 Found 74 hits with Last Name = 'kobayashi' and Initial = 'd'
Found 74 hits with Last Name = 'kobayashi' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50019290
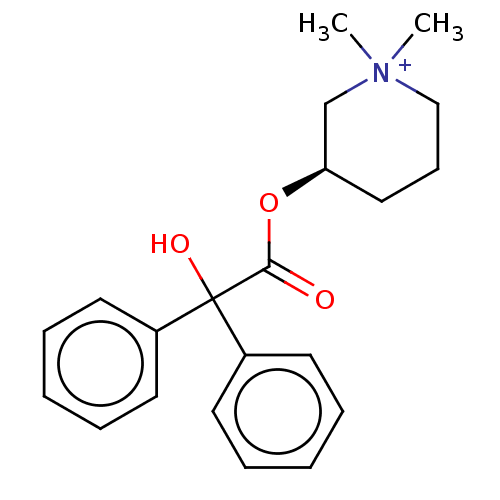
(CHEMBL3289390)Show SMILES [Br-].C[N+]1(C)CCC[C@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m1./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50377964
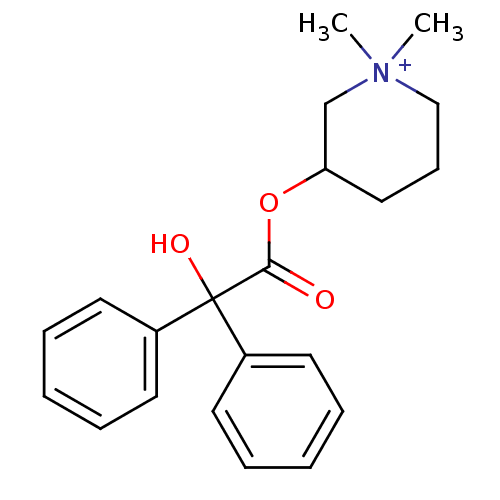
(Cantil | Glycophenylate | MEPENZOLATE BROMIDE | Me...)Show SMILES C[N+]1(C)CCCC(C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H26NO3/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3-8,10-13,19,24H,9,14-16H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50019290

(CHEMBL3289390)Show SMILES [Br-].C[N+]1(C)CCC[C@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50019289

(CHEMBL3289391)Show SMILES [Br-].C[N+]1(C)CCC[C@@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50377964

(Cantil | Glycophenylate | MEPENZOLATE BROMIDE | Me...)Show SMILES C[N+]1(C)CCCC(C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H26NO3/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18/h3-8,10-13,19,24H,9,14-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613945

(CHEMBL5289073) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50019289

(CHEMBL3289391)Show SMILES [Br-].C[N+]1(C)CCC[C@@H](C1)OC(=O)C(O)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1/t19-;/m0./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3R expressed in CHOK1 cells after 2 hrs by scintillation counting analysis |
Bioorg Med Chem 22: 3488-97 (2014)
Article DOI: 10.1016/j.bmc.2014.04.029
BindingDB Entry DOI: 10.7270/Q2VD7111 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613946
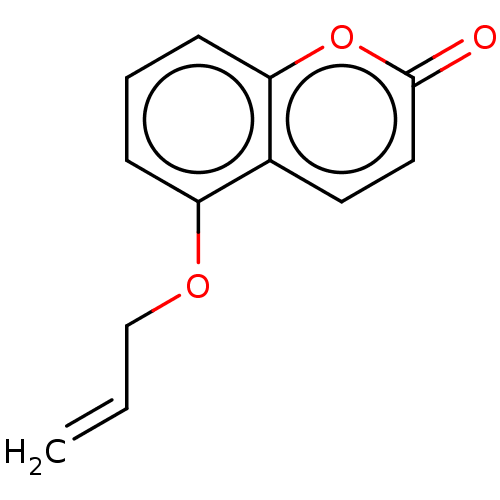
(5-Allyloxycoumarin | CHEMBL450963) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613944
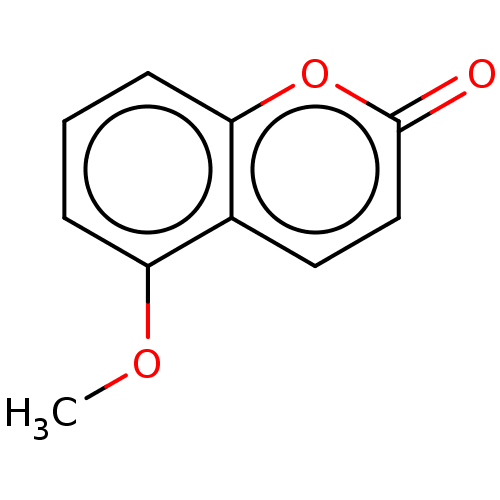
(CHEMBL502746) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613947

(CHEMBL448936) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50041234

(6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...)Show InChI InChI=1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613943
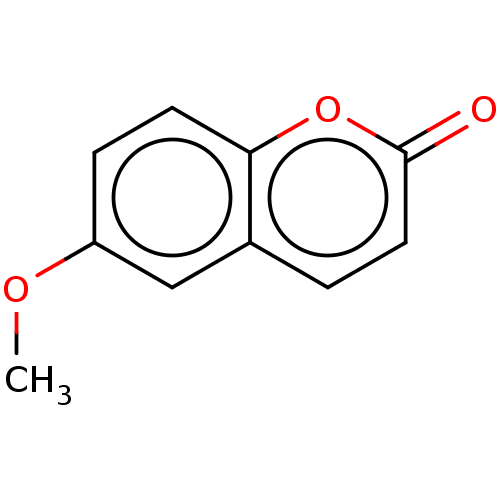
(6-Methoxycoumarin | CHEMBL496547) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50041234

(6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...)Show InChI InChI=1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613941
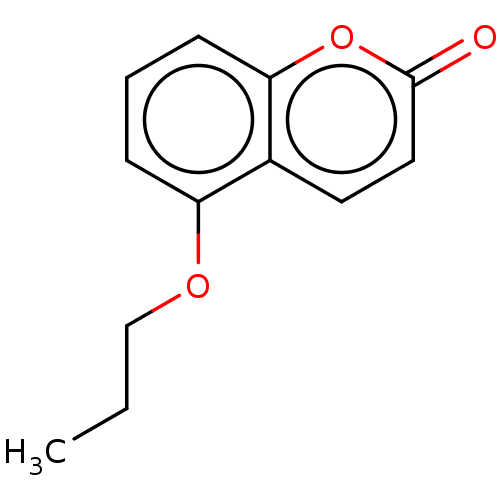
(CHEMBL451623) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50041234

(6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...)Show InChI InChI=1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613942
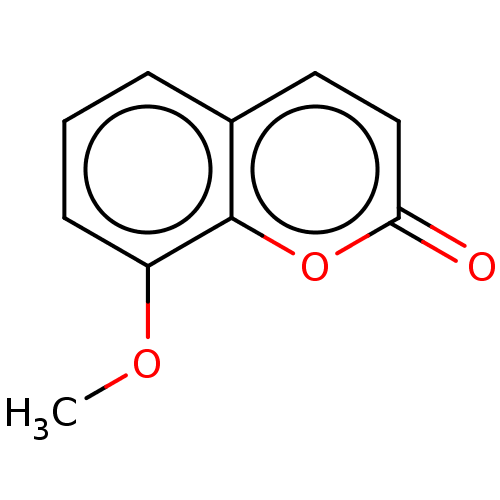
(8-Methoxycoumarin | CHEMBL503184) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50041234

(6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...)Show InChI InChI=1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50613946

(5-Allyloxycoumarin | CHEMBL450963) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM12341

(CHEMBL178681 | CHEMBL359657 | US8609708, 1 | US860...)Show InChI InChI=1S/C10H10N2S/c11-6-9-3-4-10(13-9)8-2-1-5-12-7-8/h1-5,7H,6,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50613945

(CHEMBL5289073) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50613947

(CHEMBL448936) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50613944

(CHEMBL502746) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50242734

(CHEMBL4070331)Show SMILES CCN(CC)c1ccc2cc(-c3cc4ccc(cc4oc3=O)N(CC)CC)c(=O)oc2c1 Show InChI InChI=1S/C26H28N2O4/c1-5-27(6-2)19-11-9-17-13-21(25(29)31-23(17)15-19)22-14-18-10-12-20(28(7-3)8-4)16-24(18)32-26(22)30/h9-16H,5-8H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Health Sciences University of Hokkaido
Curated by ChEMBL
| Assay Description
Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla... |
Bioorg Med Chem Lett 27: 2645-2649 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.062
BindingDB Entry DOI: 10.7270/Q2CF9SH5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50242736
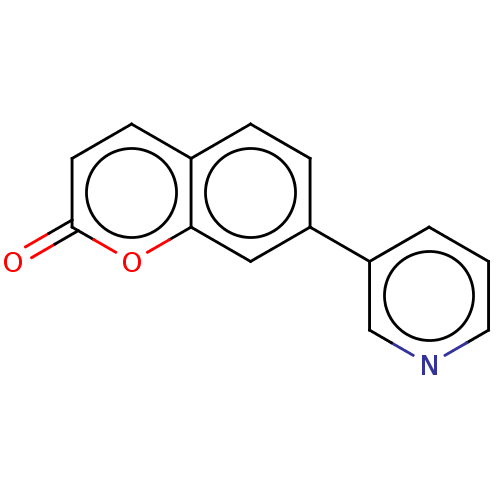
(CHEMBL4098990)Show InChI InChI=1S/C14H9NO2/c16-14-6-5-10-3-4-11(8-13(10)17-14)12-2-1-7-15-9-12/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Health Sciences University of Hokkaido
Curated by ChEMBL
| Assay Description
Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla... |
Bioorg Med Chem Lett 27: 2645-2649 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.062
BindingDB Entry DOI: 10.7270/Q2CF9SH5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613945

(CHEMBL5289073) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50398447
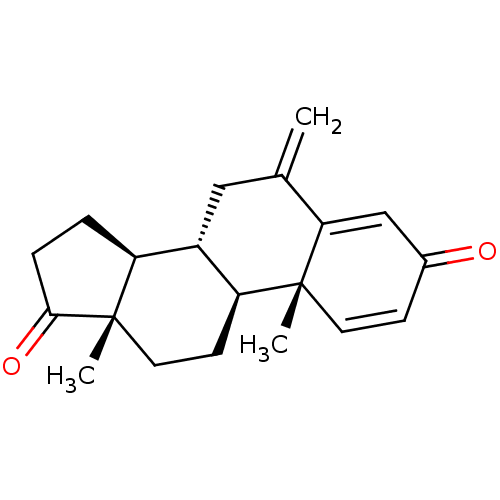
(Aromasin | EXEMESTANE)Show SMILES C[C@]12CC[C@H]3[C@@H](CC(=C)C4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O |r,c:13,t:9| Show InChI InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Health Sciences University of Hokkaido
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 reverse transcriptase |
Bioorg Med Chem Lett 27: 2645-2649 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.062
BindingDB Entry DOI: 10.7270/Q2CF9SH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613946

(5-Allyloxycoumarin | CHEMBL450963) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465930

(CHEMBL4292990)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1cc2ccccc2cc1O Show InChI InChI=1S/C24H30N4O/c1-23(2)14-18(15-24(3,4)27-23)28(5)22-11-10-20(25-26-22)19-12-16-8-6-7-9-17(16)13-21(19)29/h6-13,18,27,29H,14-15H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465915

(CHEMBL4281315)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1cc2ccccc2s1 Show InChI InChI=1S/C22H28N4S/c1-21(2)13-16(14-22(3,4)25-21)26(5)20-11-10-17(23-24-20)19-12-15-8-6-7-9-18(15)27-19/h6-12,16,25H,13-14H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613944

(CHEMBL502746) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613947

(CHEMBL448936) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613943

(6-Methoxycoumarin | CHEMBL496547) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465921
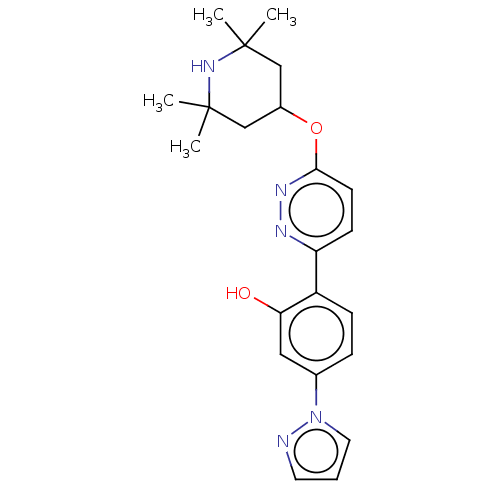
(CHEMBL4288439)Show SMILES CC1(C)CC(CC(C)(C)N1)Oc1ccc(nn1)-c1ccc(cc1O)-n1cccn1 Show InChI InChI=1S/C22H27N5O2/c1-21(2)13-16(14-22(3,4)26-21)29-20-9-8-18(24-25-20)17-7-6-15(12-19(17)28)27-11-5-10-23-27/h5-12,16,26,28H,13-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465926
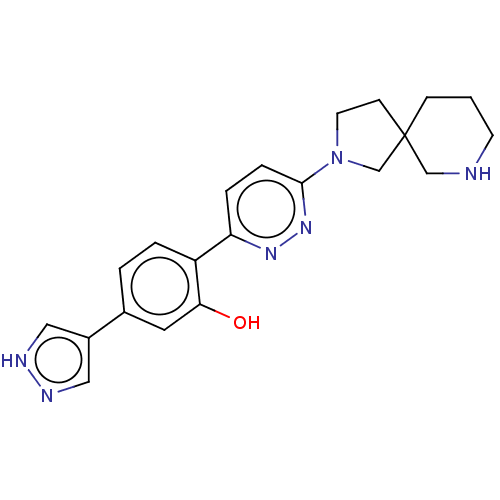
(CHEMBL4294614)Show SMILES Oc1cc(ccc1-c1ccc(nn1)N1CCC2(C1)CCCNC2)-c1cn[nH]c1 Show InChI InChI=1S/C21H24N6O/c28-19-10-15(16-11-23-24-12-16)2-3-17(19)18-4-5-20(26-25-18)27-9-7-21(14-27)6-1-8-22-13-21/h2-5,10-12,22,28H,1,6-9,13-14H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465920

(CHEMBL4280558)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(cc1O)-n1cccn1 Show InChI InChI=1S/C23H30N6O/c1-22(2)14-17(15-23(3,4)27-22)28(5)21-10-9-19(25-26-21)18-8-7-16(13-20(18)30)29-12-6-11-24-29/h6-13,17,27,30H,14-15H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465919
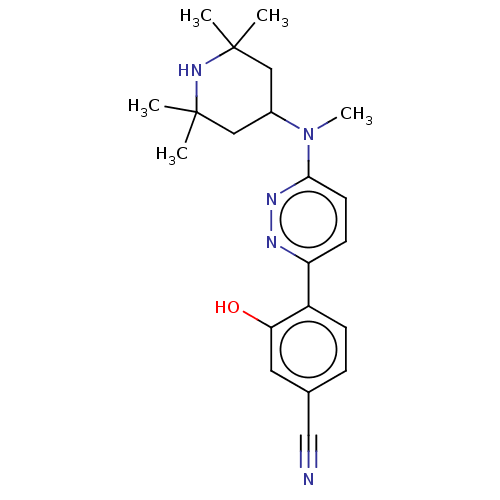
(CHEMBL4283397)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(cc1O)C#N Show InChI InChI=1S/C21H27N5O/c1-20(2)11-15(12-21(3,4)25-20)26(5)19-9-8-17(23-24-19)16-7-6-14(13-22)10-18(16)27/h6-10,15,25,27H,11-12H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50041234

(6-hydroxy-7-methoxy-5-benzofuranacrylic acid delta...)Show InChI InChI=1S/C12H8O4/c1-14-12-10-8(4-5-15-10)6-7-2-3-9(13)16-11(7)12/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465916
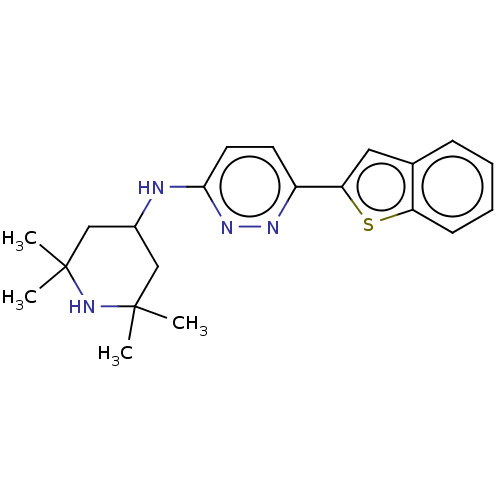
(CHEMBL4289183)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1ccc(nn1)-c1cc2ccccc2s1 Show InChI InChI=1S/C21H26N4S/c1-20(2)12-15(13-21(3,4)25-20)22-19-10-9-16(23-24-19)18-11-14-7-5-6-8-17(14)26-18/h5-11,15,25H,12-13H2,1-4H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50613941

(CHEMBL451623) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50242747
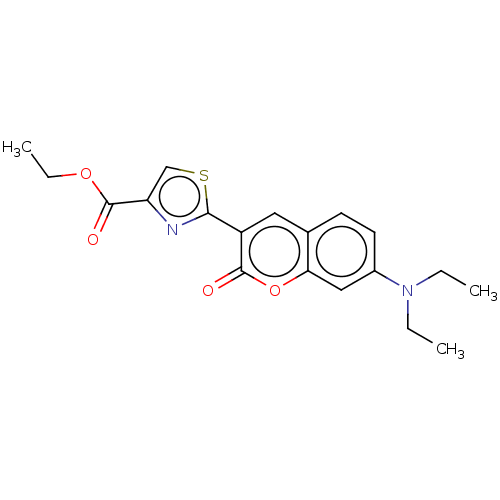
(CHEMBL4078790)Show SMILES CCOC(=O)c1csc(n1)-c1cc2ccc(cc2oc1=O)N(CC)CC Show InChI InChI=1S/C19H20N2O4S/c1-4-21(5-2)13-8-7-12-9-14(18(22)25-16(12)10-13)17-20-15(11-26-17)19(23)24-6-3/h7-11H,4-6H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health Sciences University of Hokkaido
Curated by ChEMBL
| Assay Description
Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla... |
Bioorg Med Chem Lett 27: 2645-2649 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.062
BindingDB Entry DOI: 10.7270/Q2CF9SH5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50242748
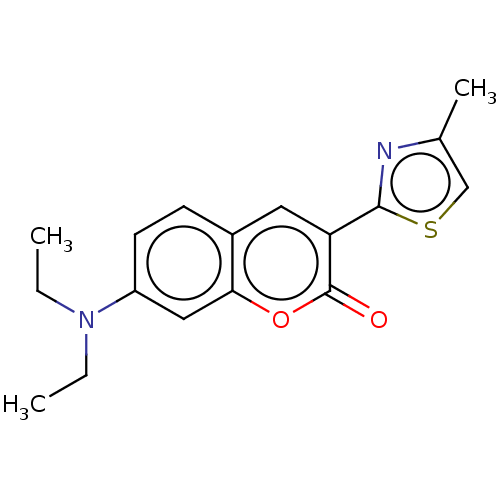
(CHEMBL4103066)Show InChI InChI=1S/C17H18N2O2S/c1-4-19(5-2)13-7-6-12-8-14(16-18-11(3)10-22-16)17(20)21-15(12)9-13/h6-10H,4-5H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health Sciences University of Hokkaido
Curated by ChEMBL
| Assay Description
Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla... |
Bioorg Med Chem Lett 27: 2645-2649 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.062
BindingDB Entry DOI: 10.7270/Q2CF9SH5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465927

(CHEMBL4285798)Show SMILES CC1(C)CC(CC(C)(C)N1)Nc1ccc(nn1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H28N4/c1-22(2)14-19(15-23(3,4)27-22)24-21-12-11-20(25-26-21)18-10-9-16-7-5-6-8-17(16)13-18/h5-13,19,27H,14-15H2,1-4H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465918

(CHEMBL4282391)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(F)cc1O Show InChI InChI=1S/C20H27FN4O/c1-19(2)11-14(12-20(3,4)24-19)25(5)18-9-8-16(22-23-18)15-7-6-13(21)10-17(15)26/h6-10,14,24,26H,11-12H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465929

(CHEMBL4286715)Show SMILES [H][C@@]12CNC[C@]1([H])CN(C2)c1ccc(nn1)-c1ccc(cc1O)-c1cn[nH]c1 |r| Show InChI InChI=1S/C19H20N6O/c26-18-5-12(13-8-21-22-9-13)1-2-16(18)17-3-4-19(24-23-17)25-10-14-6-20-7-15(14)11-25/h1-5,8-9,14-15,20,26H,6-7,10-11H2,(H,21,22)/t14-,15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50242750
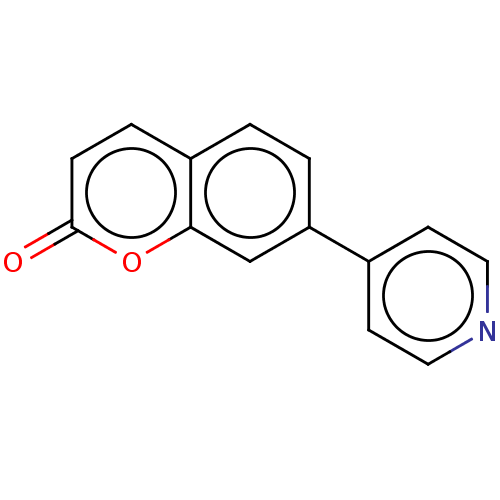
(CHEMBL4080965)Show InChI InChI=1S/C14H9NO2/c16-14-4-3-11-1-2-12(9-13(11)17-14)10-5-7-15-8-6-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health Sciences University of Hokkaido
Curated by ChEMBL
| Assay Description
Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla... |
Bioorg Med Chem Lett 27: 2645-2649 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.062
BindingDB Entry DOI: 10.7270/Q2CF9SH5 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50242745
(CHEMBL4087613)Show InChI InChI=1S/C16H16N2O3/c1-3-18(4-2)12-6-5-11-7-13(15-9-17-10-20-15)16(19)21-14(11)8-12/h5-10H,3-4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Health Sciences University of Hokkaido
Curated by ChEMBL
| Assay Description
Inhibition of CYP19 in human placental microsome using [1beta-3H]-androstenedione as substrate after 15 mins in presence of NADPH by liquid scintilla... |
Bioorg Med Chem Lett 27: 2645-2649 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.062
BindingDB Entry DOI: 10.7270/Q2CF9SH5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465922

(CHEMBL4277023)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1cc2cccnc2cc1O Show InChI InChI=1S/C23H29N5O/c1-22(2)13-16(14-23(3,4)27-22)28(5)21-9-8-18(25-26-21)17-11-15-7-6-10-24-19(15)12-20(17)29/h6-12,16,27,29H,13-14H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465931

(CHEMBL4290774)Show InChI InChI=1S/C18H19N5O2/c24-17-12-13(23-11-1-8-20-23)2-3-15(17)16-4-5-18(22-21-16)25-14-6-9-19-10-7-14/h1-5,8,11-12,14,19,24H,6-7,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465925

(CHEMBL4283367)Show SMILES CN(C1CC(C)(C)NC(C)(C)C1)c1ccc(nn1)-c1ccc(cc1O)-c1cn[nH]c1 Show InChI InChI=1S/C23H30N6O/c1-22(2)11-17(12-23(3,4)28-22)29(5)21-9-8-19(26-27-21)18-7-6-15(10-20(18)30)16-13-24-25-14-16/h6-10,13-14,17,28,30H,11-12H2,1-5H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50465914
(Branaplam | LMI-070 | LMI070)Show SMILES CC1(C)CC(CC(C)(C)N1)Oc1ccc(nn1)-c1ccc(cc1O)-c1cn[nH]c1 Show InChI InChI=1S/C22H27N5O2/c1-21(2)10-16(11-22(3,4)27-21)29-20-8-7-18(25-26-20)17-6-5-14(9-19(17)28)15-12-23-24-13-15/h5-9,12-13,16,27-28H,10-11H2,1-4H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human ERG expressed in CHO cell membranes after 90 mins by micro-beta counting |
J Med Chem 61: 11021-11036 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01291
BindingDB Entry DOI: 10.7270/Q2474DJX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data