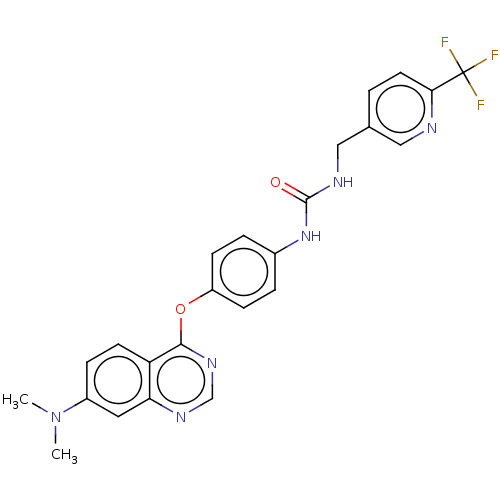 (CHEMBL4847875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild-type human partial length CSF1R (I564 to S939 residues) expressed in bacterial expression system by Kinomescan method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01006 BindingDB Entry DOI: 10.7270/Q2W3815D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530618 (CHEMBL4559807) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.636 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D816H single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 17 D820E single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT A loop exon 18 A829P single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ass... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT JM domain exon 11 V560G single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspot kinase ... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50389234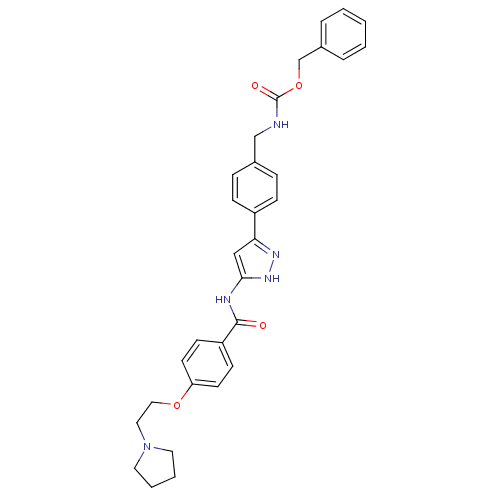 (CHEMBL2063324) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of FLT3 autophosphorylation in human MV4-11 cells after 2 hrs by Western blot analysis | Bioorg Med Chem Lett 22: 4654-9 (2012) Article DOI: 10.1016/j.bmcl.2012.05.116 BindingDB Entry DOI: 10.7270/Q2XS5WGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50579499 (CHEMBL4847875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human c-Kit by Kinomescan method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01006 BindingDB Entry DOI: 10.7270/Q2W3815D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530615 (CHEMBL4534719) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530615 (CHEMBL4534719) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50521626 (CHEMBL4448433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of human c-KIT ATP binding domain exon 13 K642E single mutant using poly (Glu,Tyr) 4:1 as substrate in presence of 33P-gamma-ATP by hotspo... | J Med Chem 62: 3940-3957 (2019) Article DOI: 10.1021/acs.jmedchem.8b01845 BindingDB Entry DOI: 10.7270/Q2TB1B92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50579496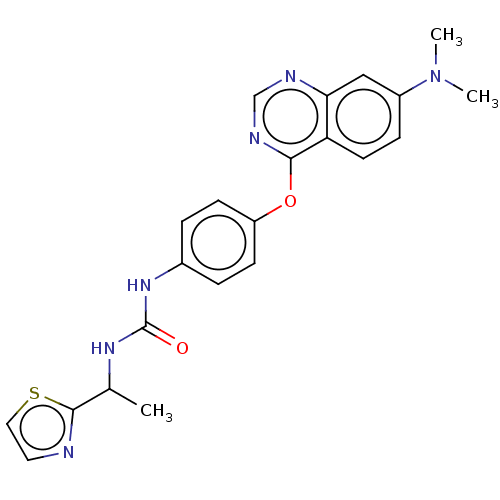 (CHEMBL4851545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant GST-CSF1R (residues L534 to C972) expressed in Sf9 insect cells using poly(Glu,Tyr) as substrate incubated for 20 min... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01006 BindingDB Entry DOI: 10.7270/Q2W3815D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50530622 (CHEMBL4514452) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of GST-tagged human EGFR D770_N771insNPG mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent k... | J Med Chem 62: 10108-10123 (2019) Article DOI: 10.1021/acs.jmedchem.9b00722 BindingDB Entry DOI: 10.7270/Q21V5JFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1835 total ) | Next | Last >> |