

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50403068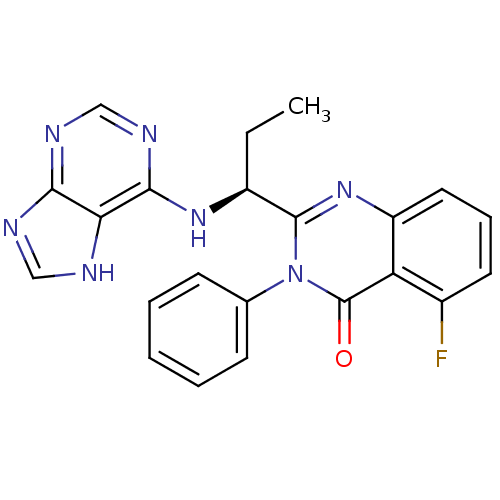 (CHEMBL2216870 | IDELALISIB | US9745321, CAL-101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kdelta (unknown origin) by Selectscreen kinase assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574856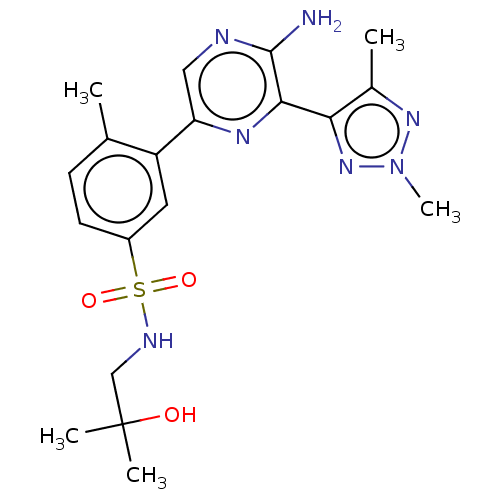 (CHEMBL4864109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574850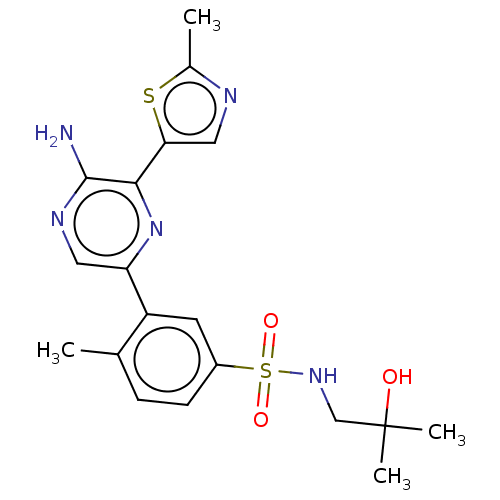 (CHEMBL4857826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574845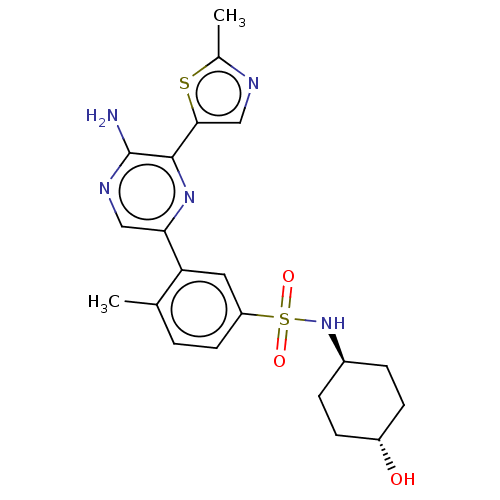 (CHEMBL4866794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574856 (CHEMBL4864109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574848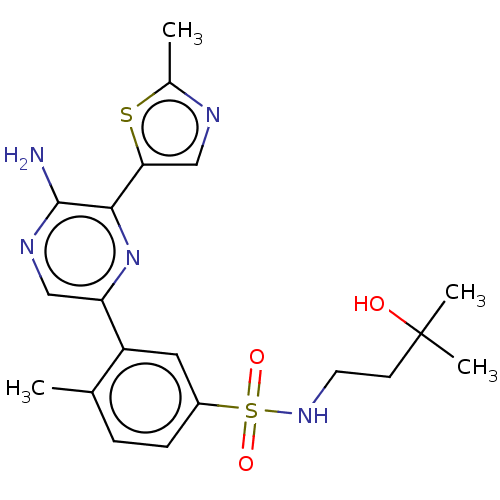 (CHEMBL4850655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50395821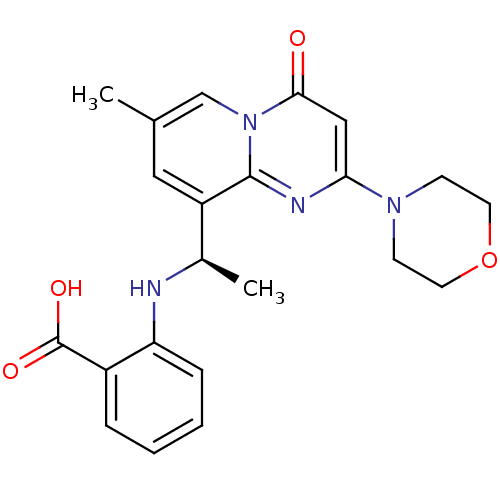 (CHEMBL2165191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human PI3Kbeta assessed as reduction in PIP3 formation by AlphaScreen assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1539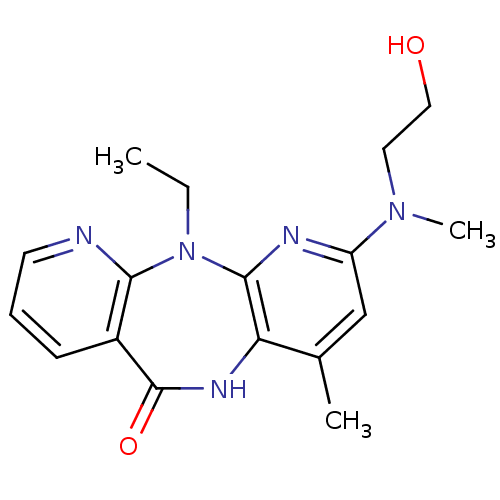 (2-ethyl-5-[(2-hydroxyethyl)(methyl)amino]-7-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1528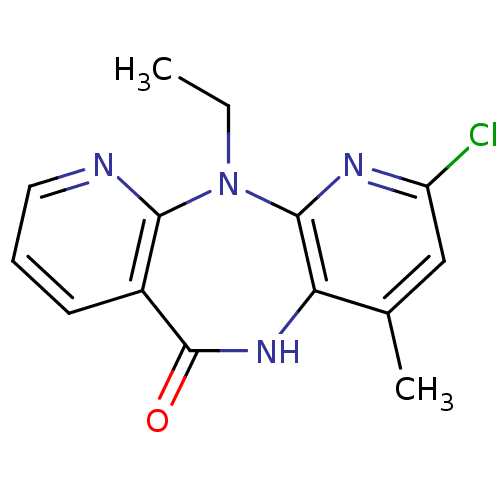 (5-chloro-2-ethyl-7-methyl-2,4,9,15-tetraazatricycl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM295061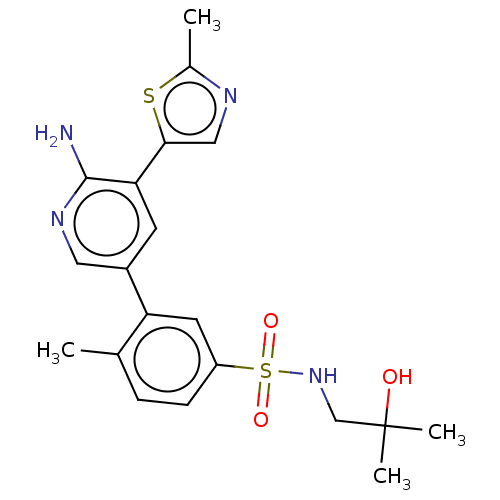 (3-[6-Amino-5-(2-methyl-thiazol-5-yl)-pyridin-3-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574847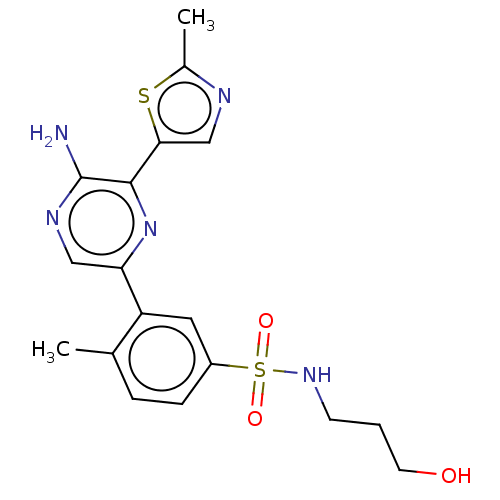 (CHEMBL4855904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574849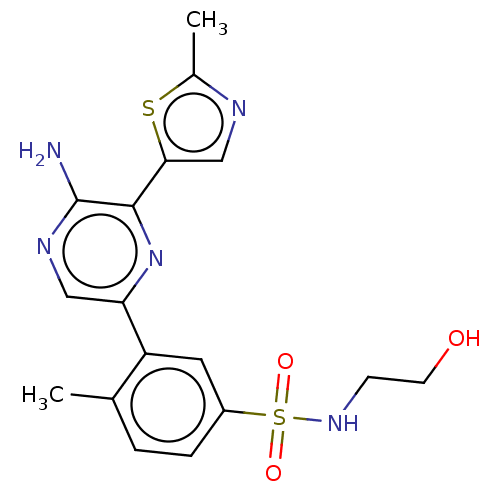 (CHEMBL4863328) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574854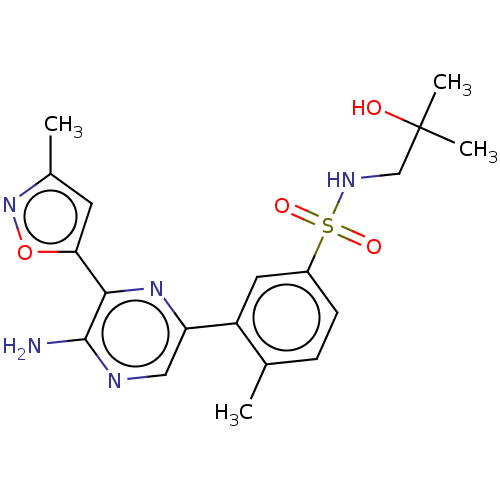 (CHEMBL4849042) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574845 (CHEMBL4866794) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574860 (CHEMBL4859017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2008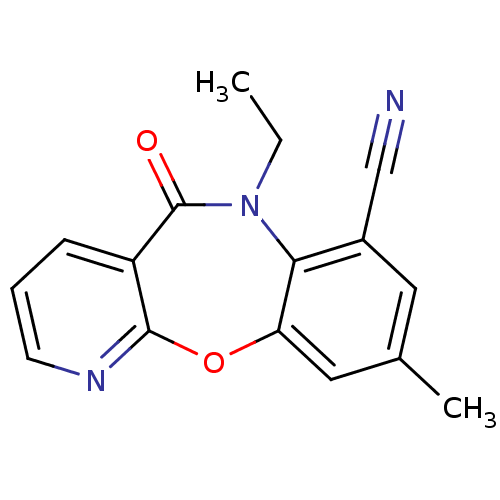 (10-ethyl-14-methyl-9-oxo-2-oxa-4,10-diazatricyclo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1520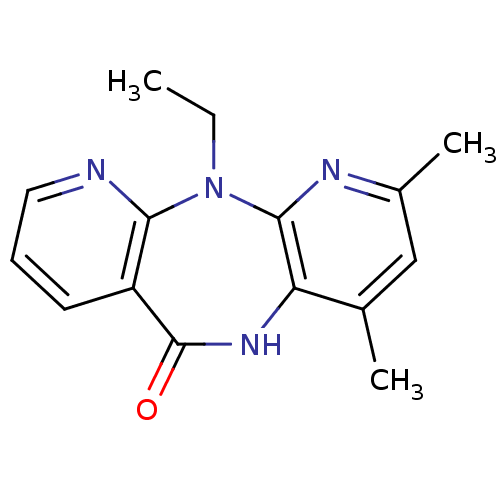 (2-ethyl-5,7-dimethyl-2,4,9,15-tetraazatricyclo[9.4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1526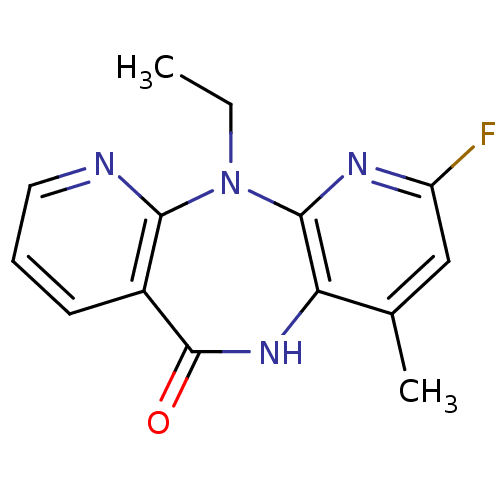 (2-ethyl-5-fluoro-7-methyl-2,4,9,15-tetraazatricycl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1527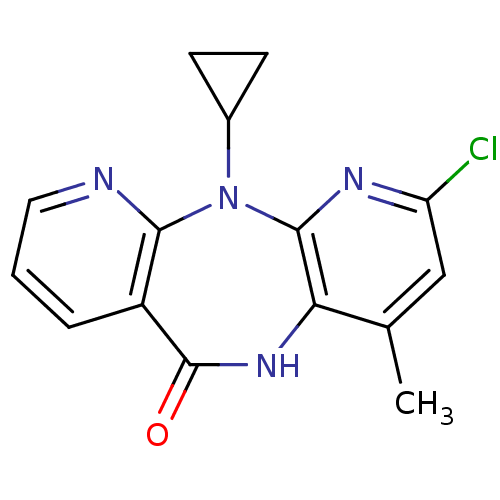 (5-chloro-2-cyclopropyl-7-methyl-2,4,9,15-tetraazat...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1987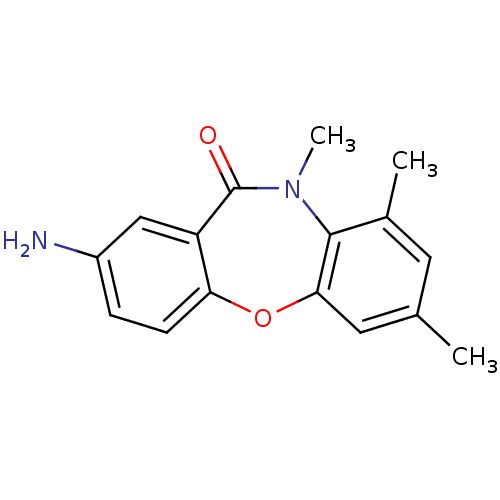 (13-amino-5,7,9-trimethyl-2-oxa-9-azatricyclo[9.4.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1549 (2-ethyl-7-methyl-5-(methylsulfanyl)-2,4,9,15-tetra...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1562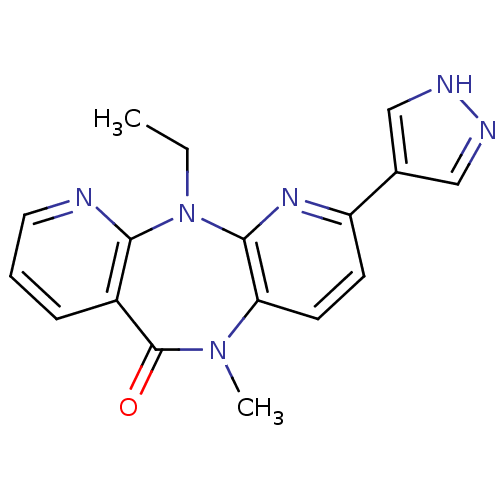 (2-ethyl-9-methyl-5-(1H-pyrazol-4-yl)-2,4,9,15-tetr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1540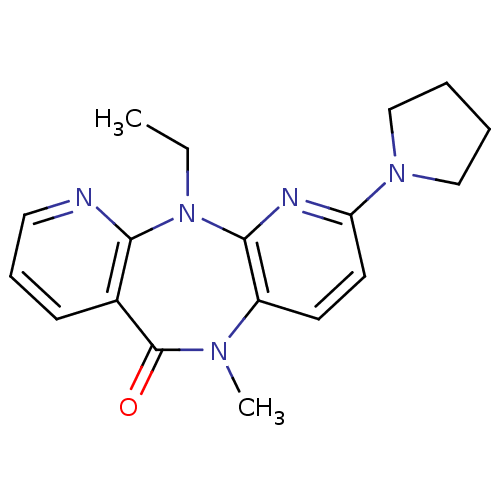 (2-ethyl-9-methyl-5-(pyrrolidin-1-yl)-2,4,9,15-tetr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2012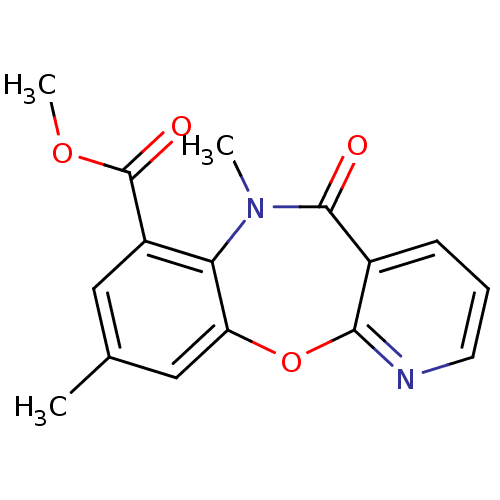 (Pyridobenzoxazepinone 80 | methyl 10,14-dimethyl-9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2010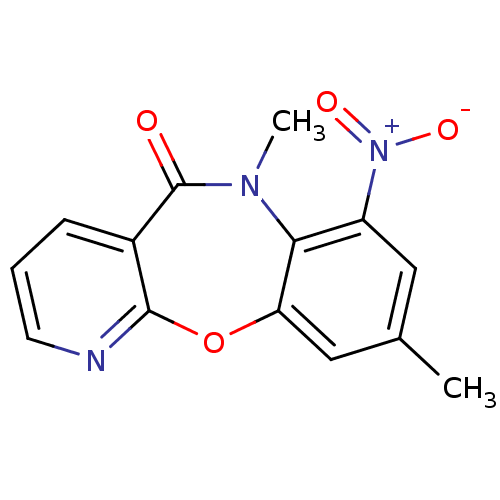 (10,14-dimethyl-12-nitro-2-oxa-4,10-diazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM295051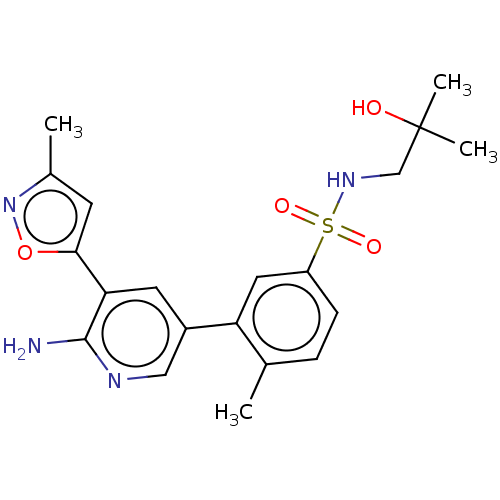 (3-(6-Amino-5-(3-methylisoxazol-5-yl)pyridin-3-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574847 (CHEMBL4855904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574860 (CHEMBL4859017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574851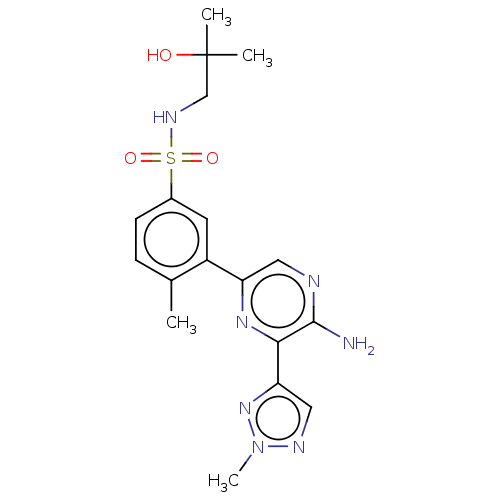 (CHEMBL4848126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574846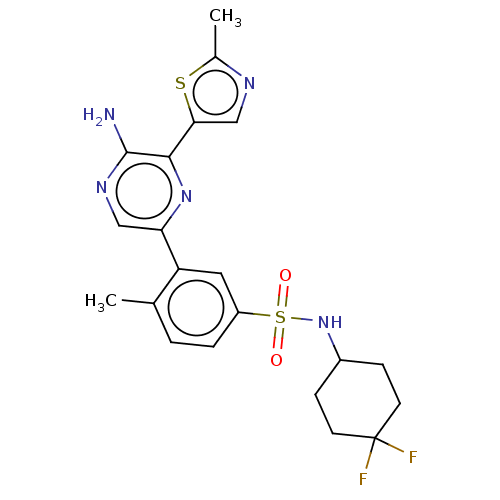 (CHEMBL4867254) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2003 (6-amino-10-ethyl-12,14-dimethyl-2-oxa-4,10-diazatr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574850 (CHEMBL4857826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma in human U937 cells assessed as reduction in AKT phosphorylation preincubated for 30 mins followed by MIP1alpha stimulation f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574855 (CHEMBL4878811) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2004 (10-ethyl-12,14-dimethyl-2-oxa-4,10-diazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1531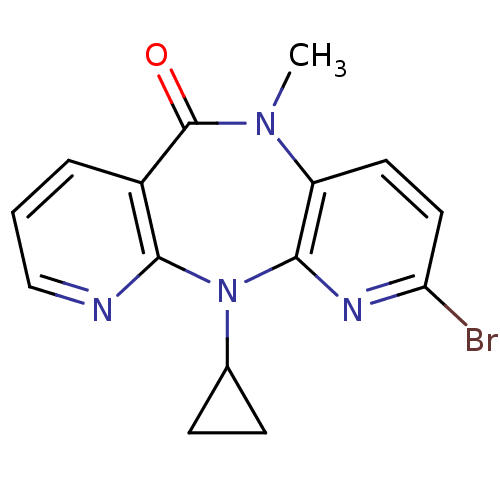 (2-Bromo-11-cyclopropyl-5-methyl-5,11-dihydro-6H-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1979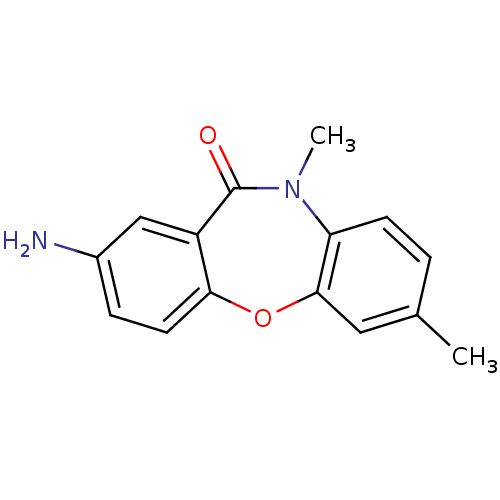 (13-amino-5,9-dimethyl-2-oxa-9-azatricyclo[9.4.0.0^...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1553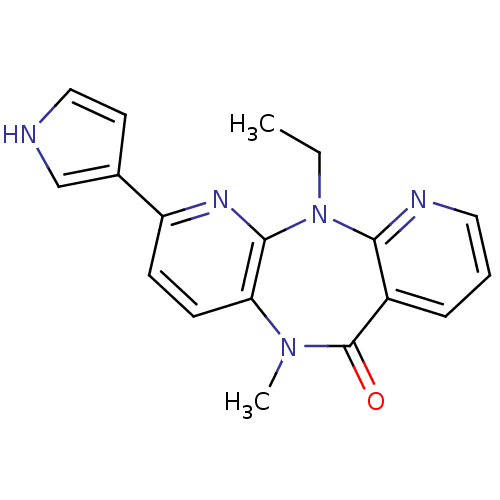 (2-ethyl-9-methyl-5-(1H-pyrrol-3-yl)-2,4,9,15-tetra...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2006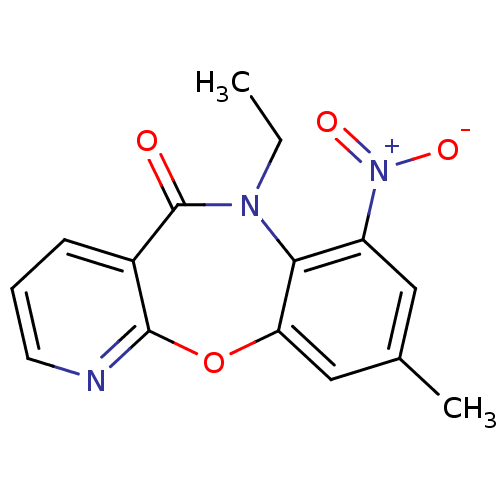 (10-ethyl-14-methyl-12-nitro-2-oxa-4,10-diazatricyc...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50574856 (CHEMBL4864109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kalpha (unknown origin) using L-alpha-phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by Kinase Glo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1517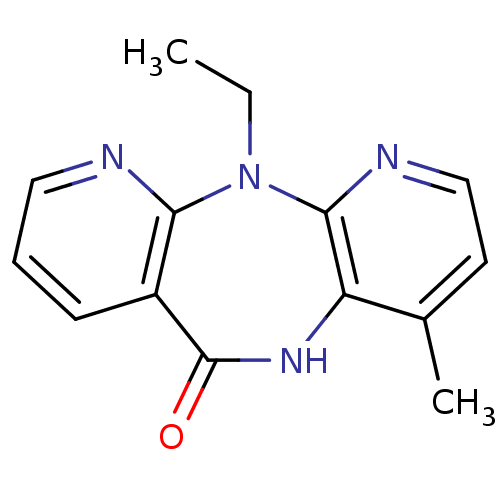 (2-ethyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50574847 (CHEMBL4855904) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kalpha (unknown origin) using L-alpha-phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by Kinase Glo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574852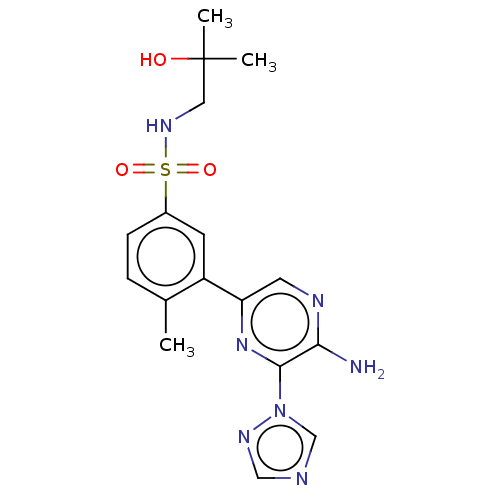 (CHEMBL4868011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1646 (2-ethyl-10,12,13-trimethyl-2,4,10-triazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50574853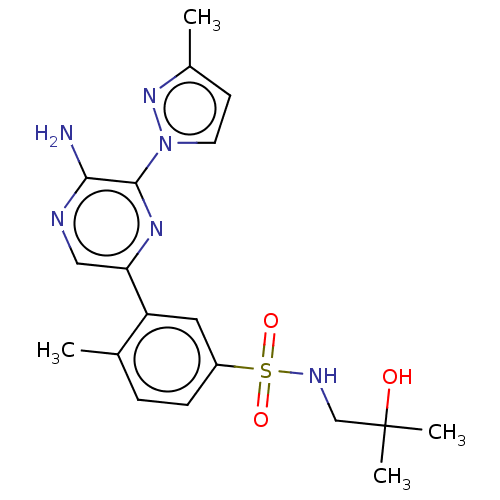 (CHEMBL4878721) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (Homo sapiens (Human)) | BDBM50574846 (CHEMBL4867254) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kalpha (unknown origin) using L-alpha-phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by Kinase Glo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1639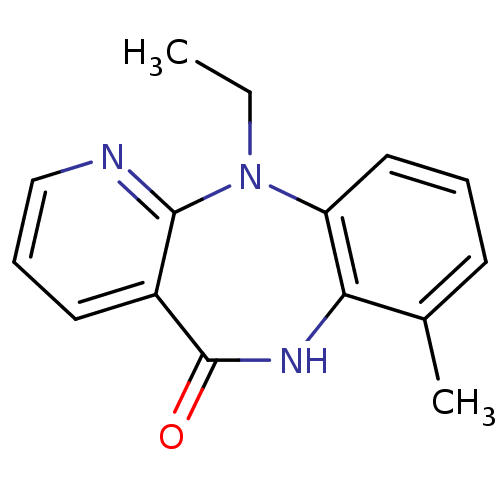 (2-ethyl-12-methyl-2,4,10-triazatricyclo[9.4.0.0^{3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM295056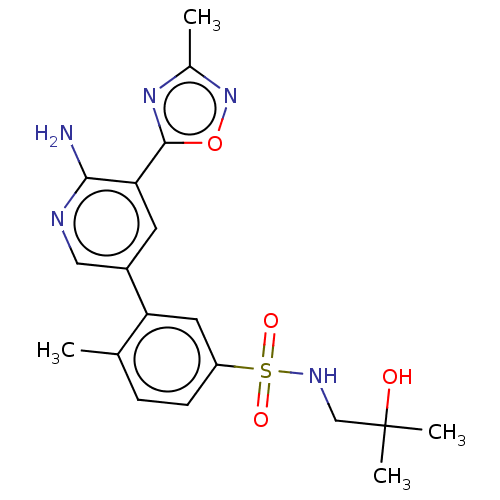 (3-(6-Amino-5-(3-methyl-1,2,4-oxadiazol-5-yl)pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM295050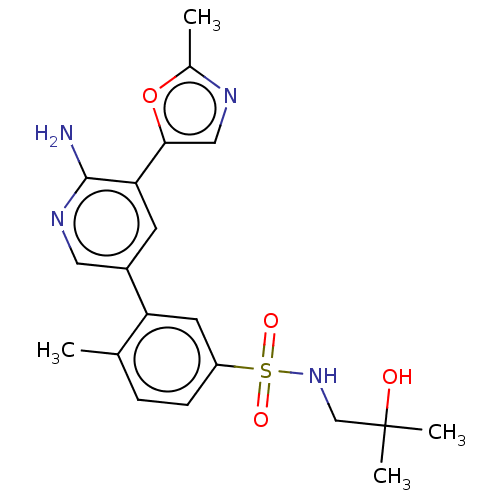 (3-(6-Amino-5-(2-methyloxazol-5-yl)pyridin-3-yl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PI3Kgamma (unknown origin) using phosphatidylinositol as substrate incubated for 30 to 60 mins in presence of ATP by TR-FRET based Adap... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00986 BindingDB Entry DOI: 10.7270/Q27M0CRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1517 (2-ethyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 25 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 38: 4830-8 (1995) Article DOI: 10.1021/jm00024a010 BindingDB Entry DOI: 10.7270/Q23B5XBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1644 (2-ethyl-10,13,14-trimethyl-2,4,10-triazatricyclo[9...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 34: 2231-41 (1991) Article DOI: 10.1021/jm00111a045 BindingDB Entry DOI: 10.7270/Q2TT4P4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 303 total ) | Next | Last >> |