

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc. Curated by ChEMBL | Assay Description Evaluated for binding affinity against alpha-1 adrenergic receptor | J Med Chem 43: 2770-4 (2000) Article DOI: 10.1021/jm990578n BindingDB Entry DOI: 10.7270/Q23R0WMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM69602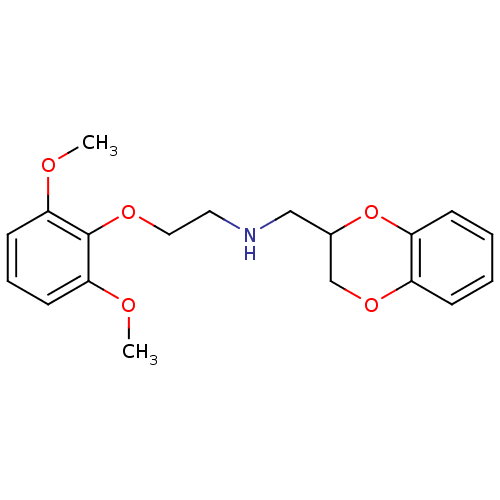 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc. Curated by ChEMBL | Assay Description Evaluated for binding affinity against alpha-1 adrenergic receptor | J Med Chem 43: 2770-4 (2000) Article DOI: 10.1021/jm990578n BindingDB Entry DOI: 10.7270/Q23R0WMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc. Curated by ChEMBL | Assay Description Evaluated for binding affinity against alpha-1 adrenergic receptor | J Med Chem 43: 2770-4 (2000) Article DOI: 10.1021/jm990578n BindingDB Entry DOI: 10.7270/Q23R0WMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc. Curated by ChEMBL | Assay Description Evaluated for binding affinity against alpha-1 adrenergic receptor | J Med Chem 43: 2770-4 (2000) Article DOI: 10.1021/jm990578n BindingDB Entry DOI: 10.7270/Q23R0WMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039664 (CHEMBL91890 | N-Biphenyl-4-yl-N-[(carbamoylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50472725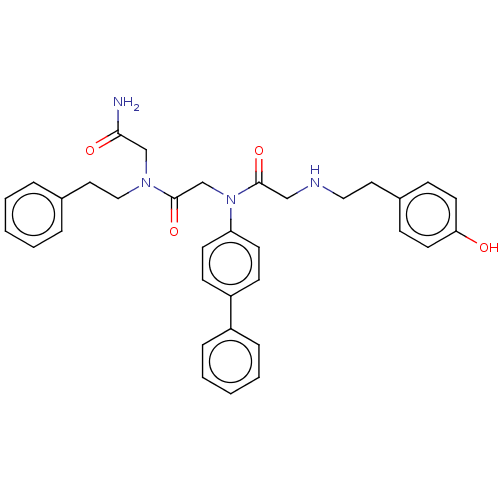 (CHEMBL90588 | CHIR-2279) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc. Curated by ChEMBL | Assay Description Evaluated for binding affinity against alpha-1 adrenergic receptor | J Med Chem 43: 2770-4 (2000) Article DOI: 10.1021/jm990578n BindingDB Entry DOI: 10.7270/Q23R0WMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039665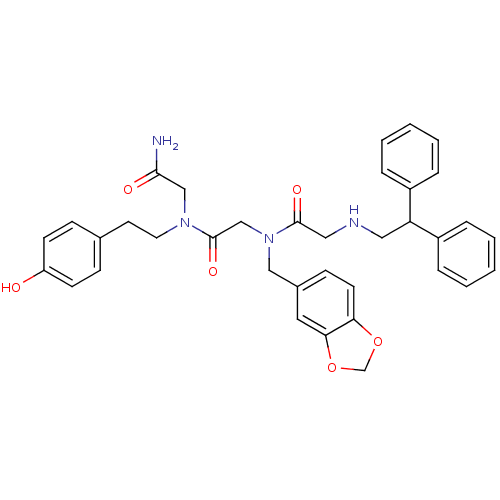 (CHEMBL90649 | CHIR-4531 | N-Benzo[1,3]dioxol-5-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGO | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50019056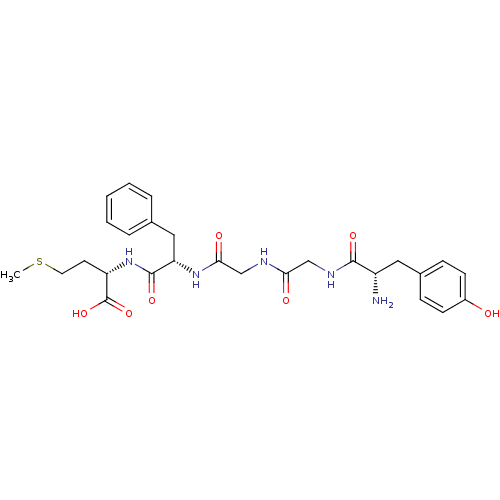 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039661 (CHEMBL327549 | CHIR-4537 | N-({Carbamoylmethyl-[2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGO | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039663 (CHEMBL89378 | CHIR-4534 | N-({Carbamoylmethyl-[2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from rat brain membrane using [3H]DAMGO | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50472726 (CHEMBL92380 | CHIR-2283) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc. Curated by ChEMBL | Assay Description Evaluated for binding affinity against alpha-1 adrenergic receptor | J Med Chem 43: 2770-4 (2000) Article DOI: 10.1021/jm990578n BindingDB Entry DOI: 10.7270/Q23R0WMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50472727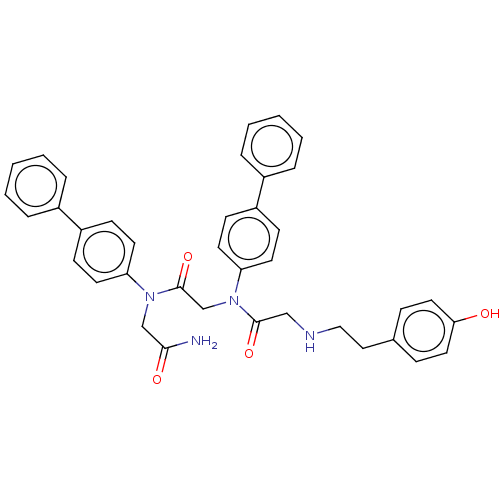 (CHEMBL92379 | CHIR-2276) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc. Curated by ChEMBL | Assay Description Evaluated for binding affinity against alpha-1 adrenergic receptor | J Med Chem 43: 2770-4 (2000) Article DOI: 10.1021/jm990578n BindingDB Entry DOI: 10.7270/Q23R0WMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50029050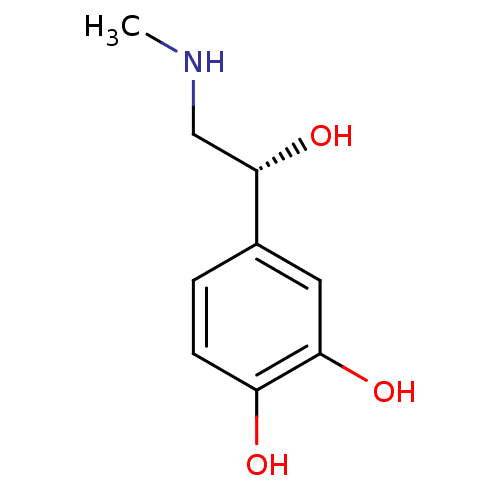 ((-)-(R)-epinephrine | (-)-3,4-dihydroxy-alpha-((me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50029051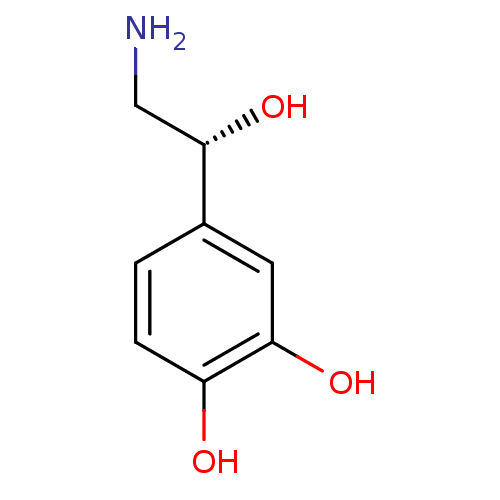 ((-)-arterenol | (-)-noradrenaline | (-)-norepineph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chiron Corporation Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards mu-specific opiate receptor from combinatorial peptoid library | J Med Chem 37: 2678-85 (1994) BindingDB Entry DOI: 10.7270/Q2P26ZSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281579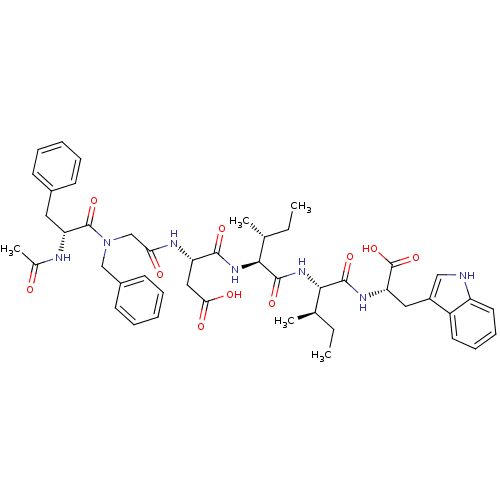 ((S)-3-{2-[((R)-2-Acetylamino-3-phenyl-propionyl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50281586 ((R)-3-{[(R)-1-((R)-2-Acetylamino-3-phenyl-propiony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The inhibitory constant of the compound was evaluated against Endothelin A receptor | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281576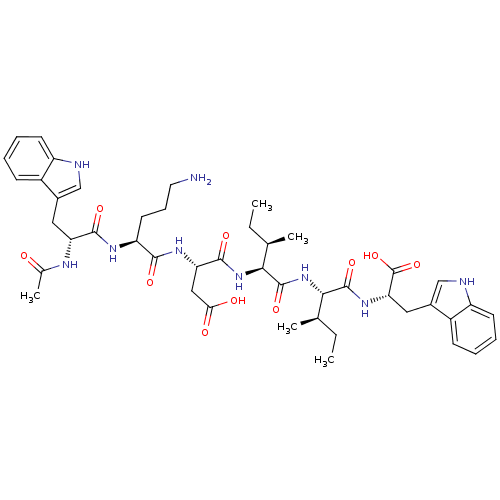 ((S)-3-{(S)-2-[(R)-2-Acetylamino-3-(1H-indol-3-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281577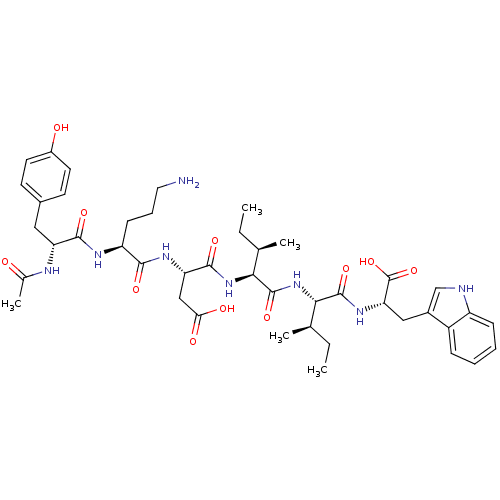 ((S)-3-{(S)-2-[(R)-2-Acetylamino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50281579 ((S)-3-{2-[((R)-2-Acetylamino-3-phenyl-propionyl)-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The inhibitory constant of the compound was evaluated against Endothelin A receptor | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281586 ((R)-3-{[(R)-1-((R)-2-Acetylamino-3-phenyl-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281580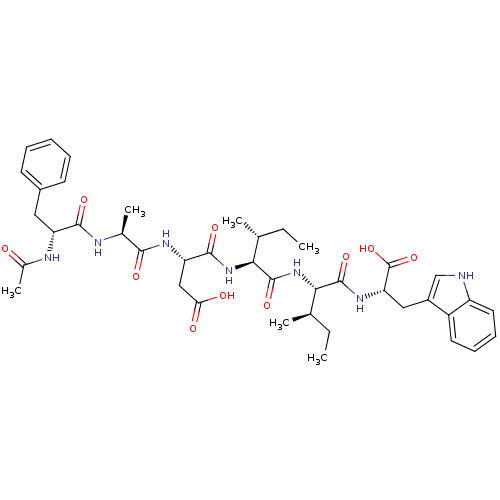 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281581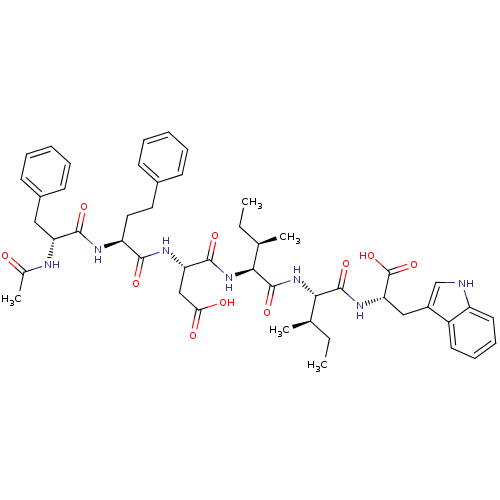 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281582 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281575 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281585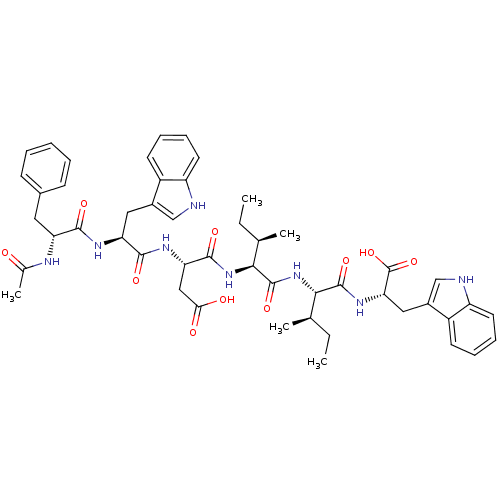 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281587 ((S)-3-[(R)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281583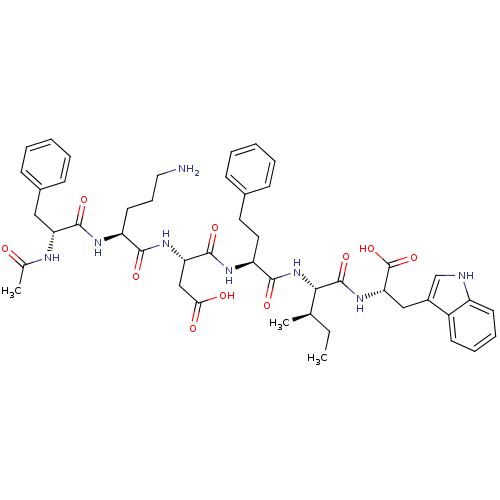 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50281576 ((S)-3-{(S)-2-[(R)-2-Acetylamino-3-(1H-indol-3-yl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The inhibitory constant of the compound was evaluated against Endothelin A receptor in rabbit renal artery tissue | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281910 ((S)-3-[(S)-5-((S)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound for Endothelin A receptor was determined in murine 3T3 cells | Bioorg Med Chem Lett 3: 1253-1256 (1993) Article DOI: 10.1016/S0960-894X(00)80326-1 BindingDB Entry DOI: 10.7270/Q2ZC82S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281584 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the binding affinity against eEndothelin A receptor in murine 3T3 cells using [125I]-ET-1 as a radioligand | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281911 ((R)-3-[(R)-5-((S)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound for Endothelin A receptor was determined in murine 3T3 cells | Bioorg Med Chem Lett 3: 1253-1256 (1993) Article DOI: 10.1016/S0960-894X(00)80326-1 BindingDB Entry DOI: 10.7270/Q2ZC82S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281578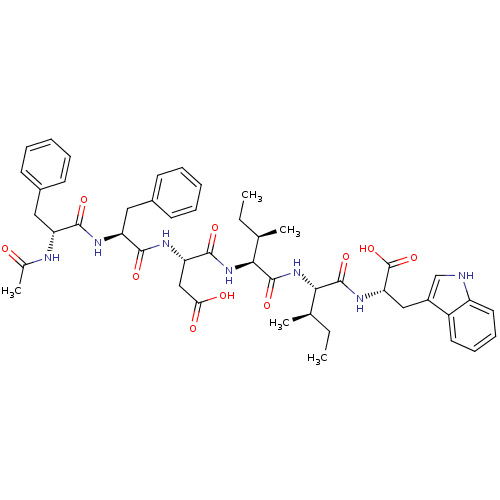 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-ET-1 binding to Endothelin A receptor of murine 3T3 cells | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50281577 ((S)-3-{(S)-2-[(R)-2-Acetylamino-3-(4-hydroxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The inhibitory constant of the compound was evaluated against Endothelin A receptor | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50281584 ((S)-3-[(S)-2-((R)-2-Acetylamino-3-phenyl-propionyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | <800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The inhibitory constant was evaluated against Endothelin A receptor | Bioorg Med Chem Lett 3: 519-524 (1993) Article DOI: 10.1016/S0960-894X(01)81219-1 BindingDB Entry DOI: 10.7270/Q27081C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Mus musculus) | BDBM50281912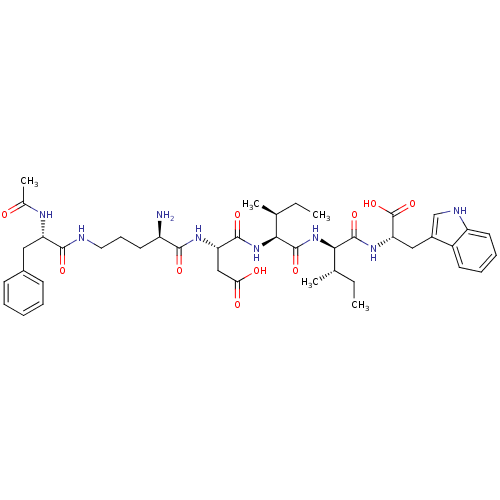 ((S)-3-[(R)-5-((S)-2-Acetylamino-3-phenyl-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound for Endothelin A receptor was determined in murine 3T3 cells | Bioorg Med Chem Lett 3: 1253-1256 (1993) Article DOI: 10.1016/S0960-894X(00)80326-1 BindingDB Entry DOI: 10.7270/Q2ZC82S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||