 Found 1038 hits with Last Name = 'dubé' and Initial = 'd'
Found 1038 hits with Last Name = 'dubé' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50174201
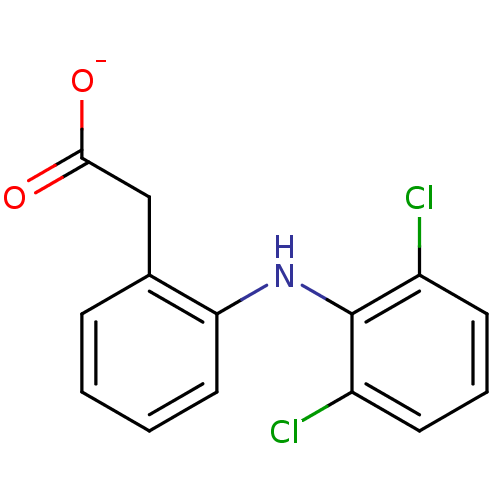
(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340424
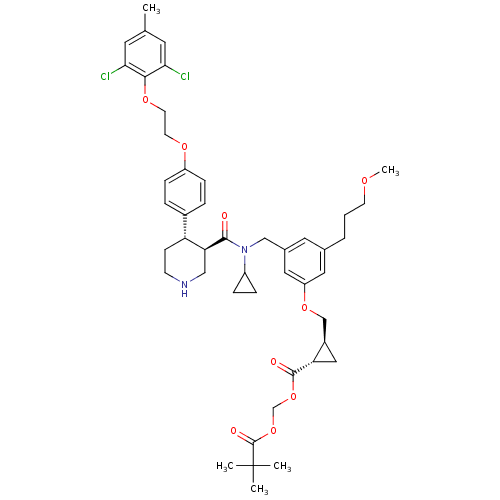
((1S,2S)-pivaloyloxymethyl 2-((3-(((3R,4S)-N-cyclop...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)OCOC(=O)C(C)(C)C)c1 |r| Show InChI InChI=1S/C46H58Cl2N2O9/c1-29-19-40(47)42(41(48)20-29)56-18-17-55-35-12-8-32(9-13-35)37-14-15-49-25-39(37)43(51)50(34-10-11-34)26-31-21-30(7-6-16-54-5)22-36(23-31)57-27-33-24-38(33)44(52)58-28-59-45(53)46(2,3)4/h8-9,12-13,19-23,33-34,37-39,49H,6-7,10-11,14-18,24-28H2,1-5H3/t33-,37-,38+,39+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50174201

(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340406
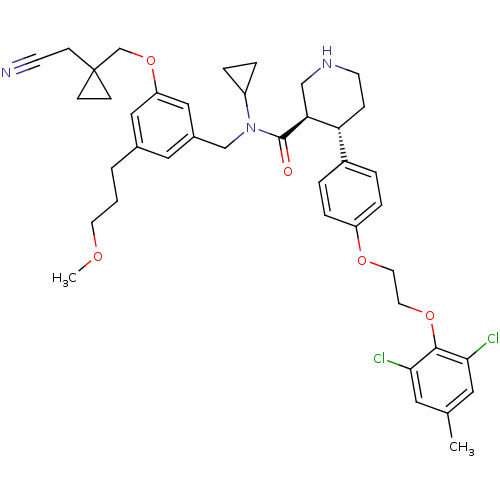
((3R,4S)-N-(3-((1-(cyanomethyl)cyclopropyl)methoxy)...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(CC#N)CC2)c1 |r| Show InChI InChI=1S/C41H49Cl2N3O5/c1-28-20-37(42)39(38(43)21-28)50-19-18-49-33-9-5-31(6-10-33)35-11-16-45-25-36(35)40(47)46(32-7-8-32)26-30-22-29(4-3-17-48-2)23-34(24-30)51-27-41(12-13-41)14-15-44/h5-6,9-10,20-24,32,35-36,45H,3-4,7-8,11-14,16-19,25-27H2,1-2H3/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50072064

(5-Chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[...)Show SMILES Cc1ccc(cn1)-c1ncc(Cl)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C18H15ClN2O2S/c1-12-3-4-14(10-20-12)18-17(9-15(19)11-21-18)13-5-7-16(8-6-13)24(2,22)23/h3-11H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340407
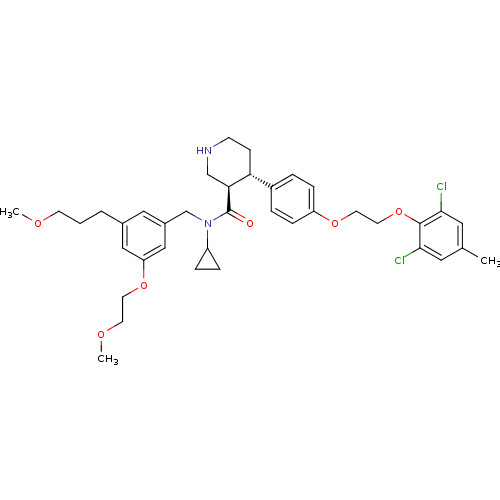
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCOC)c1 |r| Show InChI InChI=1S/C38H48Cl2N2O6/c1-26-19-35(39)37(36(40)20-26)48-18-17-46-31-10-6-29(7-11-31)33-12-13-41-24-34(33)38(43)42(30-8-9-30)25-28-21-27(5-4-14-44-2)22-32(23-28)47-16-15-45-3/h6-7,10-11,19-23,30,33-34,41H,4-5,8-9,12-18,24-25H2,1-3H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340409
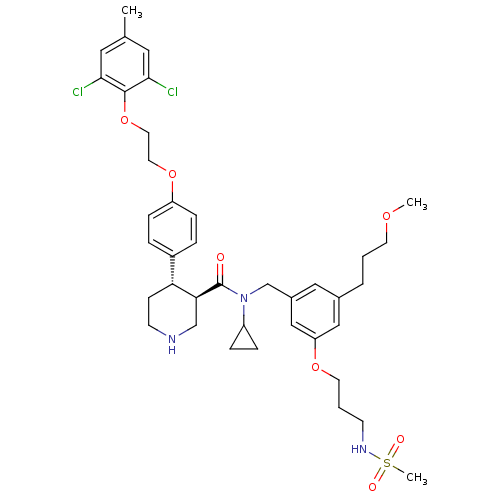
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCNS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O7S/c1-27-20-36(40)38(37(41)21-27)51-19-18-50-32-11-7-30(8-12-32)34-13-15-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-4-16-48-2)23-33(24-29)49-17-5-14-43-52(3,46)47/h7-8,11-12,20-24,31,34-35,42-43H,4-6,9-10,13-19,25-26H2,1-3H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340412
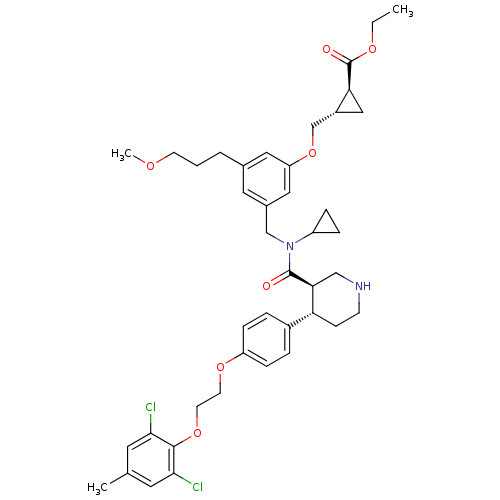
((1S,2S)-ethyl 2-((3-(((3R,4S)-N-cyclopropyl-4-(4-(...)Show SMILES CCOC(=O)[C@H]1C[C@@H]1COc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C42H52Cl2N2O7/c1-4-50-42(48)36-23-31(36)26-53-34-21-28(6-5-15-49-3)20-29(22-34)25-46(32-9-10-32)41(47)37-24-45-14-13-35(37)30-7-11-33(12-8-30)51-16-17-52-40-38(43)18-27(2)19-39(40)44/h7-8,11-12,18-22,31-32,35-37,45H,4-6,9-10,13-17,23-26H2,1-3H3/t31-,35-,36+,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50056999

(CHEMBL56367 | nimesulide)Show InChI InChI=1S/C13H12N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340411
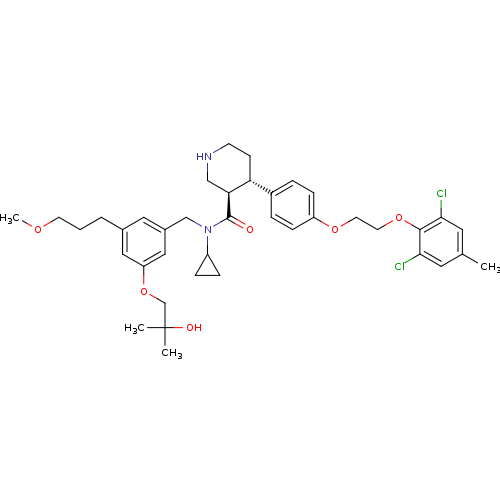
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC(C)(C)O)c1 |r| Show InChI InChI=1S/C39H50Cl2N2O6/c1-26-18-35(40)37(36(41)19-26)48-17-16-47-31-11-7-29(8-12-31)33-13-14-42-23-34(33)38(44)43(30-9-10-30)24-28-20-27(6-5-15-46-4)21-32(22-28)49-25-39(2,3)45/h7-8,11-12,18-22,30,33-34,42,45H,5-6,9-10,13-17,23-25H2,1-4H3/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50056998

(4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...)Show SMILES CN1C(C(=O)Nc2ncc(C)s2)=C(O)c2ccccc2S1(=O)=O |t:12| Show InChI InChI=1S/C14H13N3O4S2/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2/h3-7,18H,1-2H3,(H,15,16,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50174201

(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340410
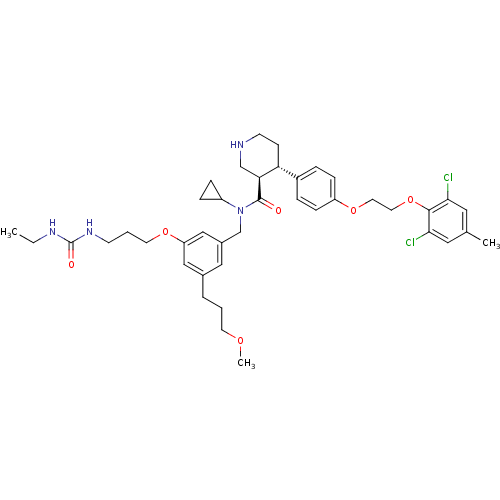
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES CCNC(=O)NCCCOc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1 |r| Show InChI InChI=1S/C41H54Cl2N4O6/c1-4-45-41(49)46-15-6-18-51-34-24-29(7-5-17-50-3)23-30(25-34)27-47(32-10-11-32)40(48)36-26-44-16-14-35(36)31-8-12-33(13-9-31)52-19-20-53-39-37(42)21-28(2)22-38(39)43/h8-9,12-13,21-25,32,35-36,44H,4-7,10-11,14-20,26-27H2,1-3H3,(H2,45,46,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355516
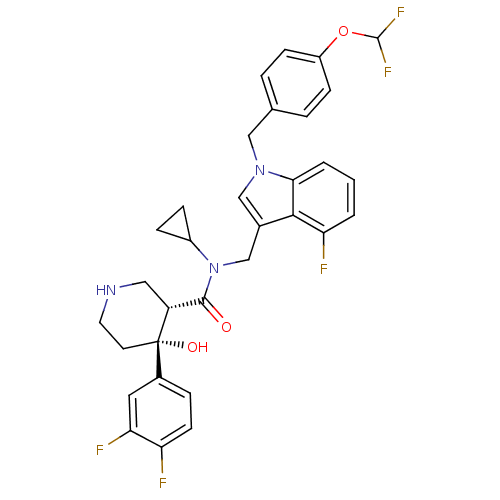
(CHEMBL1910318)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccc(OC(F)F)cc2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C32H30F5N3O3/c33-25-11-6-21(14-27(25)35)32(42)12-13-38-15-24(32)30(41)40(22-7-8-22)18-20-17-39(28-3-1-2-26(34)29(20)28)16-19-4-9-23(10-5-19)43-31(36)37/h1-6,9-11,14,17,22,24,31,38,42H,7-8,12-13,15-16,18H2/t24-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340408
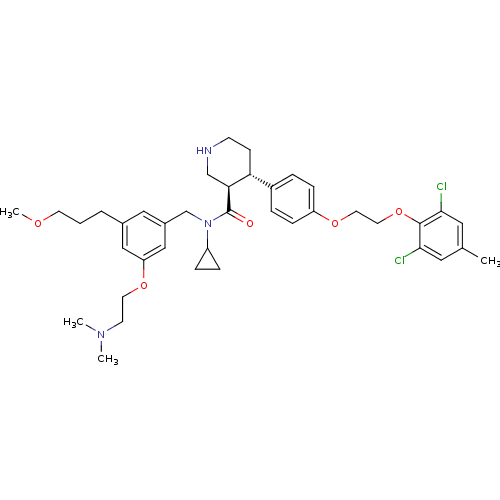
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCN(C)C)c1 |r| Show InChI InChI=1S/C39H51Cl2N3O5/c1-27-20-36(40)38(37(41)21-27)49-19-18-48-32-11-7-30(8-12-32)34-13-14-42-25-35(34)39(45)44(31-9-10-31)26-29-22-28(6-5-16-46-4)23-33(24-29)47-17-15-43(2)3/h7-8,11-12,20-24,31,34-35,42H,5-6,9-10,13-19,25-26H2,1-4H3/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340417
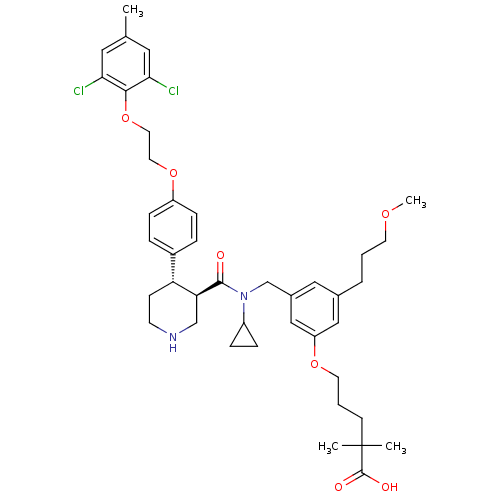
(5-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C42H54Cl2N2O7/c1-28-21-37(43)39(38(44)22-28)53-20-19-52-33-12-8-31(9-13-33)35-14-16-45-26-36(35)40(47)46(32-10-11-32)27-30-23-29(7-5-17-50-4)24-34(25-30)51-18-6-15-42(2,3)41(48)49/h8-9,12-13,21-25,32,35-36,45H,5-7,10-11,14-20,26-27H2,1-4H3,(H,48,49)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340423
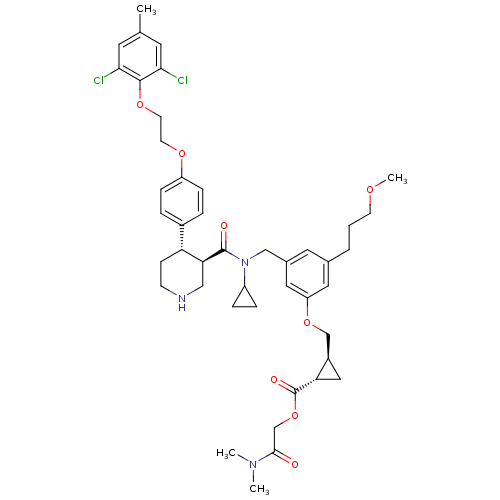
((1S,2S)-2-(dimethylamino)-2-oxoethyl 2-((3-(((3R,4...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)OCC(=O)N(C)C)c1 |r| Show InChI InChI=1S/C44H55Cl2N3O8/c1-28-18-39(45)42(40(46)19-28)55-17-16-54-34-11-7-31(8-12-34)36-13-14-47-24-38(36)43(51)49(33-9-10-33)25-30-20-29(6-5-15-53-4)21-35(22-30)56-26-32-23-37(32)44(52)57-27-41(50)48(2)3/h7-8,11-12,18-22,32-33,36-38,47H,5-6,9-10,13-17,23-27H2,1-4H3/t32-,36-,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355514
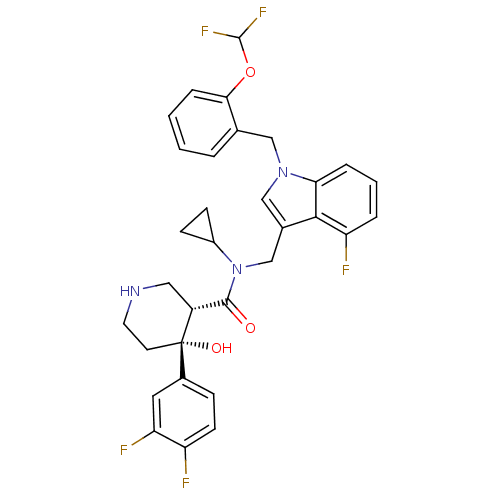
(CHEMBL1910316)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccccc2OC(F)F)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C32H30F5N3O3/c33-24-11-8-21(14-26(24)35)32(42)12-13-38-15-23(32)30(41)40(22-9-10-22)18-20-17-39(27-6-3-5-25(34)29(20)27)16-19-4-1-2-7-28(19)43-31(36)37/h1-8,11,14,17,22-23,31,38,42H,9-10,12-13,15-16,18H2/t23-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
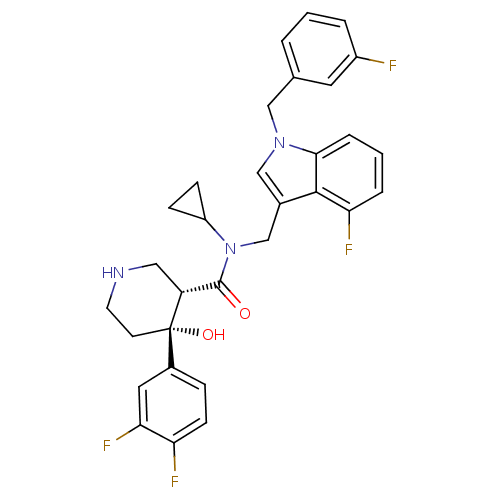
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355517
(CHEMBL1910319)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2cccc(F)c2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C31H29F4N3O2/c32-22-4-1-3-19(13-22)16-37-17-20(29-26(34)5-2-6-28(29)37)18-38(23-8-9-23)30(39)24-15-36-12-11-31(24,40)21-7-10-25(33)27(35)14-21/h1-7,10,13-14,17,23-24,36,40H,8-9,11-12,15-16,18H2/t24-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
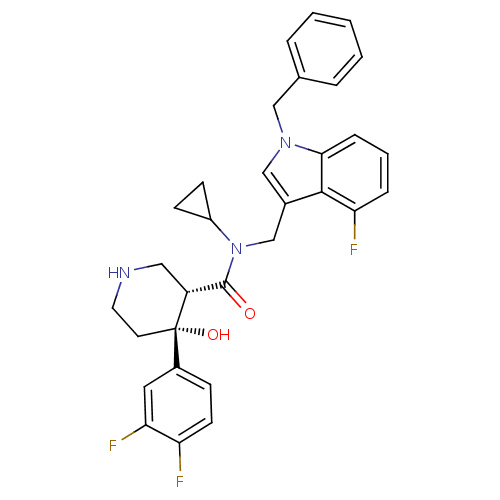
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355513
(CHEMBL1910315)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccccc2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C31H30F3N3O2/c32-25-12-9-22(15-27(25)34)31(39)13-14-35-16-24(31)30(38)37(23-10-11-23)19-21-18-36(17-20-5-2-1-3-6-20)28-8-4-7-26(33)29(21)28/h1-9,12,15,18,23-24,35,39H,10-11,13-14,16-17,19H2/t24-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
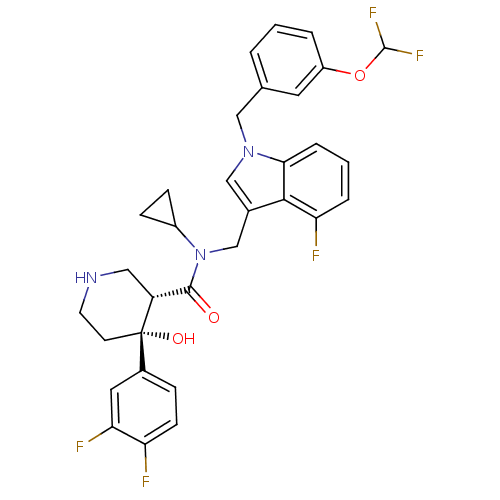
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355515
(CHEMBL1910317)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2cccc(OC(F)F)c2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C32H30F5N3O3/c33-25-10-7-21(14-27(25)35)32(42)11-12-38-15-24(32)30(41)40(22-8-9-22)18-20-17-39(28-6-2-5-26(34)29(20)28)16-19-3-1-4-23(13-19)43-31(36)37/h1-7,10,13-14,17,22,24,31,38,42H,8-9,11-12,15-16,18H2/t24-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355518
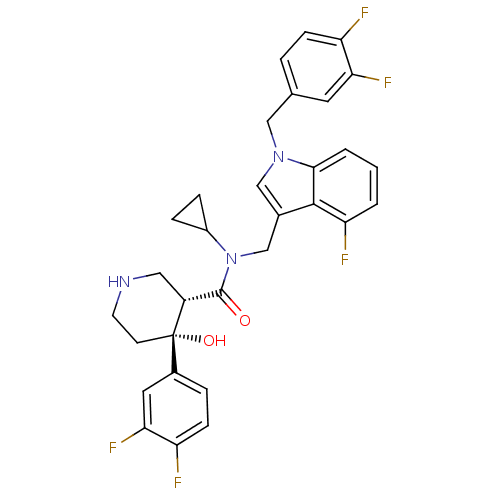
(CHEMBL1910320)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccc(F)c(F)c2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C31H28F5N3O2/c32-23-8-4-18(12-26(23)35)15-38-16-19(29-25(34)2-1-3-28(29)38)17-39(21-6-7-21)30(40)22-14-37-11-10-31(22,41)20-5-9-24(33)27(36)13-20/h1-5,8-9,12-13,16,21-22,37,41H,6-7,10-11,14-15,17H2/t22-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355519

(CHEMBL1910321)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2cccnc2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C30H29F3N4O2/c31-24-9-6-21(13-26(24)33)30(39)10-12-35-15-23(30)29(38)37(22-7-8-22)18-20-17-36(16-19-3-2-11-34-14-19)27-5-1-4-25(32)28(20)27/h1-6,9,11,13-14,17,22-23,35,39H,7-8,10,12,15-16,18H2/t23-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355521
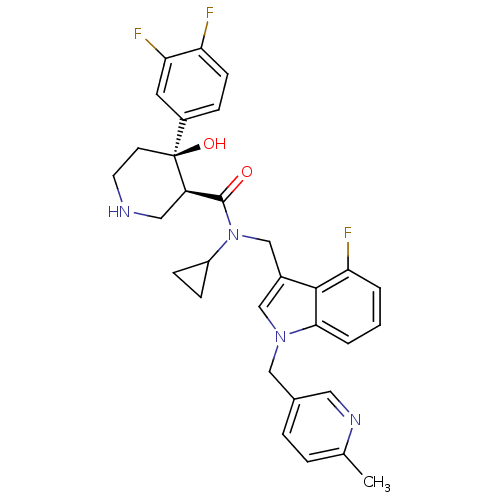
(CHEMBL1910545)Show SMILES Cc1ccc(Cn2cc(CN(C3CC3)C(=O)[C@H]3CNCC[C@]3(O)c3ccc(F)c(F)c3)c3c(F)cccc23)cn1 |r| Show InChI InChI=1S/C31H31F3N4O2/c1-19-5-6-20(14-36-19)16-37-17-21(29-26(33)3-2-4-28(29)37)18-38(23-8-9-23)30(39)24-15-35-12-11-31(24,40)22-7-10-25(32)27(34)13-22/h2-7,10,13-14,17,23-24,35,40H,8-9,11-12,15-16,18H2,1H3/t24-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340416
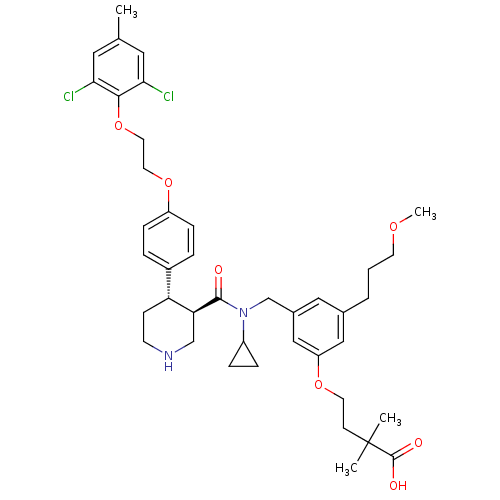
(4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C41H52Cl2N2O7/c1-27-20-36(42)38(37(43)21-27)52-19-18-51-32-11-7-30(8-12-32)34-13-15-44-25-35(34)39(46)45(31-9-10-31)26-29-22-28(6-5-16-49-4)23-33(24-29)50-17-14-41(2,3)40(47)48/h7-8,11-12,20-24,31,34-35,44H,5-6,9-10,13-19,25-26H2,1-4H3,(H,47,48)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340418
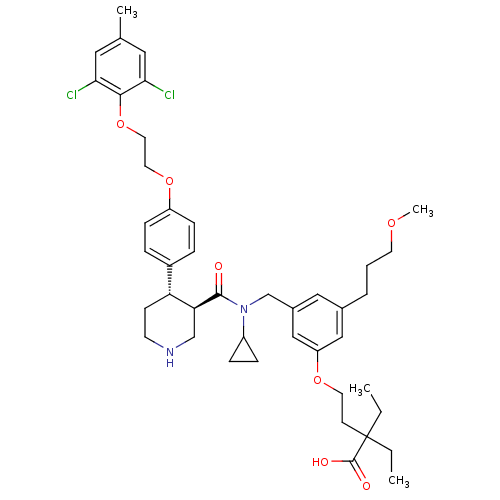
(4-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES CCC(CC)(CCOc1cc(CCCOC)cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)c1)C(O)=O |r| Show InChI InChI=1S/C43H56Cl2N2O7/c1-5-43(6-2,42(49)50)16-19-52-35-25-30(8-7-18-51-4)24-31(26-35)28-47(33-11-12-33)41(48)37-27-46-17-15-36(37)32-9-13-34(14-10-32)53-20-21-54-40-38(44)22-29(3)23-39(40)45/h9-10,13-14,22-26,33,36-37,46H,5-8,11-12,15-21,27-28H2,1-4H3,(H,49,50)/t36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
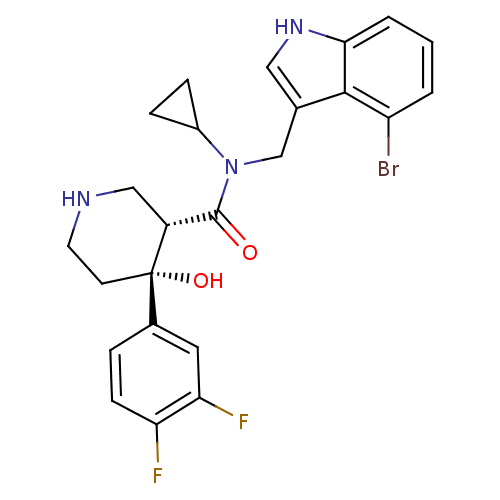
(Homo sapiens (Human)) | BDBM50355533
(CHEMBL1910299)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1c[nH]c2cccc(Br)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C24H24BrF2N3O2/c25-18-2-1-3-21-22(18)14(11-29-21)13-30(16-5-6-16)23(31)17-12-28-9-8-24(17,32)15-4-7-19(26)20(27)10-15/h1-4,7,10-11,16-17,28-29,32H,5-6,8-9,12-13H2/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
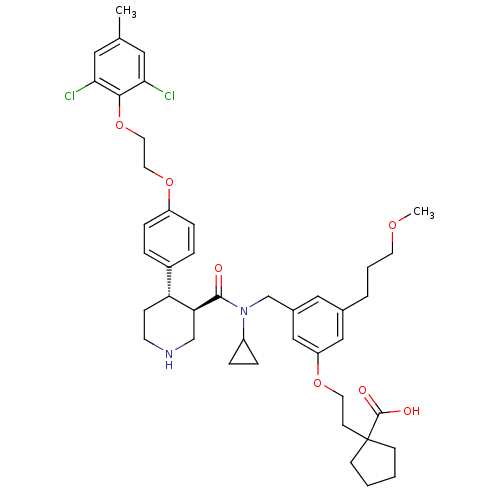
(Homo sapiens (Human)) | BDBM50340419
(1-(2-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichl...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCCC2(CCCC2)C(O)=O)c1 |r| Show InChI InChI=1S/C43H54Cl2N2O7/c1-29-22-38(44)40(39(45)23-29)54-21-20-53-34-11-7-32(8-12-34)36-13-17-46-27-37(36)41(48)47(33-9-10-33)28-31-24-30(6-5-18-51-2)25-35(26-31)52-19-16-43(42(49)50)14-3-4-15-43/h7-8,11-12,22-26,33,36-37,46H,3-6,9-10,13-21,27-28H2,1-2H3,(H,49,50)/t36-,37+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
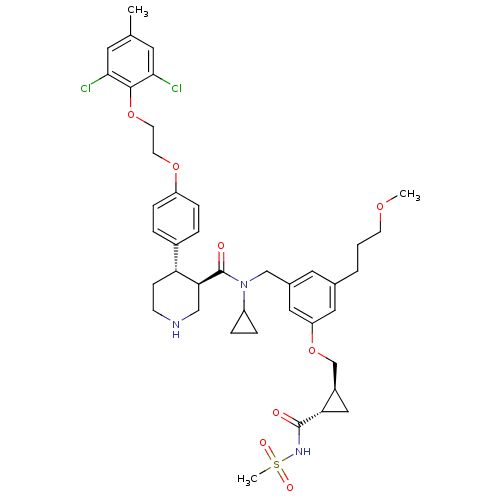
(Homo sapiens (Human)) | BDBM50340421
((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro-4-meth...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OC[C@H]2C[C@@H]2C(=O)NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C41H51Cl2N3O8S/c1-26-17-37(42)39(38(43)18-26)53-16-15-52-32-10-6-29(7-11-32)34-12-13-44-23-36(34)41(48)46(31-8-9-31)24-28-19-27(5-4-14-51-2)20-33(21-28)54-25-30-22-35(30)40(47)45-55(3,49)50/h6-7,10-11,17-21,30-31,34-36,44H,4-5,8-9,12-16,22-25H2,1-3H3,(H,45,47)/t30-,34-,35+,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
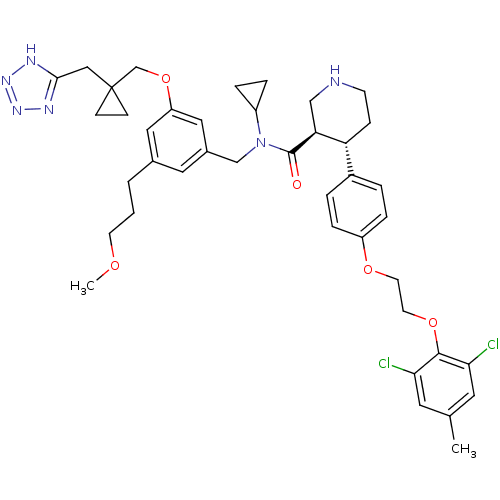
(Homo sapiens (Human)) | BDBM50340422
((3R,4S)-N-(3-((1-((2H-tetrazol-5-yl)methyl)cyclopr...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC2(Cc3nnn[nH]3)CC2)c1 |r| Show InChI InChI=1S/C41H50Cl2N6O5/c1-27-18-36(42)39(37(43)19-27)53-17-16-52-32-9-5-30(6-10-32)34-11-14-44-24-35(34)40(50)49(31-7-8-31)25-29-20-28(4-3-15-51-2)21-33(22-29)54-26-41(12-13-41)23-38-45-47-48-46-38/h5-6,9-10,18-22,31,34-35,44H,3-4,7-8,11-17,23-26H2,1-2H3,(H,45,46,47,48)/t34-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50056999

(CHEMBL56367 | nimesulide)Show InChI InChI=1S/C13H12N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h2-9,14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50340415
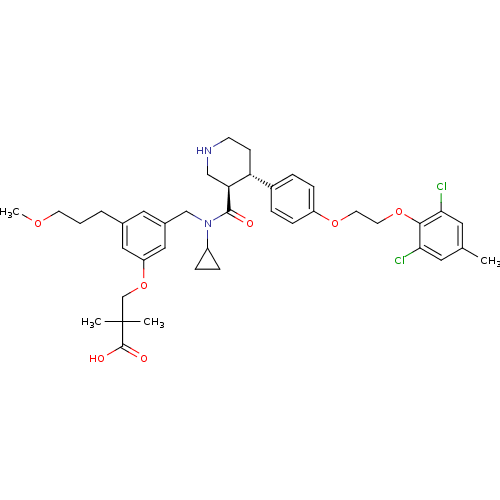
(3-(3-(((3R,4S)-N-cyclopropyl-4-(4-(2-(2,6-dichloro...)Show SMILES COCCCc1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@@H]2c2ccc(OCCOc3c(Cl)cc(C)cc3Cl)cc2)cc(OCC(C)(C)C(O)=O)c1 |r| Show InChI InChI=1S/C40H50Cl2N2O7/c1-26-18-35(41)37(36(42)19-26)50-17-16-49-31-11-7-29(8-12-31)33-13-14-43-23-34(33)38(45)44(30-9-10-30)24-28-20-27(6-5-15-48-4)21-32(22-28)51-25-40(2,3)39(46)47/h7-8,11-12,18-22,30,33-34,43H,5-6,9-10,13-17,23-25H2,1-4H3,(H,46,47)/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 2430-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.067
BindingDB Entry DOI: 10.7270/Q2G16157 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355537
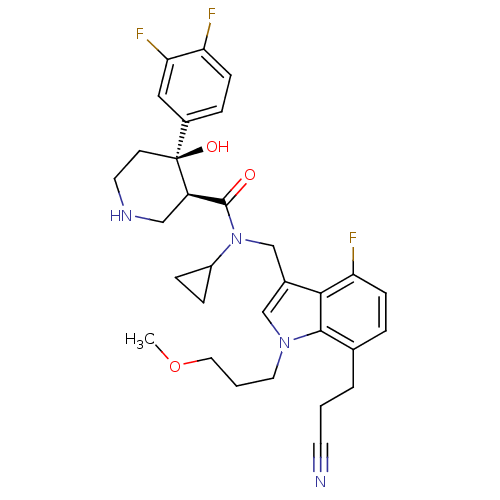
(CHEMBL1910303)Show SMILES COCCCn1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@]2(O)c2ccc(F)c(F)c2)c2c(F)ccc(CCC#N)c12 |r| Show InChI InChI=1S/C31H35F3N4O3/c1-41-15-3-14-37-18-21(28-26(33)9-5-20(29(28)37)4-2-12-35)19-38(23-7-8-23)30(39)24-17-36-13-11-31(24,40)22-6-10-25(32)27(34)16-22/h5-6,9-10,16,18,23-24,36,40H,2-4,7-8,11,13-15,17,19H2,1H3/t24-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355510
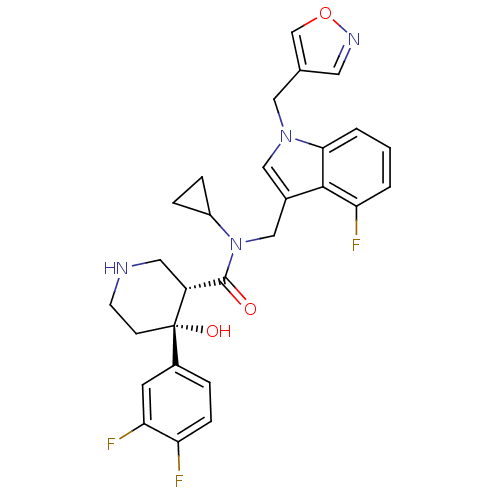
(CHEMBL1910312)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cn(Cc2cnoc2)c2cccc(F)c12)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C28H27F3N4O3/c29-22-7-4-19(10-24(22)31)28(37)8-9-32-12-21(28)27(36)35(20-5-6-20)15-18-14-34(13-17-11-33-38-16-17)25-3-1-2-23(30)26(18)25/h1-4,7,10-11,14,16,20-21,32,37H,5-6,8-9,12-13,15H2/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355528
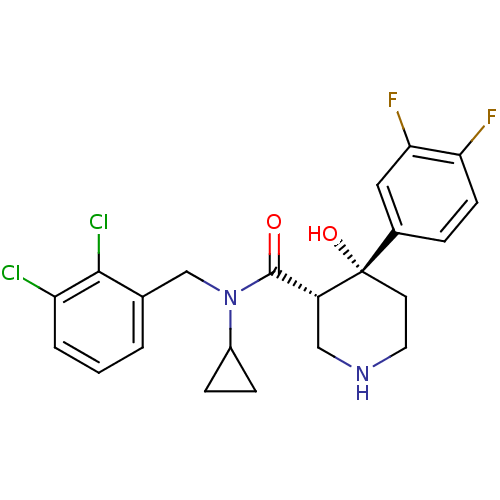
(CHEMBL1910295)Show SMILES O[C@@]1(CCNC[C@@H]1C(=O)N(Cc1cccc(Cl)c1Cl)C1CC1)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C22H22Cl2F2N2O2/c23-17-3-1-2-13(20(17)24)12-28(15-5-6-15)21(29)16-11-27-9-8-22(16,30)14-4-7-18(25)19(26)10-14/h1-4,7,10,15-16,27,30H,5-6,8-9,11-12H2/t16-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50056998

(4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...)Show SMILES CN1C(C(=O)Nc2ncc(C)s2)=C(O)c2ccccc2S1(=O)=O |t:12| Show InChI InChI=1S/C14H13N3O4S2/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2/h3-7,18H,1-2H3,(H,15,16,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50355511
(CHEMBL1910313)Show SMILES Cc1noc(C)c1Cn1cc(CN(C2CC2)C(=O)[C@H]2CNCC[C@]2(O)c2ccc(F)c(F)c2)c2c(F)cccc12 |r| Show InChI InChI=1S/C30H31F3N4O3/c1-17-22(18(2)40-35-17)16-36-14-19(28-25(32)4-3-5-27(28)36)15-37(21-7-8-21)29(38)23-13-34-11-10-30(23,39)20-6-9-24(31)26(33)12-20/h3-6,9,12,14,21,23,34,39H,7-8,10-11,13,15-16H2,1-2H3/t23-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 3976-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.014
BindingDB Entry DOI: 10.7270/Q21N81HN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50346989

(CHEMBL1796075)Show SMILES Cn1ccc(cc1=O)[C@H]1CCNC[C@@H]1C(=O)N(Cc1cn(Cc2ccc(F)cc2)c2cccc(F)c12)C1CC1 |r| Show InChI InChI=1S/C31H32F2N4O2/c1-35-14-12-21(15-29(35)38)25-11-13-34-16-26(25)31(39)37(24-9-10-24)19-22-18-36(17-20-5-7-23(32)8-6-20)28-4-2-3-27(33)30(22)28/h2-8,12,14-15,18,24-26,34H,9-11,13,16-17,19H2,1H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 21: 3970-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.013
BindingDB Entry DOI: 10.7270/Q2PN9608 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM85245
(CAS_36322-90-4 | NSC_4856 | Piroxicam)Show SMILES CN1C(C(=O)Nc2ccccn2)=C(O)c2ccccc2S1(=O)=O |t:12| Show InChI InChI=1S/C15H13N3O4S/c1-18-13(15(20)17-12-8-4-5-9-16-12)14(19)10-6-2-3-7-11(10)23(18,21)22/h2-9,19H,1H3,(H,16,17,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data