

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575344 (CHEMBL4854822) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575348 (CHEMBL4853155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575350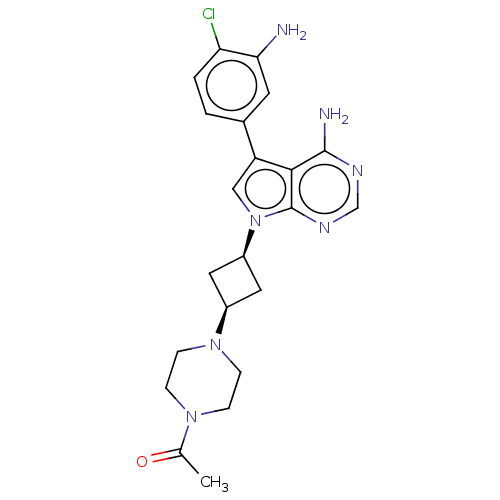 (CHEMBL4877264) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538514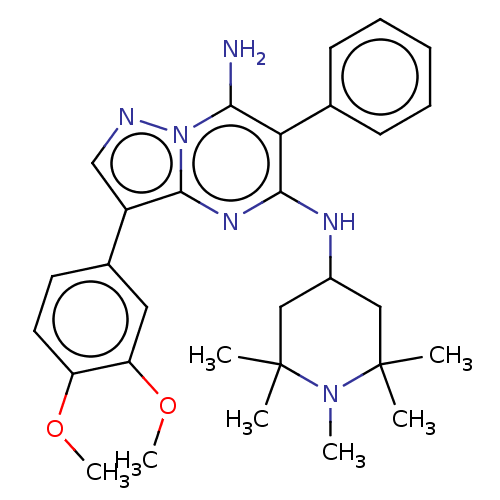 (CHEMBL4644274) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538516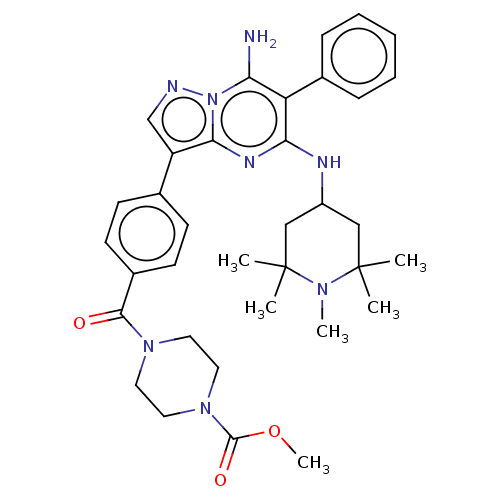 (CHEMBL4643578) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50328778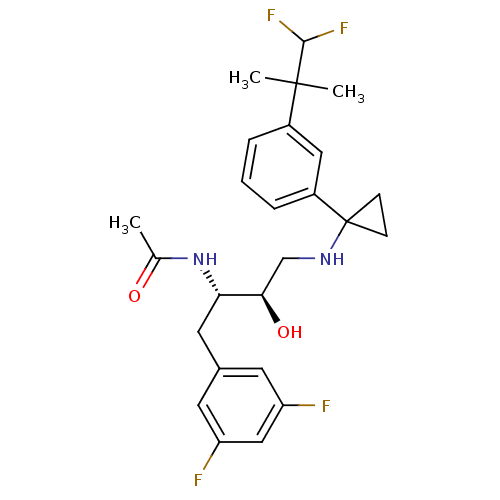 (CHEMBL1270362 | N-((2S,3R)-4-(1-(3-(1,1-difluoro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in presence of NADPH | Bioorg Med Chem Lett 20: 6231-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.102 BindingDB Entry DOI: 10.7270/Q2NZ87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50097956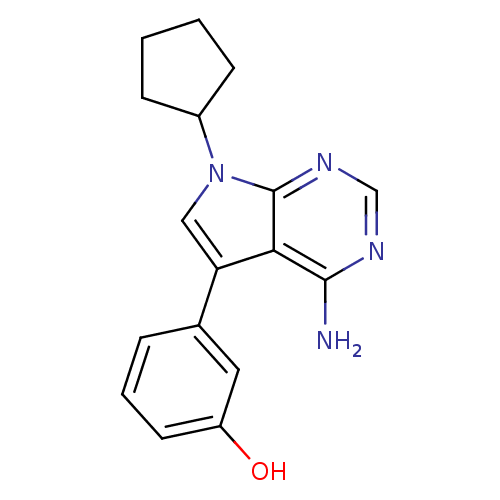 (3-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hrs by cell proliferati... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575345 (CHEMBL4848347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coiled-coil domain-containing protein 6 (Homo sapiens (Human)) | BDBM50538514 (CHEMBL4644274) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CCDC6/RET (unknown origin) transfected in human LC2/ad cells assessed as reduction in cell viability incubated for 6 days by CellTiter ... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538515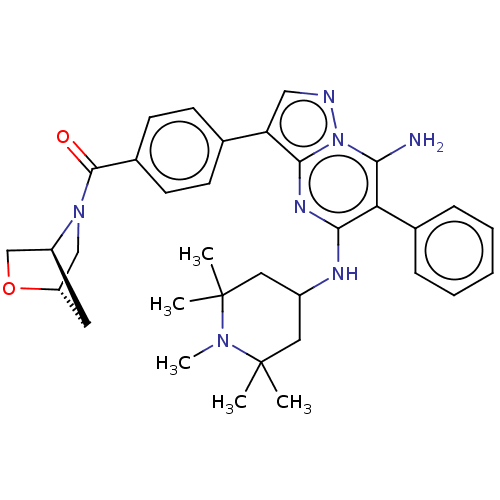 (CHEMBL4636056) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-lucifer... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545768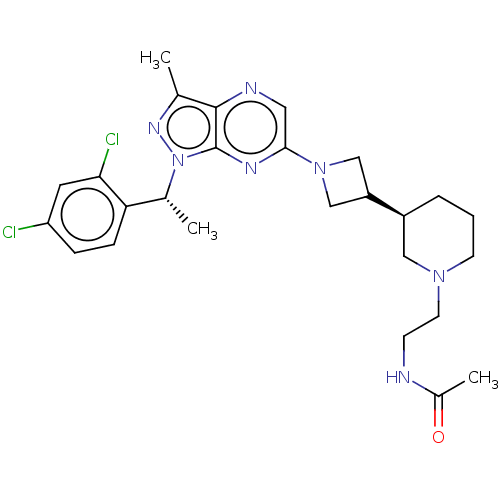 (CHEMBL4641127) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coiled-coil domain-containing protein 6 (Homo sapiens (Human)) | BDBM50538517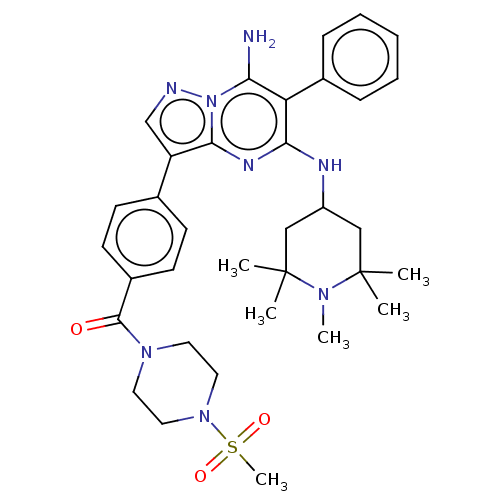 (CHEMBL4639022) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CCDC6/RET (unknown origin) transfected in human LC2/ad cells assessed as reduction in cell viability incubated for 6 days by CellTiter ... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538517 (CHEMBL4639022) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431915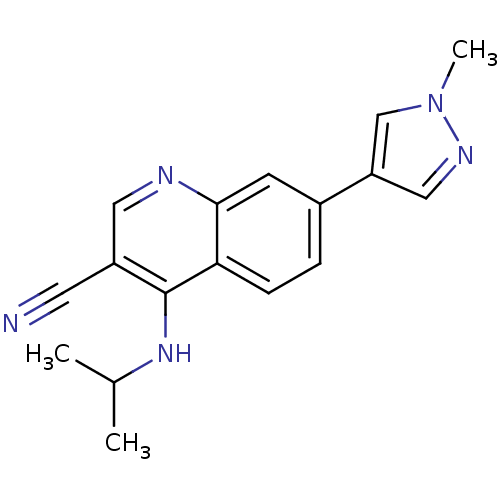 (CHEMBL2347824) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50097956 (3-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidi...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KIF5B-RET fusion protein (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 2 (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of FGFR2 (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-lucif... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545779 (CHEMBL4642563) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50538509 (CHEMBL4636995) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431914 (CHEMBL2347825) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575347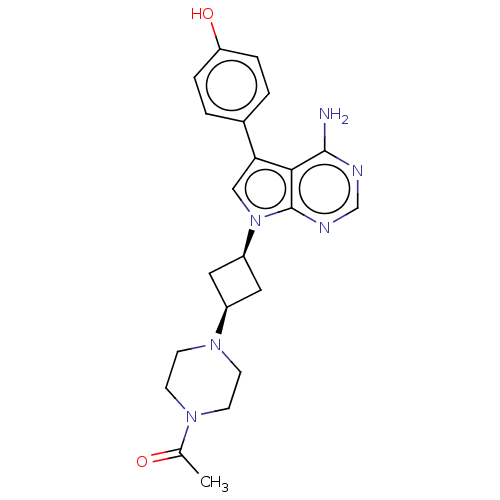 (CHEMBL4855562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545782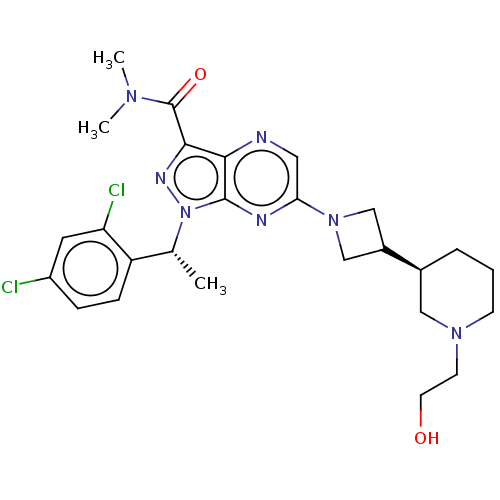 (CHEMBL4637695) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of FGFR1 (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-lucif... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431906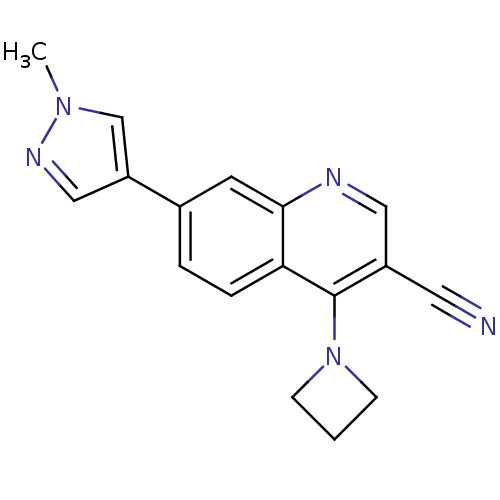 (CHEMBL2347809) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575343 (CHEMBL4858720) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KIF5B-RET fusion protein (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431911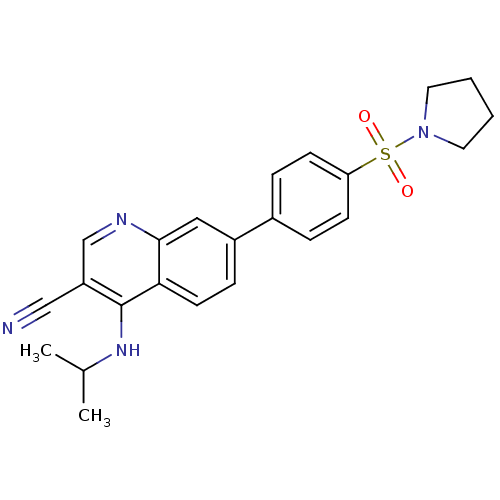 (CHEMBL2347804) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431913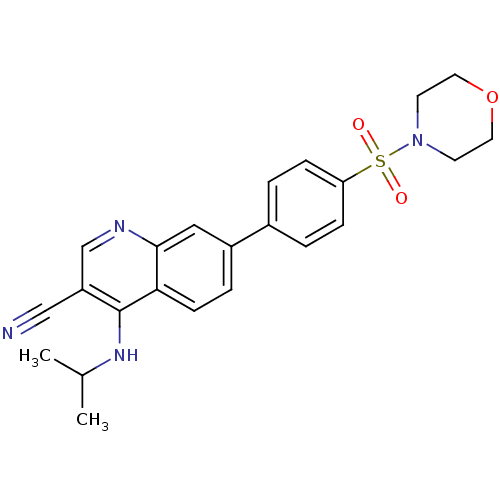 (CHEMBL2347826) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575351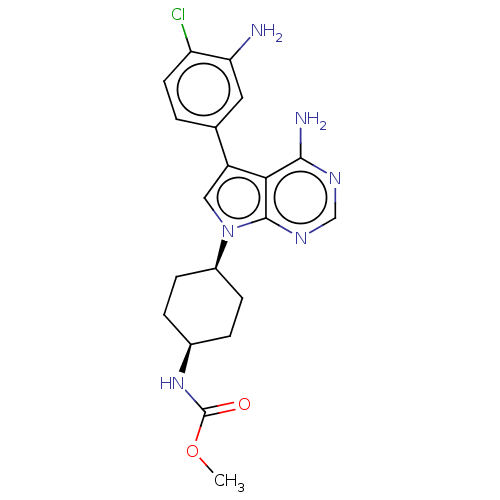 (CHEMBL4874648) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KIF5B-RET fusion protein (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coiled-coil domain-containing protein 6 (Homo sapiens (Human)) | BDBM50538516 (CHEMBL4643578) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CCDC6/RET (unknown origin) transfected in human LC2/ad cells assessed as reduction in cell viability incubated for 6 days by CellTiter ... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431905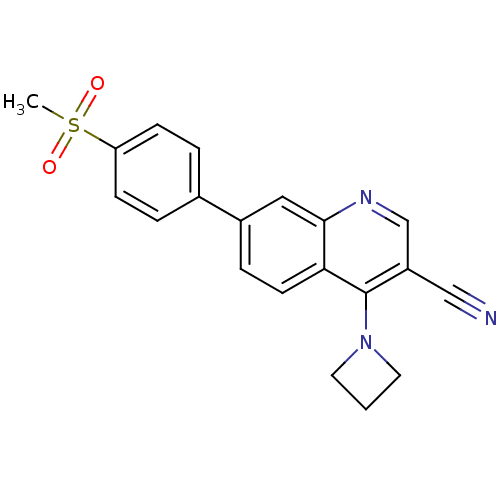 (CHEMBL2347810) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coiled-coil domain-containing protein 6 (Homo sapiens (Human)) | BDBM50538515 (CHEMBL4636056) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CCDC6/RET (unknown origin) transfected in human LC2/ad cells assessed as reduction in cell viability incubated for 6 days by CellTiter ... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545769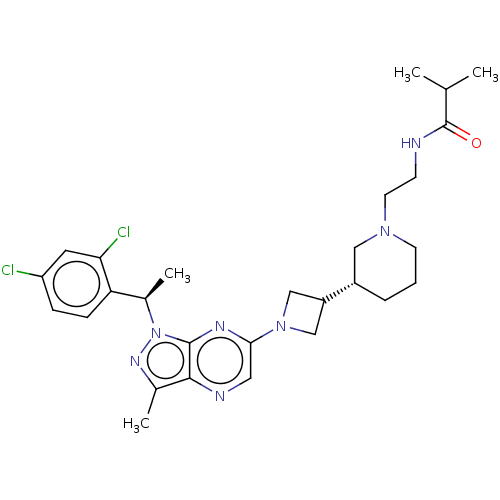 (CHEMBL4645325) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET V804M mutant (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs b... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575352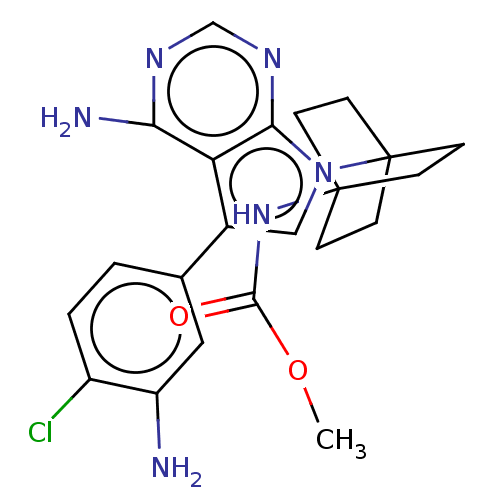 (CHEMBL4857808) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KIF5B-RET fusion protein (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIT (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-lucifer... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431904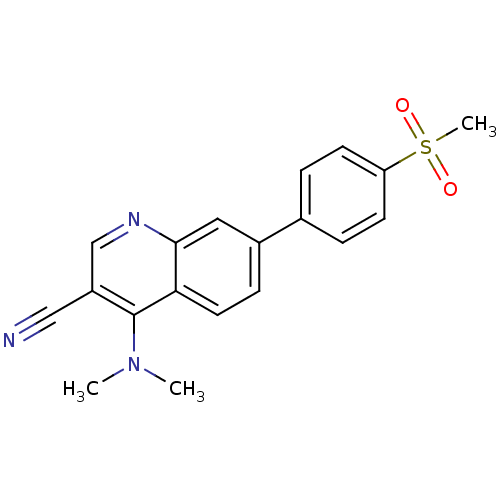 (CHEMBL2347811) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545771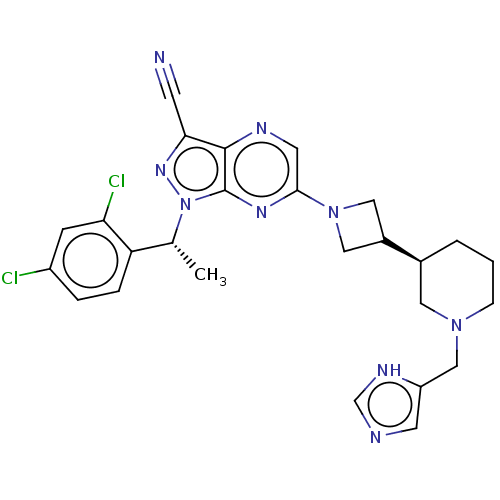 (CHEMBL4639600) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50538508 (CHEMBL4634073) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545762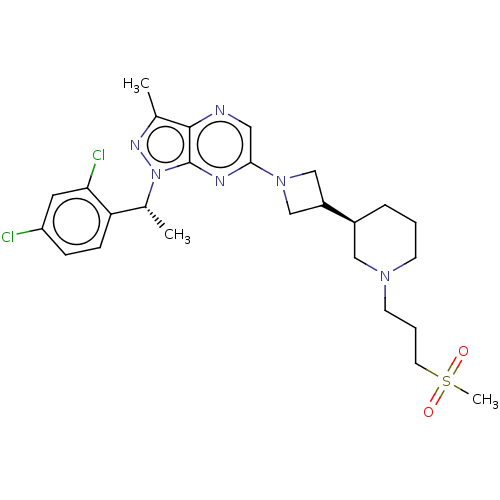 (CHEMBL4647117) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50302838 (CHEMBL572081 | N-((2S,3R)-4-(1-(3-(1H-pyrazol-1-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D | Bioorg Med Chem Lett 20: 6231-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.102 BindingDB Entry DOI: 10.7270/Q2NZ87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575353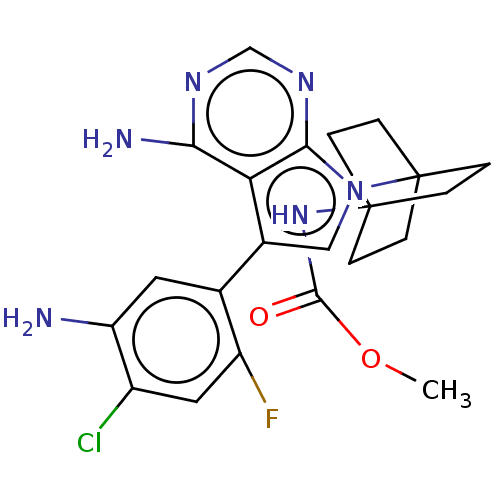 (CHEMBL4859681) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KIF5B-RET fusion protein (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hr... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545778 (CHEMBL4637143) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545770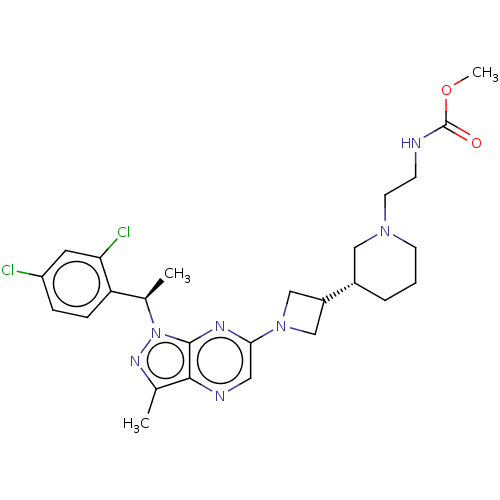 (CHEMBL4641595) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM374633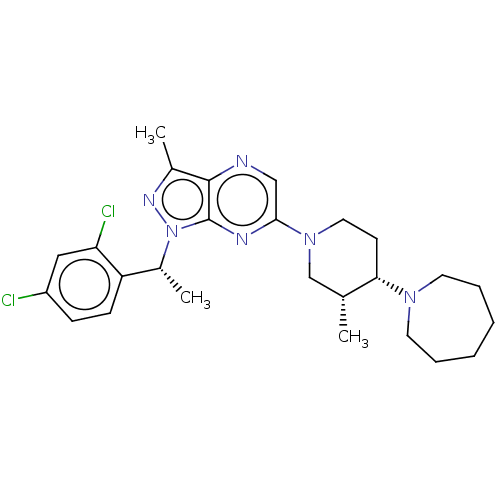 (6-((3R,4S)-4-(Azepan-1-yl)-3-methylpiperidin-1-yl)...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 62: 6190-6213 (2019) Article DOI: 10.1021/acs.jmedchem.9b00506 BindingDB Entry DOI: 10.7270/Q2571GCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50431912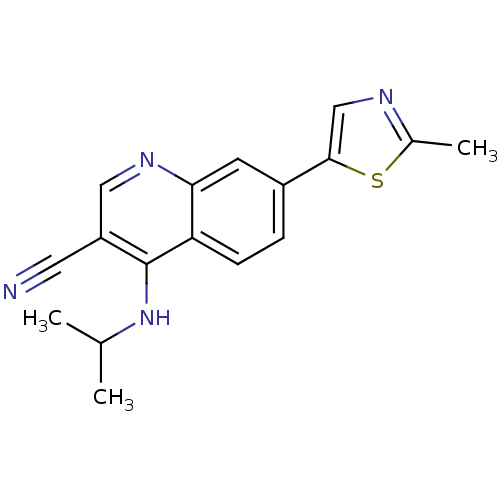 (CHEMBL2347827) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged LRRK2 (970-2527 amino acids)(unknown origin) assessed as inhibition of LRRKtide phosphorylation by HTRF assay | Bioorg Med Chem Lett 23: 1974-7 (2013) Article DOI: 10.1016/j.bmcl.2013.02.041 BindingDB Entry DOI: 10.7270/Q2FQ9XZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545777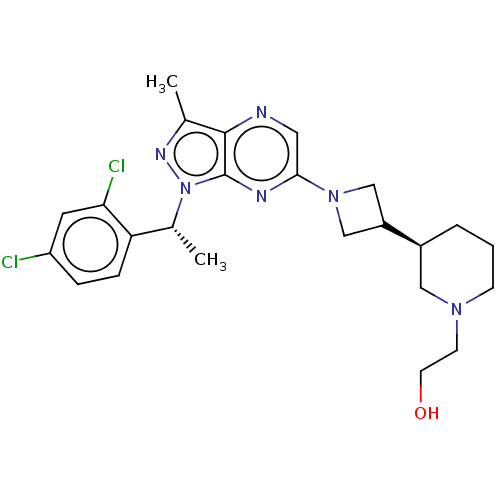 (CHEMBL4634054) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50302841 (CHEMBL571860 | N-((2S,3R)-4-(1-(3-tert-butylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin-D | Bioorg Med Chem Lett 20: 6231-6 (2010) Article DOI: 10.1016/j.bmcl.2010.08.102 BindingDB Entry DOI: 10.7270/Q2NZ87V9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50545781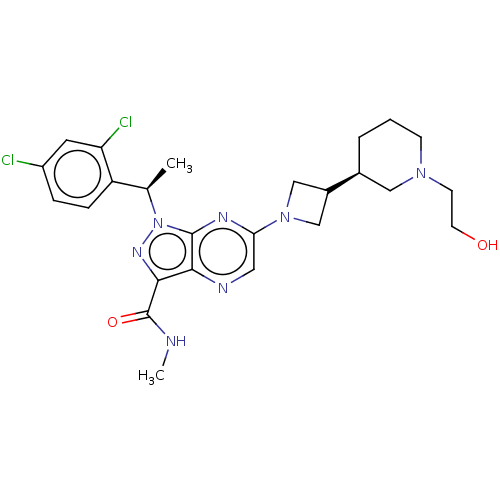 (CHEMBL4633133) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 expressed in rat chem-5 cells assessed as inhibition of CCL22-induced calcium flux measured at 2.5 secs time interv... | J Med Chem 63: 8584-8607 (2020) Article DOI: 10.1021/acs.jmedchem.0c00988 BindingDB Entry DOI: 10.7270/Q2DZ0CW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 566 total ) | Next | Last >> |