 Found 89 hits with Last Name = 'malinak' and Initial = 'd'
Found 89 hits with Last Name = 'malinak' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473
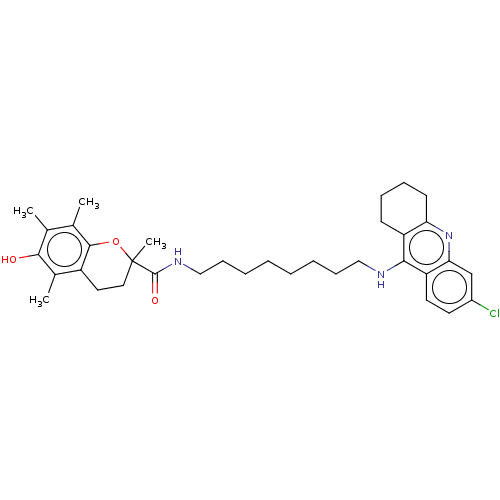
(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50465901
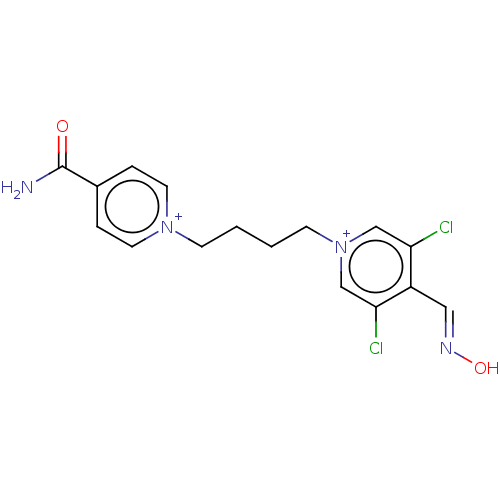
(CHEMBL4285826)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2cc(Cl)c(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H16Cl2N4O2/c17-14-10-22(11-15(18)13(14)9-20-24)6-2-1-5-21-7-3-12(4-8-21)16(19)23/h3-4,7-11H,1-2,5-6H2,(H-,19,23)/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50465900

(CHEMBL4281350)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2cc(Cl)c(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C15H14Cl2N4O2/c16-13-9-21(10-14(17)12(13)8-19-23)5-1-4-20-6-2-11(3-7-20)15(18)22/h2-3,6-10H,1,4-5H2,(H-,18,22)/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50465902

(CHEMBL4277895)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2cc(Cl)c(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H14Cl2N4O2/c17-14-10-22(11-15(18)13(14)9-20-24)6-2-1-5-21-7-3-12(4-8-21)16(19)23/h1-4,7-11H,5-6H2,(H-,19,23)/p+2/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50465903
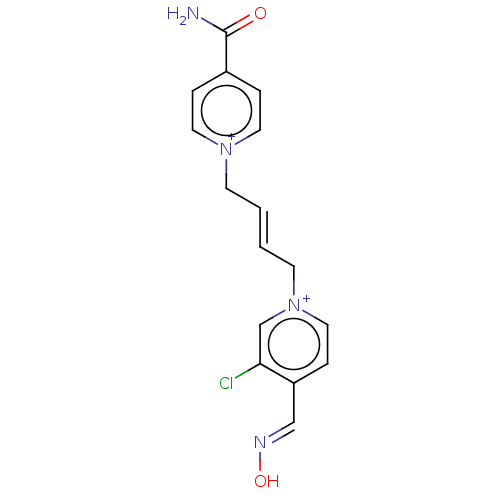
(CHEMBL4289221)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H15ClN4O2/c17-15-12-21(10-5-14(15)11-19-23)7-2-1-6-20-8-3-13(4-9-20)16(18)22/h1-5,8-12H,6-7H2,(H-,18,22)/p+2/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50020522

(1-(((4-carbamoylpyridinium-1-yl)methoxy)methyl)-2-...)Show InChI InChI=1S/C14H15N4O3/c15-14(19)12-4-7-17(8-5-12)10-21-11-18-6-2-1-3-13(18)9-16-20/h1-8H,9-11H2,(H-,15,19)/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50591828
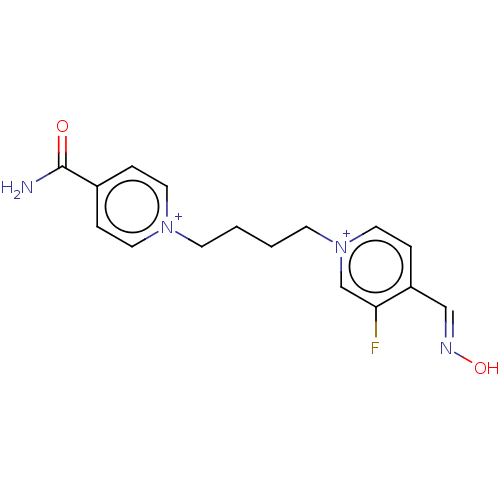
(CHEMBL5173381)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2ccc(\C=N\O)c(F)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50591827
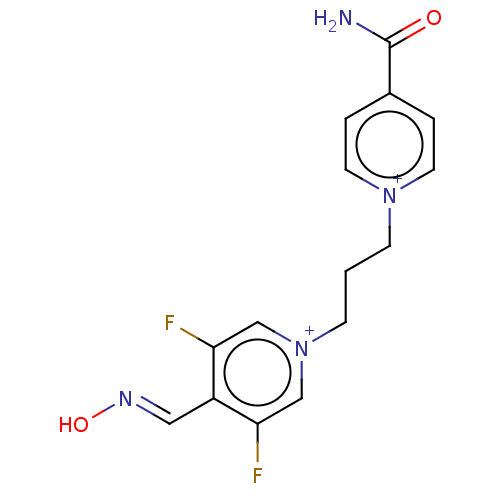
(CHEMBL5171026)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2cc(F)c(\C=N\O)c(F)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50591826

(CHEMBL5177448)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2ccc(\C=N\O)c(F)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50591830
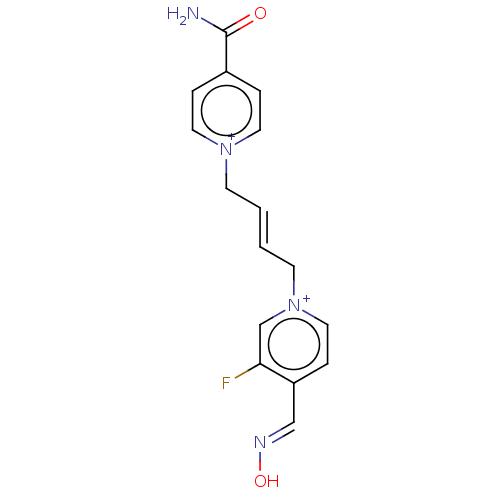
(CHEMBL595504)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(\C=N\O)c(F)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50465904
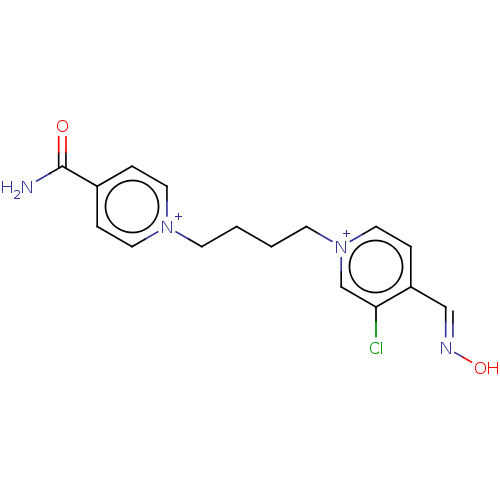
(CHEMBL4284764)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2ccc(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H17ClN4O2/c17-15-12-21(10-5-14(15)11-19-23)7-2-1-6-20-8-3-13(4-9-20)16(18)22/h3-5,8-12H,1-2,6-7H2,(H-,18,22)/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50465899

(CHEMBL4292643)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2ccc(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C15H15ClN4O2/c16-14-11-20(9-4-13(14)10-18-22)6-1-5-19-7-2-12(3-8-19)15(17)21/h2-4,7-11H,1,5-6H2,(H-,17,21)/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50591828

(CHEMBL5173381)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2ccc(\C=N\O)c(F)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50591829
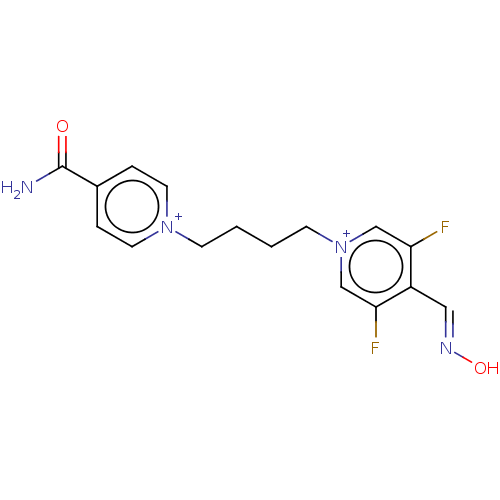
(CHEMBL5184063)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2cc(F)c(\C=N\O)c(F)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50591831
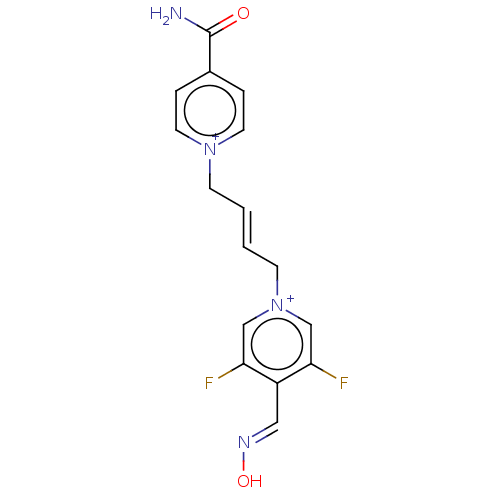
(CHEMBL5206644)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2cc(F)c(\C=N\O)c(F)c2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50333782

(4-(aminocarbonyl)-1-(3-{4-[(E)-(hydroxyimino)methy...)Show InChI InChI=1S/C15H17N4O2/c16-15(20)14-4-10-19(11-5-14)7-1-6-18-8-2-13(3-9-18)12-17-21/h2-5,8-11H,1,6-7,12H2,(H-,16,20)/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50333782

(4-(aminocarbonyl)-1-(3-{4-[(E)-(hydroxyimino)methy...)Show InChI InChI=1S/C15H17N4O2/c16-15(20)14-4-10-19(11-5-14)7-1-6-18-8-2-13(3-9-18)12-17-21/h2-5,8-11H,1,6-7,12H2,(H-,16,20)/q+1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human erythrocytic AChE using acetylthiocholine iodide as substrate measured up to 2 mins by spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50333783
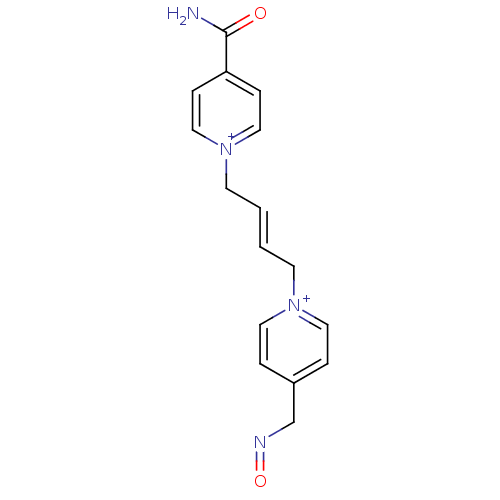
((E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomet...)Show SMILES NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(CN=O)cc2)cc1 Show InChI InChI=1S/C16H17N4O2/c17-16(21)15-5-11-20(12-6-15)8-2-1-7-19-9-3-14(4-10-19)13-18-22/h1-6,9-12H,7-8,13H2,(H-,17,21)/q+1/p+1/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human erythrocytic AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50333783

((E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomet...)Show SMILES NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(CN=O)cc2)cc1 Show InChI InChI=1S/C16H17N4O2/c17-16(21)15-5-11-20(12-6-15)8-2-1-7-19-9-3-14(4-10-19)13-18-22/h1-6,9-12H,7-8,13H2,(H-,17,21)/q+1/p+1/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025010

(CHEMBL116379)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2ccc(\C=N\O)cc2)cc1 Show InChI InChI=1S/C16H18N4O2.2BrH/c17-16(21)15-5-11-20(12-6-15)8-2-1-7-19-9-3-14(4-10-19)13-18-22;;/h3-6,9-13H,1-2,7-8H2,(H-,17,21);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human erythrocytic AChE using acetylthiocholine iodide as substrate measured up to 2 mins by spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50025010

(CHEMBL116379)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2ccc(\C=N\O)cc2)cc1 Show InChI InChI=1S/C16H18N4O2.2BrH/c17-16(21)15-5-11-20(12-6-15)8-2-1-7-19-9-3-14(4-10-19)13-18-22;;/h3-6,9-13H,1-2,7-8H2,(H-,17,21);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50591830

(CHEMBL595504)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(\C=N\O)c(F)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50465901

(CHEMBL4285826)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2cc(Cl)c(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H16Cl2N4O2/c17-14-10-22(11-15(18)13(14)9-20-24)6-2-1-5-21-7-3-12(4-8-21)16(19)23/h3-4,7-11H,1-2,5-6H2,(H-,19,23)/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM234367
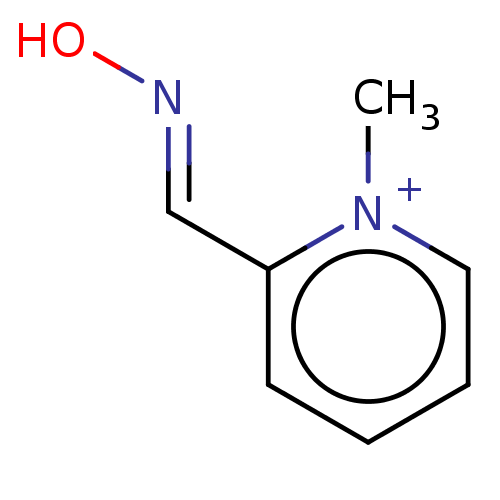
(Pralidoxime (3))Show InChI InChI=1S/C7H8N2O/c1-9-5-3-2-4-7(9)6-8-10/h2-6H,1H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
| DrugBank
Article
PubMed
| 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human recombinant AChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50465902

(CHEMBL4277895)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2cc(Cl)c(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H14Cl2N4O2/c17-14-10-22(11-15(18)13(14)9-20-24)6-2-1-5-21-7-3-12(4-8-21)16(19)23/h1-4,7-11H,5-6H2,(H-,19,23)/p+2/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50465900

(CHEMBL4281350)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2cc(Cl)c(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C15H14Cl2N4O2/c16-13-9-21(10-14(17)12(13)8-19-23)5-1-4-20-6-2-11(3-7-20)15(18)22/h2-3,6-10H,1,4-5H2,(H-,18,22)/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50020522

(1-(((4-carbamoylpyridinium-1-yl)methoxy)methyl)-2-...)Show InChI InChI=1S/C14H15N4O3/c15-14(19)12-4-7-17(8-5-12)10-21-11-18-6-2-1-3-13(18)9-16-20/h1-8H,9-11H2,(H-,15,19)/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50591831

(CHEMBL5206644)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2cc(F)c(\C=N\O)c(F)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50465904

(CHEMBL4284764)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2ccc(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H17ClN4O2/c17-15-12-21(10-5-14(15)11-19-23)7-2-1-6-20-8-3-13(4-9-20)16(18)22/h3-5,8-12H,1-2,6-7H2,(H-,18,22)/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50465899

(CHEMBL4292643)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2ccc(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C15H15ClN4O2/c16-14-11-20(9-4-13(14)10-18-22)6-1-5-19-7-2-12(3-8-19)15(17)21/h2-4,7-11H,1,5-6H2,(H-,17,21)/p+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50591827

(CHEMBL5171026)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2cc(F)c(\C=N\O)c(F)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50591829

(CHEMBL5184063)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2cc(F)c(\C=N\O)c(F)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50465903

(CHEMBL4289221)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(\C=N\O)c(Cl)c2)cc1 Show InChI InChI=1S/C16H15ClN4O2/c17-15-12-21(10-5-14(15)11-19-23)7-2-1-6-20-8-3-13(4-9-20)16(18)22/h1-5,8-12H,6-7H2,(H-,18,22)/p+2/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM234367

(Pralidoxime (3))Show InChI InChI=1S/C7H8N2O/c1-9-5-3-2-4-7(9)6-8-10/h2-6H,1H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
| DrugBank
Article
PubMed
| 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50025010

(CHEMBL116379)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCCC[n+]2ccc(\C=N\O)cc2)cc1 Show InChI InChI=1S/C16H18N4O2.2BrH/c17-16(21)15-5-11-20(12-6-15)8-2-1-7-19-9-3-14(4-10-19)13-18-22;;/h3-6,9-13H,1-2,7-8H2,(H-,17,21);2*1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50591826

(CHEMBL5177448)Show SMILES [Br-].[Br-].NC(=O)c1cc[n+](CCC[n+]2ccc(\C=N\O)c(F)c2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50333782

(4-(aminocarbonyl)-1-(3-{4-[(E)-(hydroxyimino)methy...)Show InChI InChI=1S/C15H17N4O2/c16-15(20)14-4-10-19(11-5-14)7-1-6-18-8-2-13(3-9-18)12-17-21/h2-5,8-11H,1,6-7,12H2,(H-,16,20)/q+1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50333783

((E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomet...)Show SMILES NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(CN=O)cc2)cc1 Show InChI InChI=1S/C16H17N4O2/c17-16(21)15-5-11-20(12-6-15)8-2-1-7-19-9-3-14(4-10-19)13-18-22/h1-6,9-12H,7-8,13H2,(H-,17,21)/q+1/p+1/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114377
BindingDB Entry DOI: 10.7270/Q2WW7NP4 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50333783

((E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomet...)Show SMILES NC(=O)c1cc[n+](C\C=C\C[n+]2ccc(CN=O)cc2)cc1 Show InChI InChI=1S/C16H17N4O2/c17-16(21)15-5-11-20(12-6-15)8-2-1-7-19-9-3-14(4-10-19)13-18-22/h1-6,9-12H,7-8,13H2,(H-,17,21)/q+1/p+1/b2-1+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research and Occupational Health
Curated by ChEMBL
| Assay Description
Reversible inhibition of human plasma BChE using acetylthiocholine iodide as substrate by Ellman spectrophotometric method |
J Med Chem 61: 10753-10766 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01398
BindingDB Entry DOI: 10.7270/Q2HD7Z9P |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133449
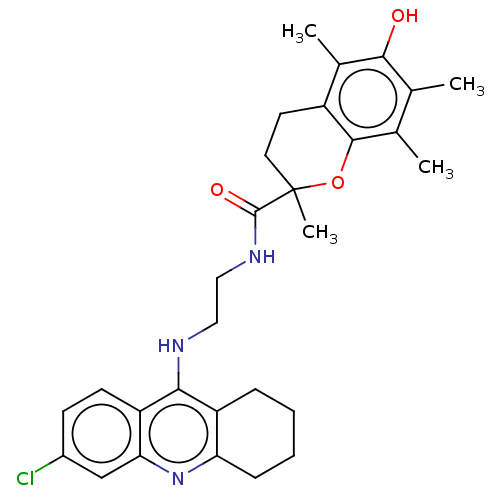
(CHEMBL3632988)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C29H34ClN3O3/c1-16-17(2)27-20(18(3)26(16)34)11-12-29(4,36-27)28(35)32-14-13-31-25-21-7-5-6-8-23(21)33-24-15-19(30)9-10-22(24)25/h9-10,15,34H,5-8,11-14H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073117
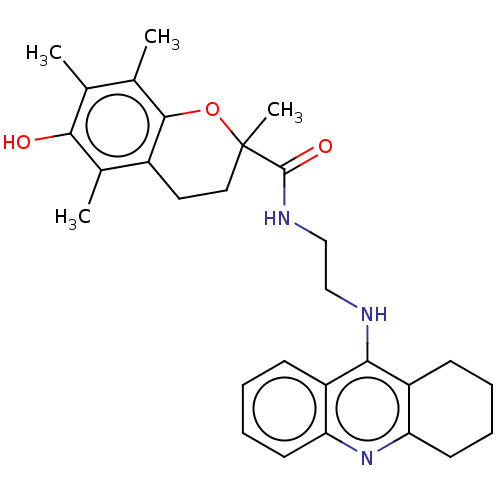
(CHEMBL3410951)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C29H35N3O3/c1-17-18(2)27-20(19(3)26(17)33)13-14-29(4,35-27)28(34)31-16-15-30-25-21-9-5-7-11-23(21)32-24-12-8-6-10-22(24)25/h5,7,9,11,33H,6,8,10,12-16H2,1-4H3,(H,30,32)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133473

(CHEMBL3632994)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C35H46ClN3O3/c1-22-23(2)33-26(24(3)32(22)40)17-18-35(4,42-33)34(41)38-20-12-8-6-5-7-11-19-37-31-27-13-9-10-14-29(27)39-30-21-25(36)15-16-28(30)31/h15-16,21,40H,5-14,17-20H2,1-4H3,(H,37,39)(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133450
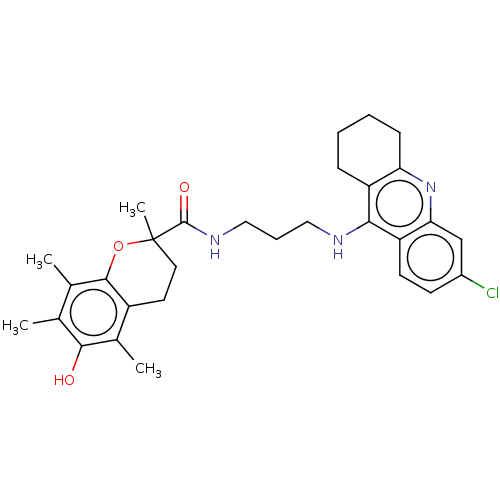
(CHEMBL3632989)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C30H36ClN3O3/c1-17-18(2)28-21(19(3)27(17)35)12-13-30(4,37-28)29(36)33-15-7-14-32-26-22-8-5-6-9-24(22)34-25-16-20(31)10-11-23(25)26/h10-11,16,35H,5-9,12-15H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50073116
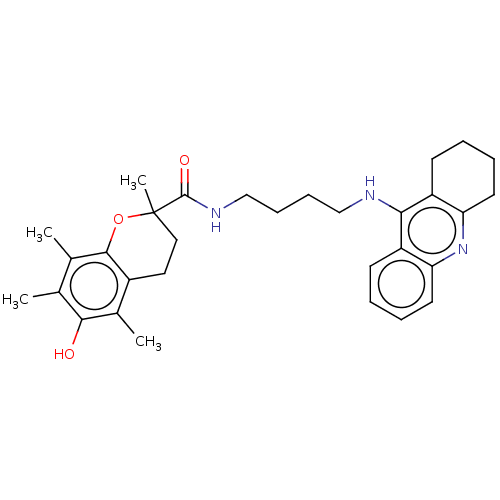
(CHEMBL3410952)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C31H39N3O3/c1-19-20(2)29-22(21(3)28(19)35)15-16-31(4,37-29)30(36)33-18-10-9-17-32-27-23-11-5-7-13-25(23)34-26-14-8-6-12-24(26)27/h5,7,11,13,35H,6,8-10,12,14-18H2,1-4H3,(H,32,34)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133421
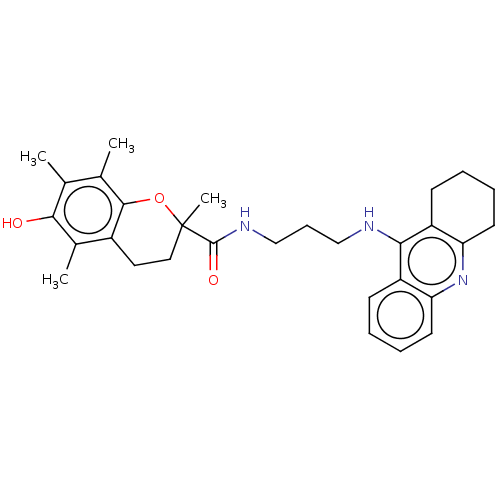
(CHEMBL3632987)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C30H37N3O3/c1-18-19(2)28-21(20(3)27(18)34)14-15-30(4,36-28)29(35)32-17-9-16-31-26-22-10-5-7-12-24(22)33-25-13-8-6-11-23(25)26/h5,7,10,12,34H,6,8-9,11,13-17H2,1-4H3,(H,31,33)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133472
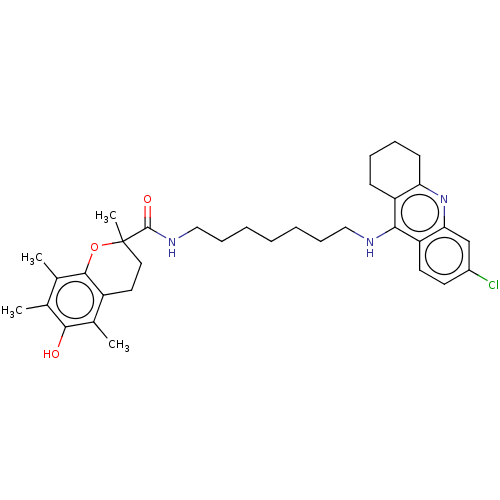
(CHEMBL3632993)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C34H44ClN3O3/c1-21-22(2)32-25(23(3)31(21)39)16-17-34(4,41-32)33(40)37-19-11-7-5-6-10-18-36-30-26-12-8-9-13-28(26)38-29-20-24(35)14-15-27(29)30/h14-15,20,39H,5-13,16-19H2,1-4H3,(H,36,38)(H,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50133469
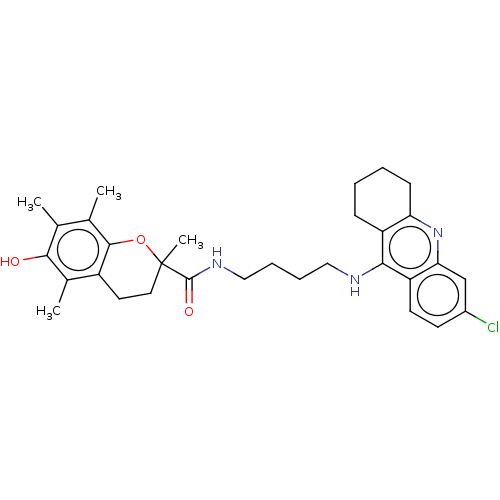
(CHEMBL3632990)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C31H38ClN3O3/c1-18-19(2)29-22(20(3)28(18)36)13-14-31(4,38-29)30(37)34-16-8-7-15-33-27-23-9-5-6-10-25(23)35-26-17-21(32)11-12-24(26)27/h11-12,17,36H,5-10,13-16H2,1-4H3,(H,33,35)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50133471
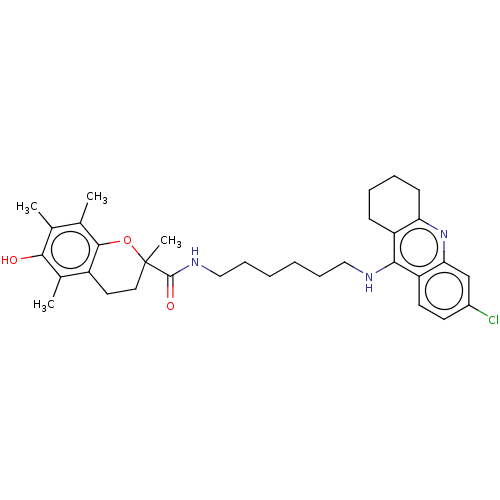
(CHEMBL3632992)Show SMILES Cc1c(C)c2OC(C)(CCc2c(C)c1O)C(=O)NCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 Show InChI InChI=1S/C33H42ClN3O3/c1-20-21(2)31-24(22(3)30(20)38)15-16-33(4,40-31)32(39)36-18-10-6-5-9-17-35-29-25-11-7-8-12-27(25)37-28-19-23(34)13-14-26(28)29/h13-14,19,38H,5-12,15-18H2,1-4H3,(H,35,37)(H,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... |
J Med Chem 58: 8985-9003 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01325
BindingDB Entry DOI: 10.7270/Q29P33GZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data