

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480493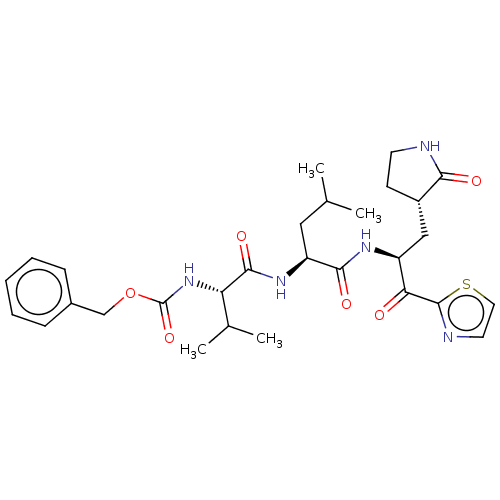 (CHEMBL539209 | acs.jmedchem.1c00409_ST.302 | jm5b0...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50453878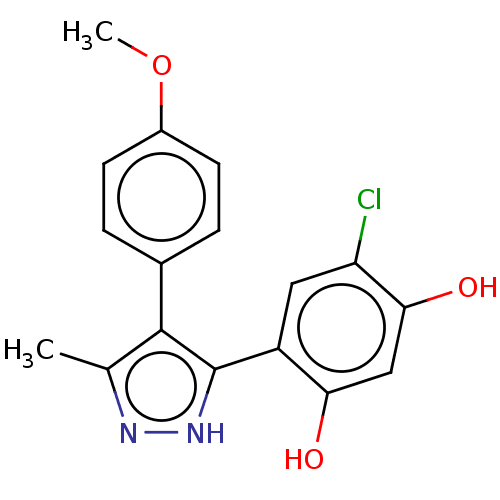 (CHEMBL371380) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Competitive inhibition of Caulobacter vibrioides full length wild type cell cycle histidine kinase CckA deltaTM Escherichia coli BL21-AI in presence ... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480488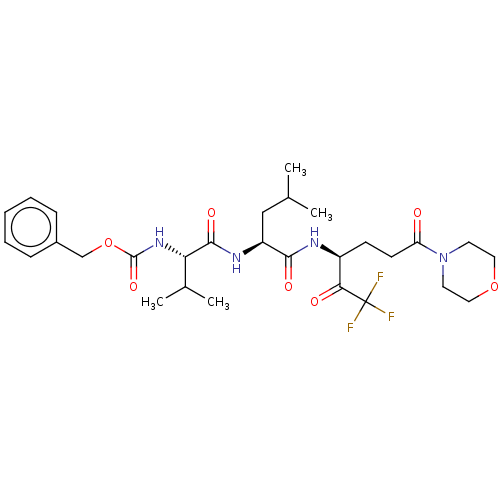 (CHEMBL555061 | acs.jmedchem.1c00409_ST.606 | med.2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480495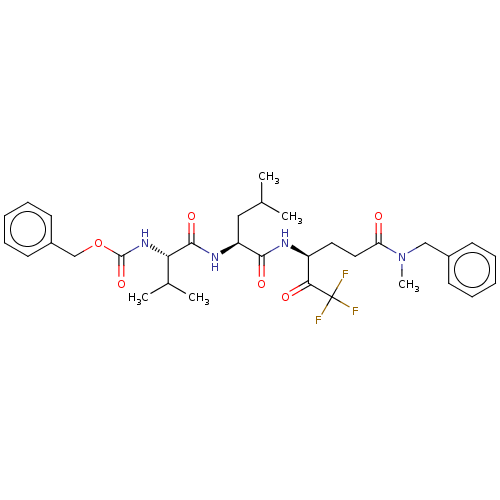 (CHEMBL555220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480499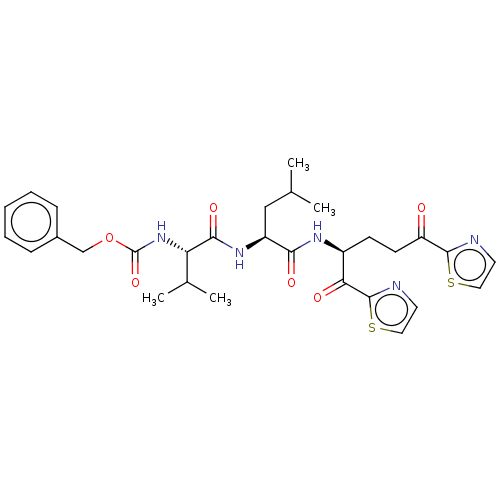 (CHEMBL541707 | acs.jmedchem.1c00409_ST.696) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480500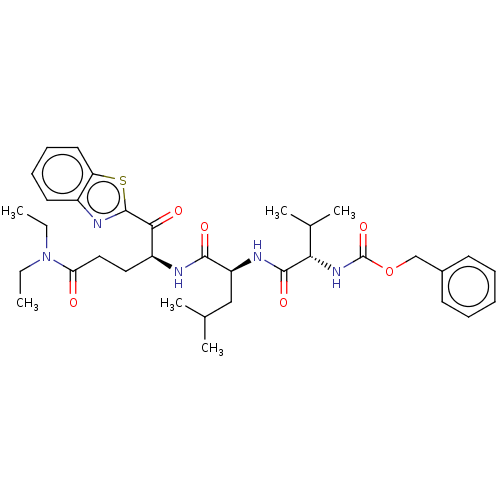 (CHEMBL538957 | acs.jmedchem.1c00409_ST.704) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480496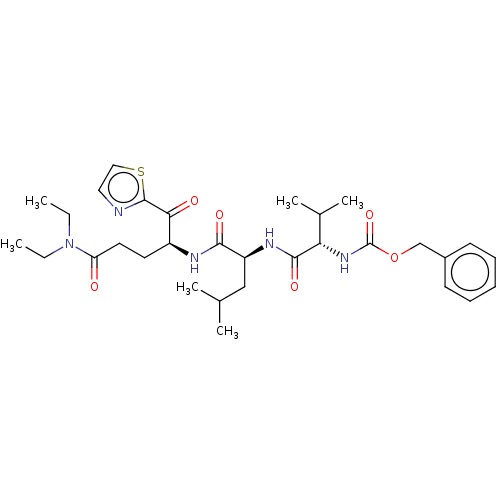 (CHEMBL555221) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480487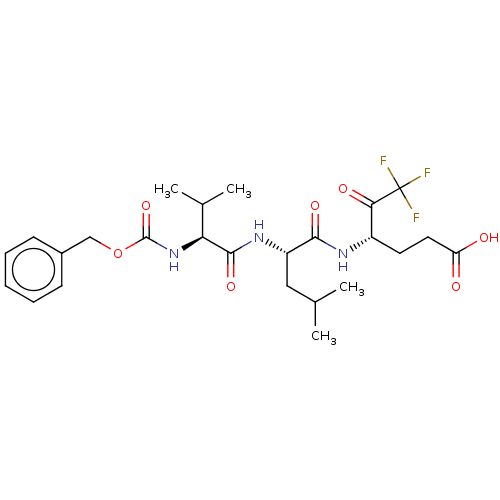 (CHEMBL551569 | acs.jmedchem.1c00409_ST.759 | med.2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480501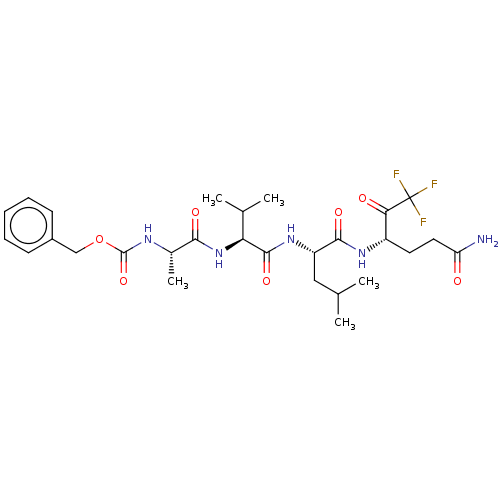 (CHEMBL553172 | acs.jmedchem.1c00409_ST.765) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480489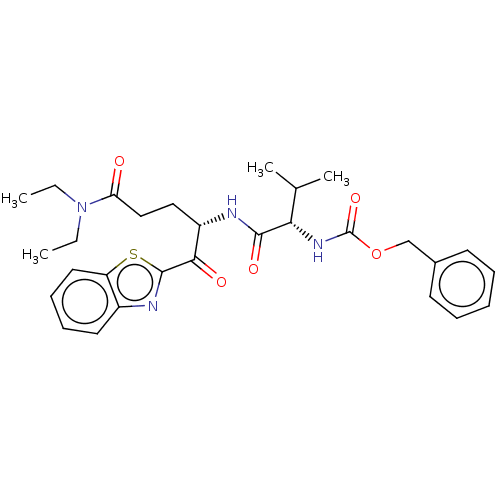 (CHEMBL557699 | acs.jmedchem.1c00409_ST.767) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480490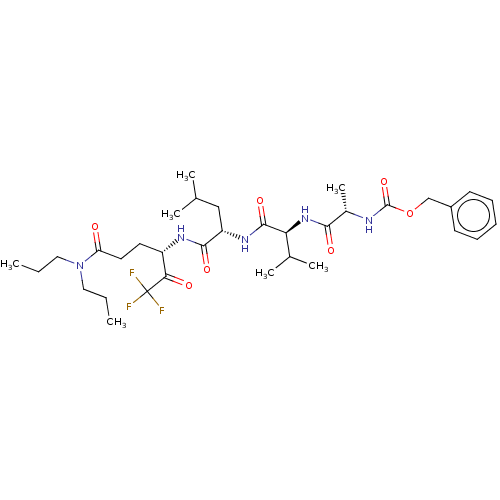 (CHEMBL537916 | acs.jmedchem.1c00409_ST.792) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480498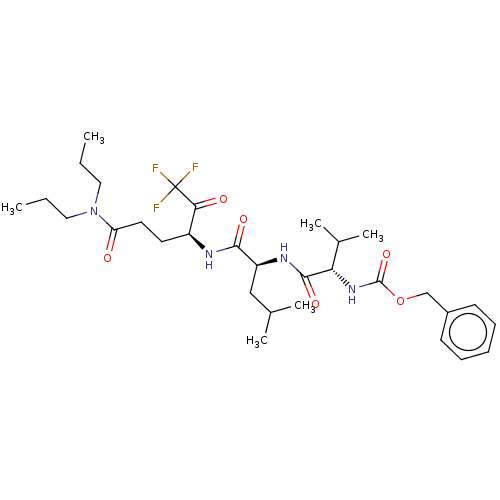 (CHEMBL539208 | acs.jmedchem.1c00409_ST.799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480494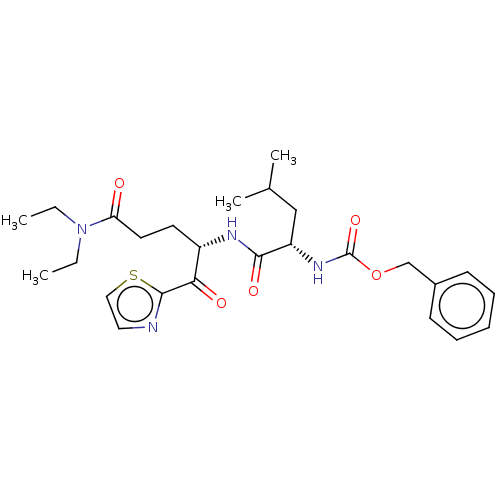 (CHEMBL551504 | acs.jmedchem.1c00409_ST.804) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480492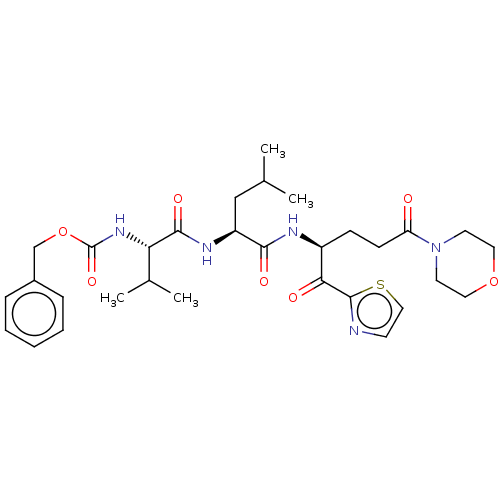 (CHEMBL555062 | acs.jmedchem.1c00409_ST.805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480491 (CHEMBL551181 | acs.jmedchem.1c00409_ST.808) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM50480497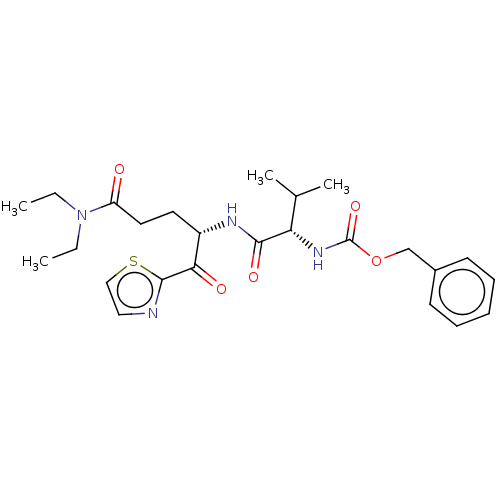 (CHEMBL557291 | acs.jmedchem.1c00409_ST.809) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of SARS coronavirus 3CL protease pretreated for 10 mins before substrate addition | Bioorg Med Chem Lett 19: 2722-7 (2009) Article DOI: 10.1016/j.bmcl.2009.03.118 BindingDB Entry DOI: 10.7270/Q2QC06BW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50277598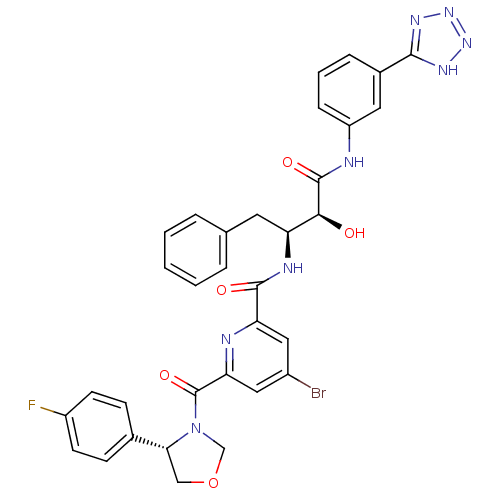 (CHEMBL453211 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50277599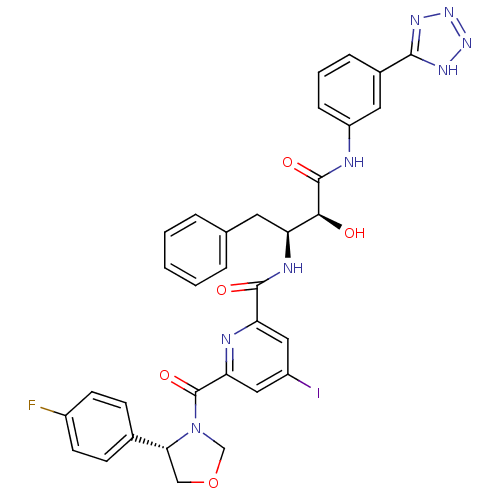 (CHEMBL507541 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50277597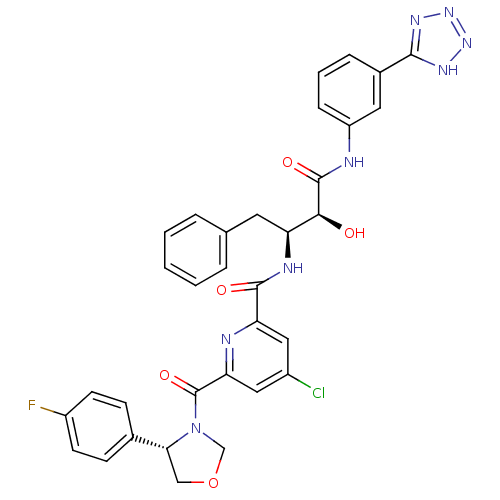 (CHEMBL501242 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50277595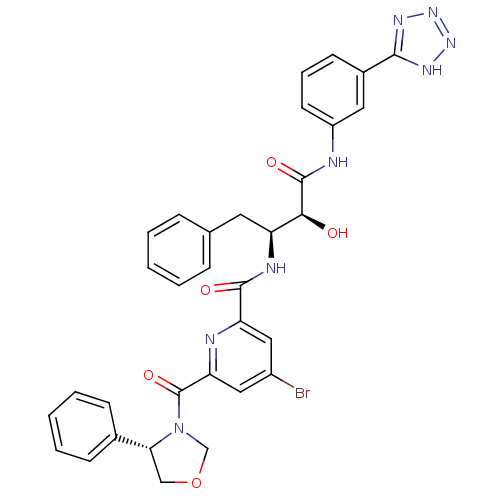 (CHEMBL445281 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50277594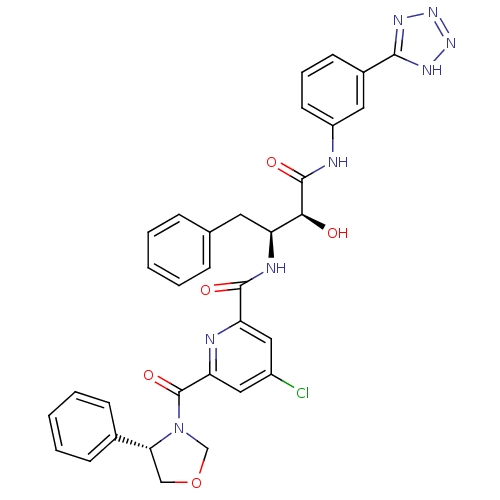 (CHEMBL448015 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50277596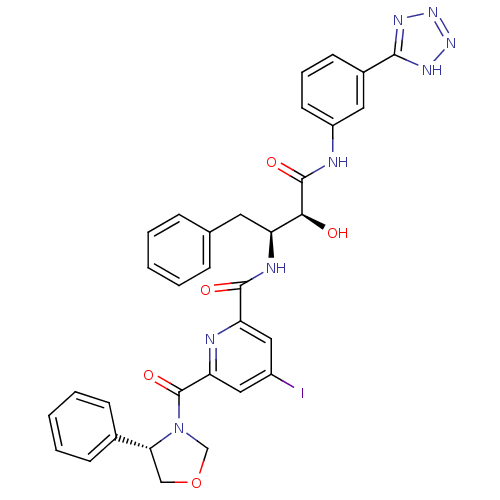 (CHEMBL504917 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50234644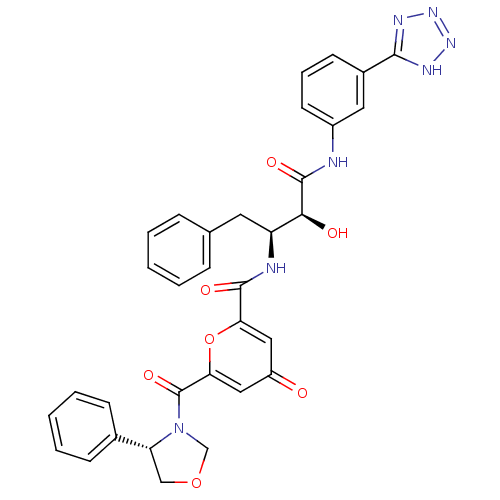 (CHEMBL400043 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50495215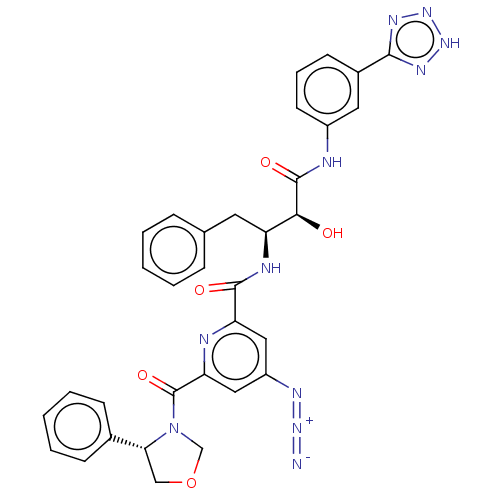 (CHEMBL3105743) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using (7-methoxycoumarin-4-yl)acetyl-SEVNL*DAEFRK(2,4-dinitrophenyl)-RR-NH2) as substrate by FRET assay | Bioorg Med Chem Lett 24: 618-23 (2014) Article DOI: 10.1016/j.bmcl.2013.12.007 BindingDB Entry DOI: 10.7270/Q2BG2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50495216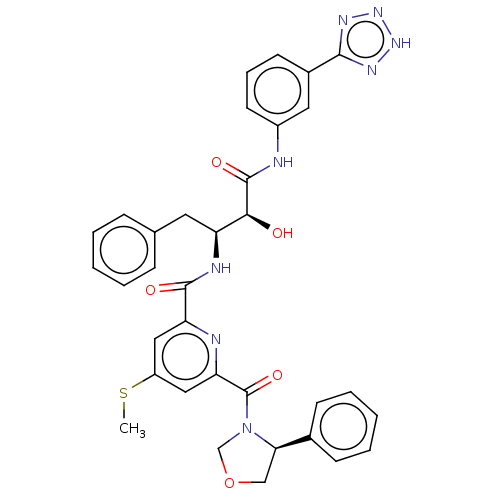 (CHEMBL3105741) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using (7-methoxycoumarin-4-yl)acetyl-SEVNL*DAEFRK(2,4-dinitrophenyl)-RR-NH2) as substrate by FRET assay | Bioorg Med Chem Lett 24: 618-23 (2014) Article DOI: 10.1016/j.bmcl.2013.12.007 BindingDB Entry DOI: 10.7270/Q2BG2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50234646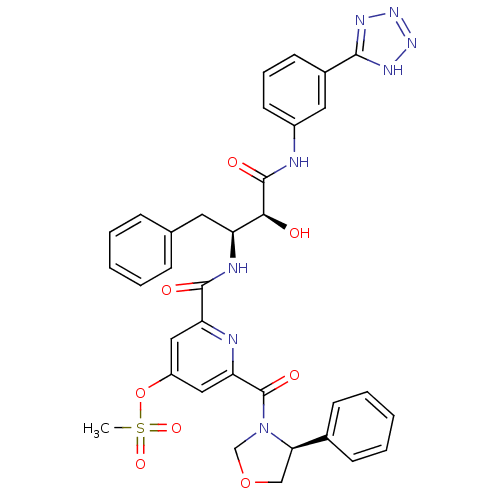 (2-(((2S,3S)-4-(3-(2H-tetrazol-5-yl)phenylamino)-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50234643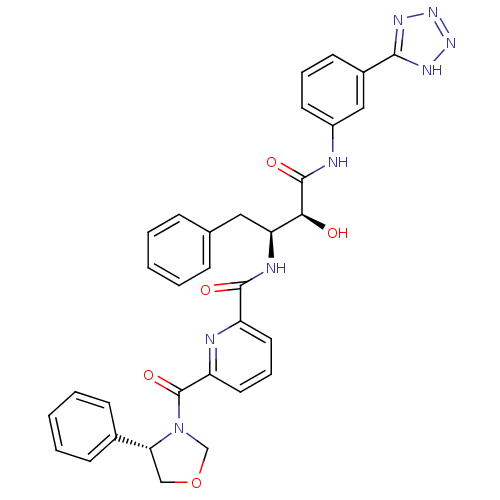 (CHEMBL399839 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50495217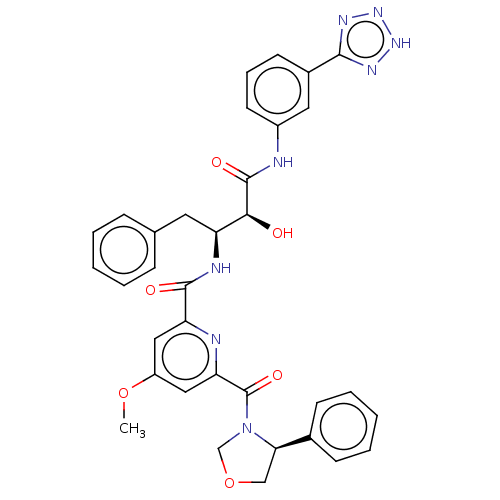 (CHEMBL3105738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Kobe Gakuin University Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using (7-methoxycoumarin-4-yl)acetyl-SEVNL*DAEFRK(2,4-dinitrophenyl)-RR-NH2) as substrate by FRET assay | Bioorg Med Chem Lett 24: 618-23 (2014) Article DOI: 10.1016/j.bmcl.2013.12.007 BindingDB Entry DOI: 10.7270/Q2BG2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50234645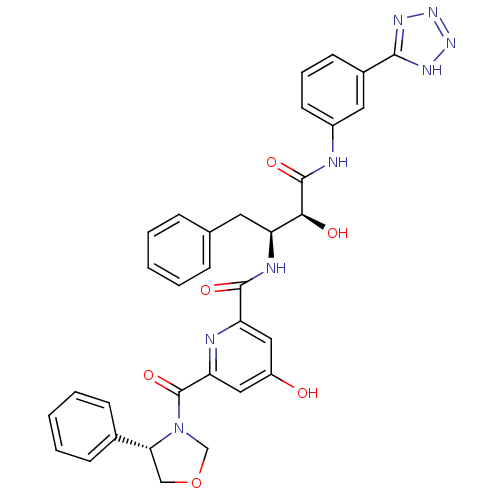 (CHEMBL253865 | N-((2S,3S)-4-(3-(2H-tetrazol-5-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human recombinant Arg235 region of BACE1 by FRET assay | Bioorg Med Chem Lett 19: 2435-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.049 BindingDB Entry DOI: 10.7270/Q27D2V1G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM20926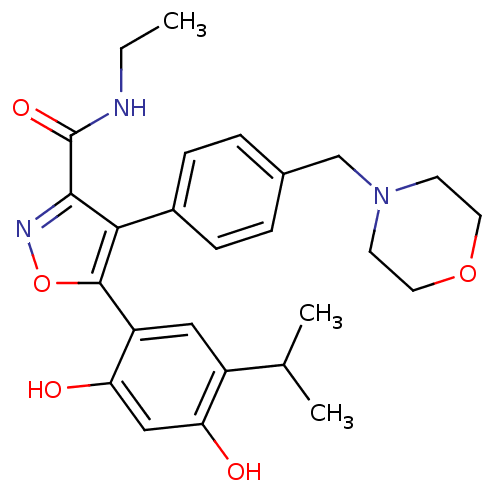 (5-[2,4-dihydroxy-5-(propan-2-yl)phenyl]-N-ethyl-4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM15364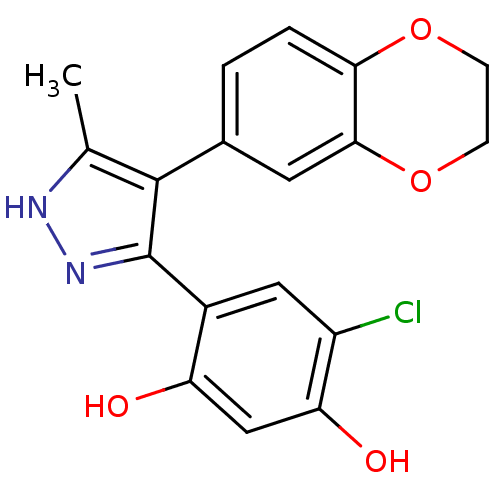 (4-chloro-6-[4-(2,3-dihydro-1,4-benzodioxin-6-yl)-5...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50453878 (CHEMBL371380) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphate regulon sensor protein PhoR (Escherichia coli (strain K12)) | BDBM50453879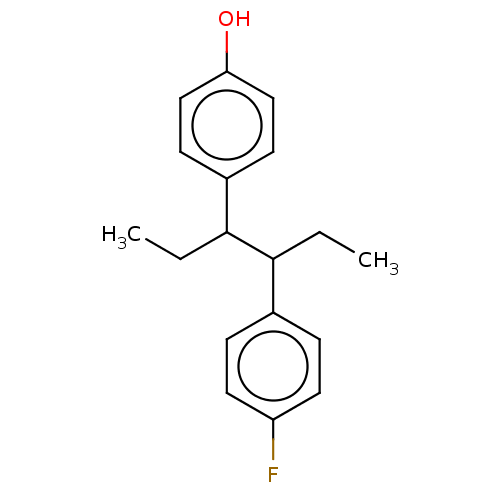 (CHEMBL4202687) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DHp-catalytic domain PhoR autophosphorylation expressed in Escherichia coli RIL in presence of gamma-32P-ATP by SDS-PA... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50409992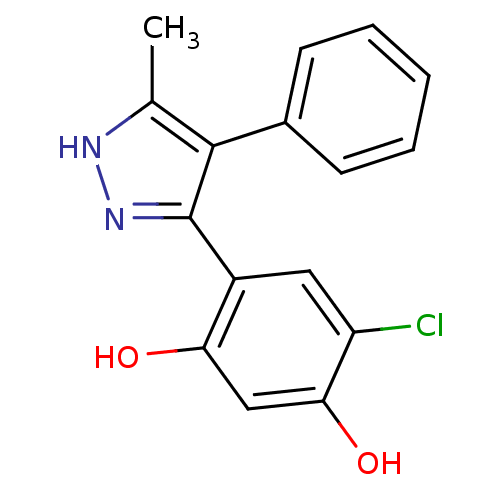 (CHEMBL364882) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50453882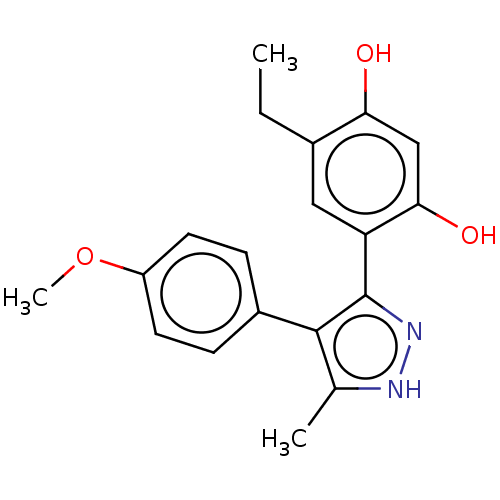 (CHEMBL4218027) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensor protein kinase WalK (Staphylococcus epidermidis (strain ATCC 35984 / RP...) | BDBM50453880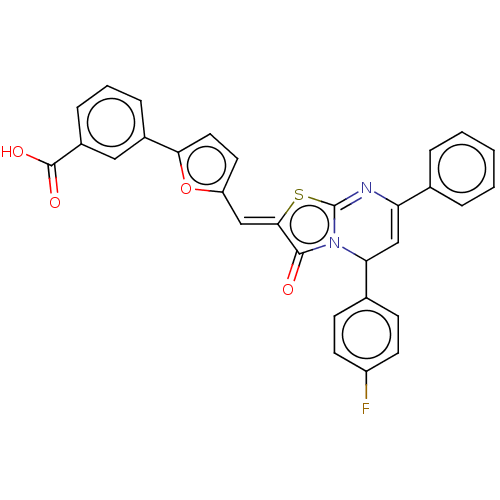 (CHEMBL3343945) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus epidermidis ATCC 35984 N-terminal GB1-tagged YycG expressed in Escherichia coli BL21 (DE3) preincubated for ... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensor protein kinase WalK (Staphylococcus epidermidis (strain ATCC 35984 / RP...) | BDBM50453886 (CHEMBL4215525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Staphylococcus epidermidis ATCC 35984 N-terminal GB1-tagged YycG expressed in Escherichia coli BL21 (DE3) preincubated for ... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50453881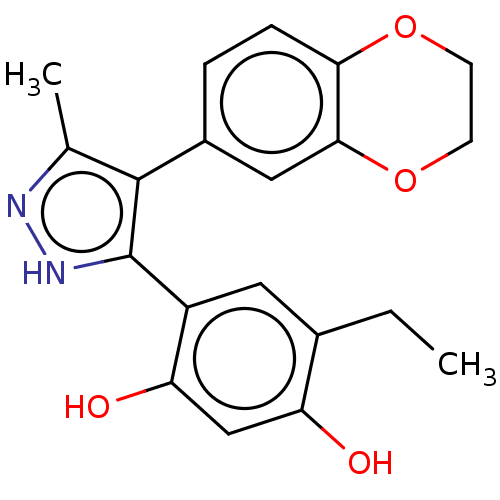 (CCT018159 | CHEBI:41656 | CHEMBL399530) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50409996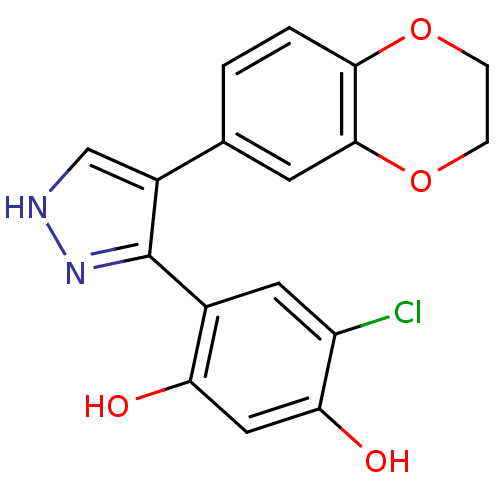 (CHEMBL191228) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50453884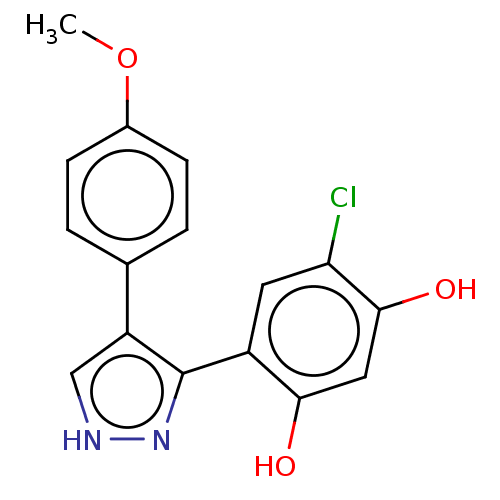 (CHEMBL4217100) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50453883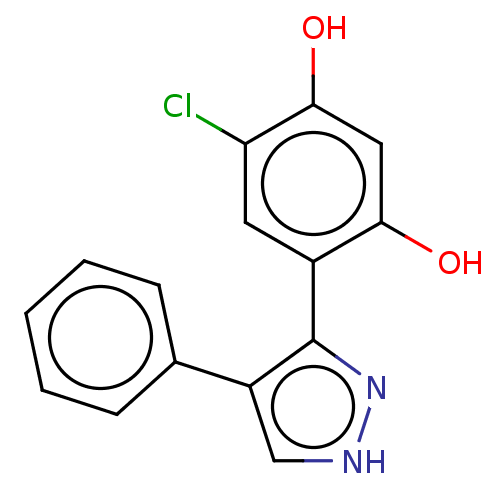 (CHEMBL4204469) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM15365 (3-(2,4-Dihydroxyphenyl)-4-(4-methoxyphenyl)-5-meth...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM50453887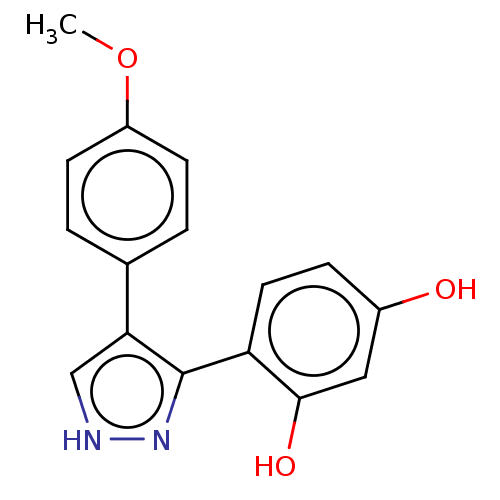 (CHEMBL1551662) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Streptococcus pneumoniae PCS8203) | BDBM50453885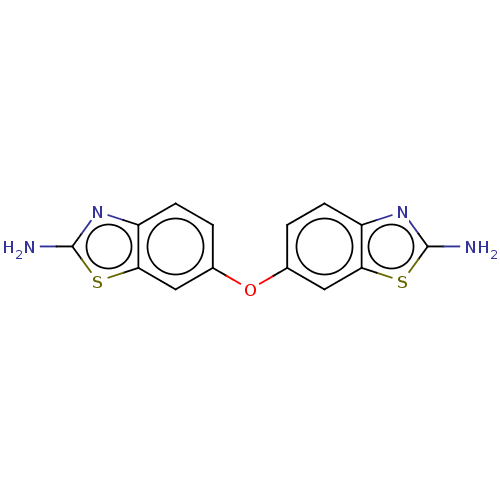 (CHEMBL4209595) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae VicK preincubated for 30 mins followed by [gamma-33P]ATP addition after 30 mins by SDS-PAGE method | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chemotaxis protein CheA (Escherichia coli (strain K12)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.11E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of Escherichia coli CheA preincubated for 30 mins followed by [gamma-33P]ATP addition after 30 mins by SDS-PAGE method | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Caulobacter vibrioides) | BDBM227589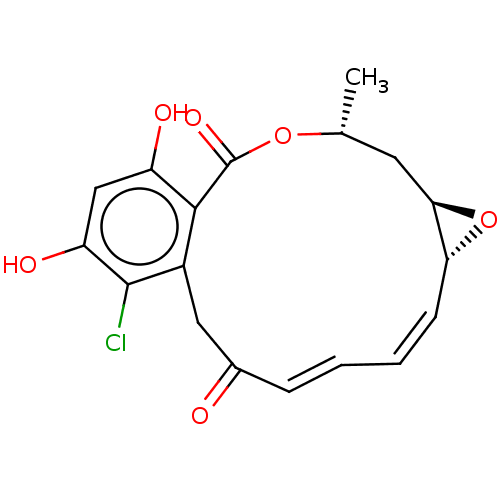 (Radicicol) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Caulobacter vibrioides cell cycle histidine kinase CckA deltaTM mutant DHp domain (70 to 691 residues) expressed in Escheri... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sensor protein kinase WalK (Staphylococcus aureus subsp. aureus CN1) | BDBM50453879 (CHEMBL4202687) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHp-catalytic domain PhoR autophosphorylation expressed in Escherichia coli RIL in presence of gamma-32P-ATP by S... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histidine kinase (Streptococcus pneumoniae PCS8203) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae VicK preincubated for 30 mins followed by [gamma-33P]ATP addition after 30 mins by SDS-PAGE method | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Virulence sensor histidine kinase PhoQ (Salmonella typhimurium (strain LT2 / SGSC1412 / AT...) | BDBM50453878 (CHEMBL371380) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Salmonella typhimurium N-terminal His6-SUMO-tagged DHp-catalytic domain PhoQ (257 to 487 residues) autophosphorylation expr... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Virulence sensor histidine kinase PhoQ (Salmonella typhimurium (strain LT2 / SGSC1412 / AT...) | BDBM50453881 (CCT018159 | CHEBI:41656 | CHEMBL399530) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Williams College Curated by ChEMBL | Assay Description Inhibition of recombinant Salmonella typhimurium N-terminal His6-SUMO-tagged DHp-catalytic domain PhoQ (257 to 487 residues) autophosphorylation expr... | Bioorg Med Chem Lett 27: 5235-5244 (2017) Article DOI: 10.1016/j.bmcl.2017.10.036 BindingDB Entry DOI: 10.7270/Q20G3NQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |