 Found 25703 hits with Last Name = 'ho' and Initial = 'e'
Found 25703 hits with Last Name = 'ho' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50170654
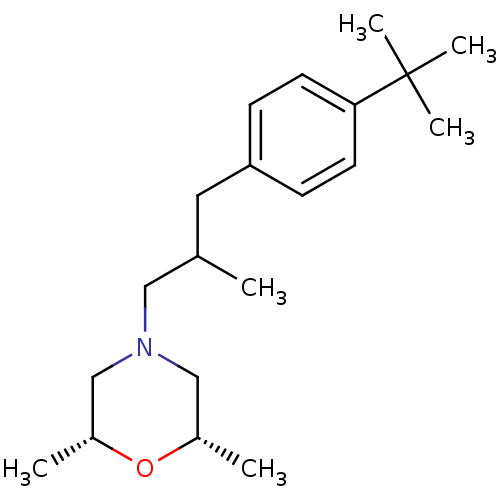
((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...)Show SMILES CC(CN1C[C@H](C)O[C@H](C)C1)Cc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3/t15?,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin School of Medicine and Public Health
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in guinea pig brain microsomes |
Bioorg Med Chem 18: 4397-404 (2010)
Article DOI: 10.1016/j.bmc.2010.04.078
BindingDB Entry DOI: 10.7270/Q20C4VZT |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM209097
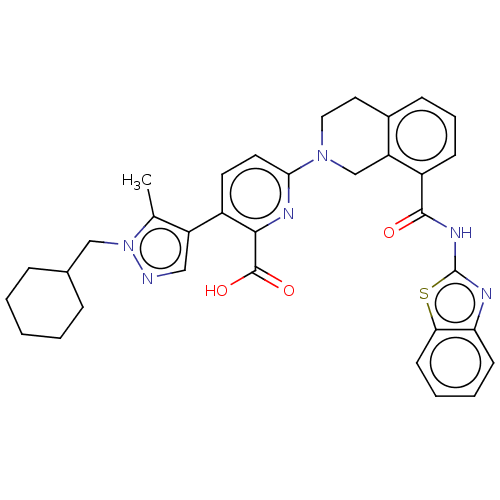
(US9266877, 43)Show SMILES Cc1c(cnn1CC1CCCCC1)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C34H34N6O3S/c1-21-26(18-35-40(21)19-22-8-3-2-4-9-22)24-14-15-30(37-31(24)33(42)43)39-17-16-23-10-7-11-25(27(23)20-39)32(41)38-34-36-28-12-5-6-13-29(28)44-34/h5-7,10-15,18,22H,2-4,8-9,16-17,19-20H2,1H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

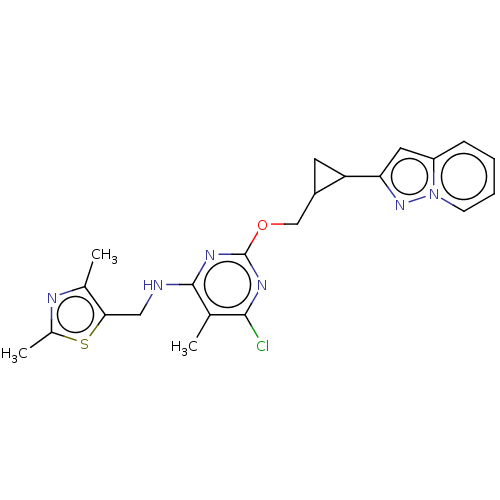
(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105
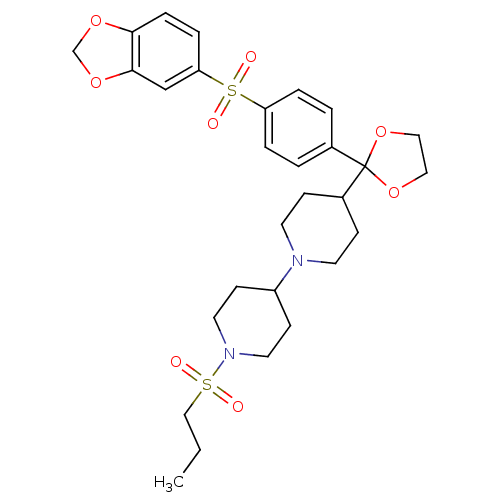
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 11: 2311-4 (2001)
BindingDB Entry DOI: 10.7270/Q28S4P7Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105

(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Galanin receptor type 1
(Homo sapiens (Human)) | BDBM50273370
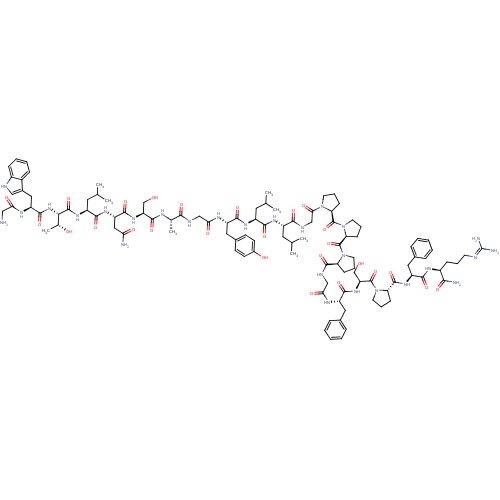
(CHEMBL503473 | GWTLNSAGYLLGPPPGFSPFR-CONH2 | Galan...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN)[C@@H](C)O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:106.112,4.4,8.12,32.33,37.40,43.48,51.56,63.76,wD:149.157,138.145,134.142,125.131,114.119,99.104,92.96,16.25,59.60,79.83,(16.53,-9.13,;15.19,-9.9,;15.2,-11.44,;13.86,-9.13,;12.52,-9.9,;11.19,-9.13,;11.19,-7.59,;12.53,-6.82,;9.86,-6.82,;9.86,-5.28,;11.2,-4.51,;12.52,-5.28,;11.2,-2.97,;8.13,-7.82,;6.39,-6.82,;6.39,-5.28,;4.66,-7.83,;4.66,-9.36,;5.99,-10.13,;5.99,-11.67,;7.32,-12.44,;8.66,-11.67,;9.99,-12.44,;8.66,-10.13,;7.33,-9.36,;2.92,-6.82,;2.92,-5.28,;4.26,-4.52,;1.19,-4.28,;1.19,-2.75,;2.52,-1.97,;3.86,-2.74,;2.52,-.43,;3.86,.34,;1.2,.34,;1.19,1.88,;2.53,2.65,;-.14,2.65,;-.14,4.19,;1.19,4.96,;-1.48,1.88,;-2.81,2.65,;-2.81,4.19,;-4.14,1.88,;-5.48,2.64,;-5.47,4.19,;-6.81,4.96,;-4.14,4.96,;-4.15,.34,;-5.48,-.43,;-6.81,.34,;-5.48,-1.97,;-6.81,-2.74,;-8.14,-1.97,;-8.14,-.43,;-9.47,-2.74,;-4.14,-2.74,;-4.14,-4.28,;-5.48,-5.05,;-2.81,-5.05,;-2.81,-6.59,;-1.48,-7.36,;-.15,-6.59,;-1.48,-8.9,;-2.85,-9.59,;-4.14,-8.76,;-4.22,-7.22,;-5.71,-6.82,;-6.55,-8.12,;-8.07,-8.35,;-8.62,-9.79,;-7.65,-10.99,;-6.13,-10.75,;-5.58,-9.31,;-.14,-9.67,;1.19,-8.9,;1.19,-7.36,;2.53,-9.67,;2.53,-11.21,;-1.47,-4.28,;-.15,-5.05,;-1.47,-2.74,;12.53,-11.44,;13.86,-12.21,;11.19,-12.21,;11.19,-13.75,;12.53,-14.52,;13.86,-13.75,;12.53,-16.06,;11.28,-16.96,;11.76,-18.43,;13.29,-18.43,;13.77,-16.97,;15.24,-16.49,;15.64,-15,;16.65,-17.91,;16.41,-19.42,;17.78,-20.13,;18.87,-19.04,;18.18,-17.67,;19.08,-15.88,;20.62,-15.8,;18.31,-14.55,;16.78,-14.38,;16.46,-12.88,;17.8,-12.12,;18.94,-13.14,;20.31,-12.44,;21.65,-13.21,;20.31,-10.9,;21.64,-10.13,;22.97,-10.89,;24.3,-10.12,;22.98,-12.43,;24.31,-13.2,;24.32,-14.74,;25.65,-15.5,;25.66,-17.04,;26.99,-17.81,;28.33,-17.04,;28.32,-15.5,;26.99,-14.73,;25.64,-12.43,;25.64,-10.89,;26.98,-13.19,;28.31,-12.42,;29.65,-13.18,;30.98,-12.41,;28.31,-10.88,;26.97,-10.11,;29.64,-10.1,;31.05,-10.72,;32.07,-9.57,;31.3,-8.24,;29.8,-8.57,;28.51,-7.72,;27.17,-8.47,;28.53,-6.17,;29.88,-5.42,;31.2,-6.21,;32.54,-5.45,;32.56,-3.91,;33.91,-3.16,;35.23,-3.95,;35.21,-5.49,;33.87,-6.24,;29.9,-3.88,;31.24,-3.12,;28.57,-3.1,;28.59,-1.55,;29.93,-.8,;29.95,.74,;28.63,1.53,;28.65,3.07,;29.99,3.82,;30.01,5.36,;31.32,3.03,;27.27,-.77,;27.29,.77,;25.92,-1.52,)| Show InChI InChI=1S/C107H153N27O26/c1-57(2)42-70(123-93(147)71(43-58(3)4)124-95(149)74(47-64-33-35-66(138)36-34-64)121-86(141)52-115-91(145)60(7)118-100(154)78(55-135)128-98(152)77(49-84(109)139)125-94(148)72(44-59(5)6)127-103(157)89(61(8)137)130-99(153)76(119-85(140)50-108)48-65-51-114-68-27-16-15-26-67(65)68)92(146)117-54-88(143)131-38-20-31-82(131)105(159)134-41-21-32-83(134)106(160)133-40-18-29-80(133)101(155)116-53-87(142)120-73(45-62-22-11-9-12-23-62)96(150)129-79(56-136)104(158)132-39-19-30-81(132)102(156)126-75(46-63-24-13-10-14-25-63)97(151)122-69(90(110)144)28-17-37-113-107(111)112/h9-16,22-27,33-36,51,57-61,69-83,89,114,135-138H,17-21,28-32,37-50,52-56,108H2,1-8H3,(H2,109,139)(H2,110,144)(H,115,145)(H,116,155)(H,117,146)(H,118,154)(H,119,140)(H,120,142)(H,121,141)(H,122,151)(H,123,147)(H,124,149)(H,125,148)(H,126,156)(H,127,157)(H,128,152)(H,129,150)(H,130,153)(H4,111,112,113)/t60-,61+,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Binding affinity to human GalR1 |
J Med Chem 53: 1871-5 (2010)
Article DOI: 10.1021/jm9018349
BindingDB Entry DOI: 10.7270/Q26D5TXJ |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM162
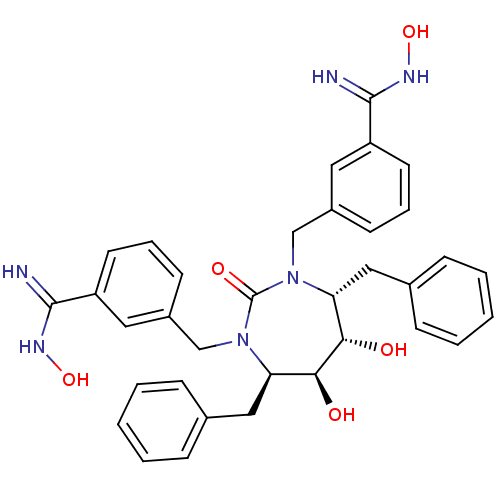
(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...)Show SMILES ONC(=N)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=N)NO)C2=O)c1 Show InChI InChI=1S/C35H38N6O5/c36-33(38-45)27-15-7-13-25(17-27)21-40-29(19-23-9-3-1-4-10-23)31(42)32(43)30(20-24-11-5-2-6-12-24)41(35(40)44)22-26-14-8-16-28(18-26)34(37)39-46/h1-18,29-32,42-43,45-46H,19-22H2,(H2,36,38)(H2,37,39)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company
| |
J Med Chem 39: 3514-25 (1996)
Article DOI: 10.1021/jm9602571
BindingDB Entry DOI: 10.7270/Q2ZW1J3M |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM177
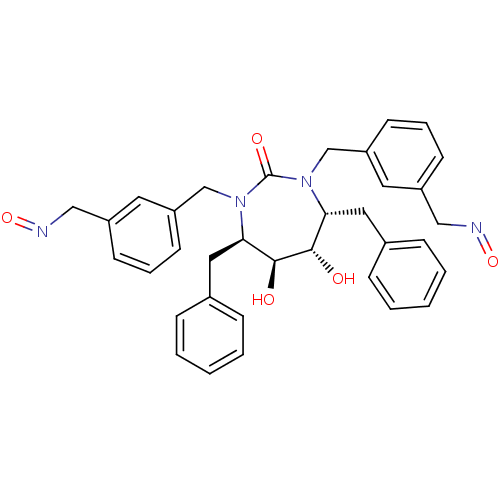
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(CN=O)c2)C(=O)N(Cc2cccc(CN=O)c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H36N4O5/c40-33-31(19-25-9-3-1-4-10-25)38(23-29-15-7-13-27(17-29)21-36-43)35(42)39(24-30-16-8-14-28(18-30)22-37-44)32(34(33)41)20-26-11-5-2-6-12-26/h1-18,31-34,40-41H,19-24H2/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company
| Assay Description
Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... |
J Med Chem 39: 3514-25 (1996)
Article DOI: 10.1021/jm9602571
BindingDB Entry DOI: 10.7270/Q2ZW1J3M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM299749

(4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...)Show SMILES Cn1cnc(c1)-c1cnc(N)c(OCC2CCN(CC2)c2nc(OCC3(CC3)C#N)nc(n2)C(=O)NC23CC(C2)C3)c1 Show InChI InChI=1S/C29H34N10O3/c1-38-13-21(33-17-38)20-8-22(23(31)32-12-20)41-14-18-2-6-39(7-3-18)26-34-24(25(40)37-29-9-19(10-29)11-29)35-27(36-26)42-16-28(15-30)4-5-28/h8,12-13,17-19H,2-7,9-11,14,16H2,1H3,(H2,31,32)(H,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50162797
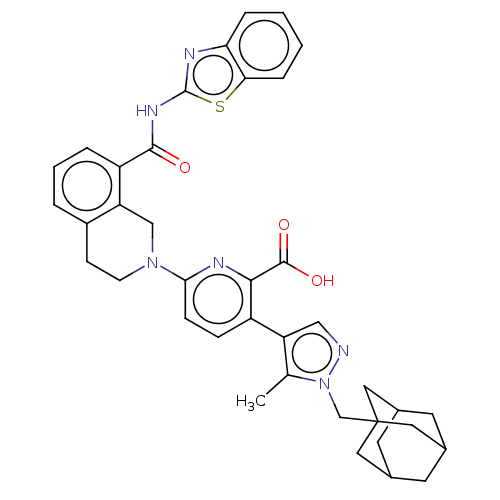
(CHEMBL3793424)Show SMILES Cc1c(cnn1CC12CC3CC(CC(C3)C1)C2)-c1ccc(nc1C(O)=O)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 |TLB:14:9:16:12.13.15,14:13:16:10.9.8,THB:12:11:8:14.13.15,12:13:10.11.16:8| Show InChI InChI=1S/C38H38N6O3S/c1-22-29(19-39-44(22)21-38-16-23-13-24(17-38)15-25(14-23)18-38)27-9-10-33(41-34(27)36(46)47)43-12-11-26-5-4-6-28(30(26)20-43)35(45)42-37-40-31-7-2-3-8-32(31)48-37/h2-10,19,23-25H,11-18,20-21H2,1H3,(H,46,47)(H,40,42,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50561528
(CHEMBL4762875)Show SMILES COC12CC3CC(CC(Cn4ncc(c4C)-c4ccc(nc4C(O)=O)N4CCc5cccc(C(=O)Nc6nc7ccccc7s6)c5C4)(C3)C1)C2 |TLB:1:2:5.4.47:7,5:6:4.3.47:48,THB:5:4:6.49.7:48,3:4:7:49.2.48,3:2:5.4.47:7| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00568
BindingDB Entry DOI: 10.7270/Q2542S8D |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50170654

((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...)Show SMILES CC(CN1C[C@H](C)O[C@H](C)C1)Cc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3/t15?,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin School of Medicine and Public Health
Curated by ChEMBL
| Assay Description
Displacement of (+)-[3H]pentazocine from sigma 1 receptor in guinea pig liver microsomes |
Bioorg Med Chem 18: 4397-404 (2010)
Article DOI: 10.1016/j.bmc.2010.04.078
BindingDB Entry DOI: 10.7270/Q20C4VZT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM299902

(6-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...)Show SMILES C[C@H](CO)NC(=O)c1nc(OC[C@H]2CCCO2)nc(n1)N1CCC(COc2cc(cnc2N)-c2cn(C)cn2)CC1 |r| Show InChI InChI=1S/C27H37N9O5/c1-17(13-37)31-25(38)24-32-26(34-27(33-24)41-15-20-4-3-9-39-20)36-7-5-18(6-8-36)14-40-22-10-19(11-29-23(22)28)21-12-35(2)16-30-21/h10-12,16-18,20,37H,3-9,13-15H2,1-2H3,(H2,28,29)(H,31,38)/t17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM299975

(4-[4-({[2-amino-5- (1-methyl-1H- imidazol-4- yl)py...)Show SMILES Cn1cnc(c1)-c1cnc(N)c(OCC2CCN(CC2)c2nc(OCC3(CC3)C#N)nc(n2)C(=O)NC2(C)CCC2)c1 Show InChI InChI=1S/C29H36N10O3/c1-28(6-3-7-28)37-25(40)24-34-26(36-27(35-24)42-17-29(16-30)8-9-29)39-10-4-19(5-11-39)15-41-22-12-20(13-32-23(22)31)21-14-38(2)18-33-21/h12-14,18-19H,3-11,15,17H2,1-2H3,(H2,31,32)(H,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
AXL enzyme inhibition (% inhibition, Kiapp and Ki values) by small molecule inhibitors was evaluated using a fluorescence-based microfluidic mobility... |
US Patent US9593097 (2017)
BindingDB Entry DOI: 10.7270/Q2GM89BD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598031

((R)-Tetrahydrofuran-3-yl(8-amino-7- | US11612606, ...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@@H]3CCOC3)ncc2c(N)c1F |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598192

((1s,3r)-3-(Cyanomethyl)cyclobutyl(8- amino-7-fluor...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@@H]3C[C@H](CC#N)C3)ncc2c(N)c1F |r,wU:20.21,22.24,(2.55,-1.22,;3.88,-.45,;5.21,-1.22,;5.21,-2.76,;6.55,-3.53,;7.88,-2.76,;7.88,-1.22,;6.55,-.45,;6.55,1.09,;5.21,1.86,;3.88,1.09,;2.55,1.86,;1.21,1.09,;-.12,1.86,;-1.46,1.09,;-2.79,1.86,;-4.12,1.09,;-5.46,1.86,;-5.46,3.4,;-6.79,1.09,;-6.79,-.45,;-7.88,-1.54,;-6.79,-2.63,;-6.79,-4.17,;-5.46,-4.94,;-4.12,-5.71,;-5.7,-1.54,;-2.79,3.4,;-1.46,4.17,;-.12,3.4,;1.21,4.17,;1.21,5.71,;2.55,3.4,;3.88,4.17,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598194
((1r,3r)-3-Cyano-3-methylcyclobutyl(8- amino-7-fluo...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3C[C@](C)(C3)C#N)ncc2c(N)c1F |r,wU:22.24,wD:20.21,22.27,(2.55,-1.43,;3.88,-.66,;5.21,-1.43,;5.21,-2.97,;6.55,-3.74,;7.88,-2.97,;7.88,-1.43,;6.55,-.66,;6.55,.88,;5.21,1.65,;3.88,.88,;2.55,1.65,;1.21,.88,;-.12,1.65,;-1.46,.88,;-2.79,1.65,;-4.12,.88,;-5.46,1.65,;-5.46,3.19,;-6.79,.88,;-6.79,-.66,;-7.88,-1.75,;-6.79,-2.84,;-7.56,-4.17,;-5.7,-1.75,;-6.02,-4.17,;-5.25,-5.5,;-2.79,3.19,;-1.46,3.96,;-.12,3.19,;1.21,3.96,;1.21,5.5,;2.55,3.19,;3.88,3.96,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598056
(2-Methyl-2-azaspiro[3.3]heptan-6-yl(8- amino-7-flu...)Show SMILES CN1CC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598057
((R)-1-Acetylpyrrolidin-3-yl(8-amino-7- | US1161260...)Show SMILES CC(=O)N1CC[C@H](C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598059
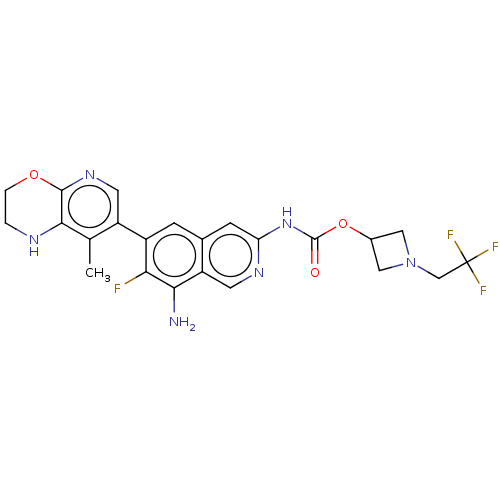
(1-(2,2,2-Trifluoroethyl)azetidin-3-yl(8- amino-7-f...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CN(CC(F)(F)F)C3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598060
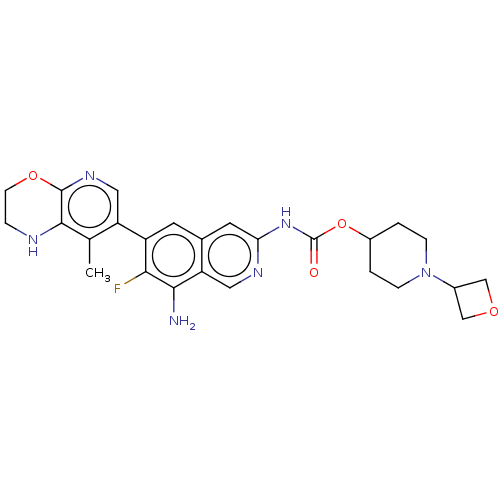
(1-(Oxetan-3-yl)piperidin-4-yl(8-amino- 7-fluoro-6-...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CCN(CC3)C3COC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598062

(7-Methyl-7-azaspiro[3.5]nonan-2-yl(8- amino-7-fluo...)Show SMILES CN1CCC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3CCCNc3c2C)CC1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598063

(2-Acetyl-2-azaspiro[3.3]heptan-6-yl(8- amino-7-flu...)Show SMILES CC(=O)N1CC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598064
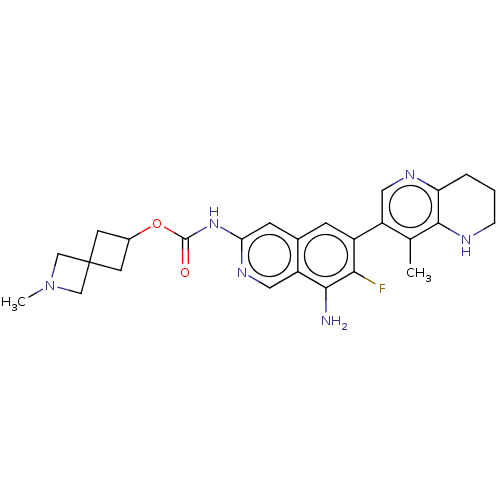
(2-Methyl-2-azaspiro[3.3]heptan-6-yl(8- amino-7-flu...)Show SMILES CN1CC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3CCCNc3c2C)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598065

(1-Acetylazetidin-3-yl(8-amino-7-fluoro- 6-(8-methy...)Show SMILES CC(=O)N1CC(C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598066

(7-Methyl-7-azaspiro[3.5]nonan-2-yl(8- amino-7-fluo...)Show SMILES CN1CCC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)CC1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598067
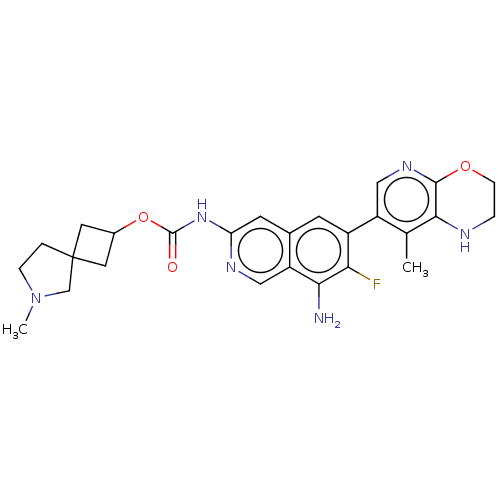
(6-Methyl-6-azaspiro[3.4]octan-2-yl(8- amino-7-fluo...)Show SMILES CN1CCC2(CC(C2)OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C1 |(-5.17,-6.02,;-5.94,-4.69,;-7.48,-4.69,;-7.96,-3.22,;-6.71,-2.32,;-7.8,-1.23,;-6.71,-.14,;-5.62,-1.23,;-6.71,1.4,;-5.38,2.17,;-5.38,3.71,;-4.04,1.4,;-2.71,2.17,;-1.38,1.4,;-.04,2.17,;1.29,1.4,;2.62,2.17,;2.62,3.71,;3.96,4.48,;1.29,4.48,;1.29,6.02,;-.04,3.71,;-1.38,4.48,;-2.71,3.71,;3.96,1.4,;5.29,2.17,;6.62,1.4,;6.62,-.14,;7.96,-.91,;7.96,-2.45,;6.62,-3.22,;5.29,-2.45,;5.29,-.91,;3.96,-.14,;2.62,-.91,;-5.47,-3.22,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598069

(7-(Oxetan-3-yl)-7-azaspiro[3.5]nonan-2- yl(8-amino...)Show SMILES Cc1c2NCCCc2ncc1-c1cc2cc(NC(=O)OC3CC4(C3)CCN(CC4)C3COC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598249

((1s,3s)-3-(Hydroxymethyl)cyclobutyl(8- amino-7-flu...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@@H]3C[C@H](CO)C3)ncc2c(N)c1F |r,wD:20.21,22.24,(2.55,-1.61,;3.88,-.84,;5.21,-1.61,;5.21,-3.15,;6.55,-3.92,;7.88,-3.15,;7.88,-1.61,;6.55,-.84,;6.55,.7,;5.21,1.47,;3.88,.7,;2.55,1.47,;1.21,.7,;-.12,1.47,;-1.46,.7,;-2.79,1.47,;-4.12,.7,;-5.46,1.47,;-5.46,3.01,;-6.79,.7,;-6.79,-.84,;-5.7,-1.93,;-6.79,-3.01,;-6.79,-4.55,;-5.46,-5.32,;-7.88,-1.93,;-2.79,3.01,;-1.46,3.78,;-.12,3.01,;1.21,3.78,;1.21,5.32,;2.55,3.01,;3.88,3.78,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598250
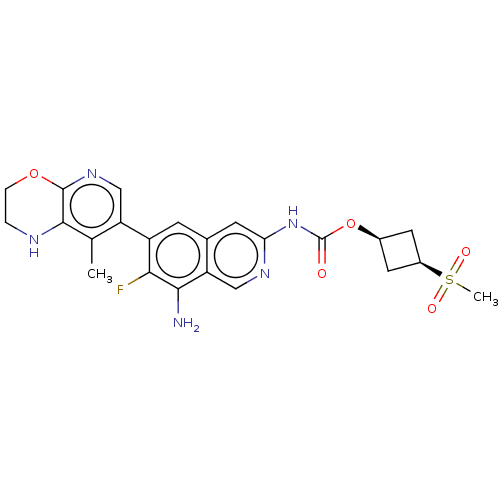
((1s,3s)-3-(Methylsulfonyl)cyclobutyl(8- | US116126...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3C[C@H](C3)S(C)(=O)=O)ncc2c(N)c1F |r,wU:20.21,22.26,(2.1,-.07,;3.44,.7,;4.77,-.07,;4.77,-1.61,;6.1,-2.38,;7.44,-1.61,;7.44,-.07,;6.1,.7,;6.1,2.24,;4.77,3.01,;3.44,2.24,;2.1,3.01,;.77,2.24,;-.56,3.01,;-1.9,2.24,;-3.23,3.01,;-4.56,2.24,;-4.56,.7,;-3.23,-.07,;-5.9,-.07,;-5.9,-1.61,;-4.81,-2.69,;-5.9,-3.78,;-6.99,-2.69,;-5.9,-5.32,;-5.9,-6.86,;-4.36,-5.32,;-7.44,-5.32,;-3.23,4.55,;-1.9,5.32,;-.56,4.55,;.77,5.32,;.77,6.86,;2.1,4.55,;3.44,5.32,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598254

((1R,3s)-3-((S)-1- Hydroxyethyl)cyclobutyl(8-amino-...)Show SMILES C[C@H](O)[C@H]1C[C@@H](C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r,wU:3.2,1.1,wD:5.7,(-8,-5.32,;-6.67,-4.55,;-5.33,-5.32,;-6.67,-3.01,;-7.76,-1.93,;-6.67,-.84,;-5.58,-1.93,;-6.67,.7,;-5.33,1.47,;-5.33,3.01,;-4,.7,;-2.67,1.47,;-1.33,.7,;,1.47,;1.33,.7,;2.67,1.47,;2.67,3.01,;4,3.78,;1.33,3.78,;1.33,5.32,;,3.01,;-1.33,3.78,;-2.67,3.01,;4,.7,;5.33,1.47,;6.67,.7,;6.67,-.84,;8,-1.61,;8,-3.15,;6.67,-3.92,;5.33,-3.15,;5.33,-1.61,;4,-.84,;2.67,-1.61,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598256
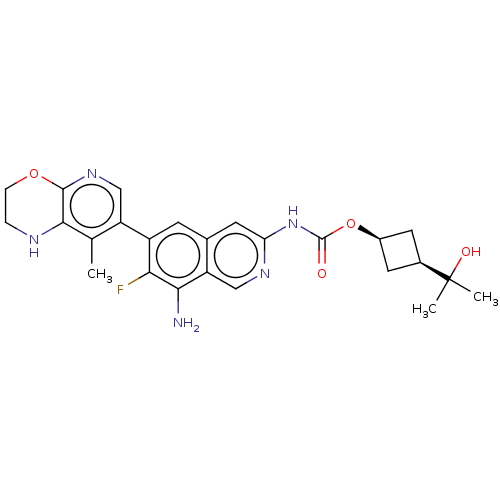
((1s,3s)-3-(2-Hydroxypropan-2- yl)cyclobutyl(8-amin...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3C[C@H](C3)C(C)(C)O)ncc2c(N)c1F |r,wD:20.21,22.26,(2.67,-1.22,;4,-.45,;5.33,-1.22,;5.33,-2.76,;6.67,-3.53,;8,-2.76,;8,-1.22,;6.67,-.45,;6.67,1.09,;5.33,1.86,;4,1.09,;2.67,1.86,;1.33,1.09,;,1.86,;-1.33,1.09,;-2.67,1.86,;-4,1.09,;-5.33,1.86,;-5.33,3.4,;-6.67,1.09,;-6.67,-.45,;-7.76,-1.54,;-6.67,-2.63,;-5.58,-1.54,;-6.67,-4.17,;-5.33,-4.94,;-8,-4.94,;-6.67,-5.71,;-2.67,3.4,;-1.33,4.17,;,3.4,;1.33,4.17,;1.33,5.71,;2.67,3.4,;4,4.17,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598257
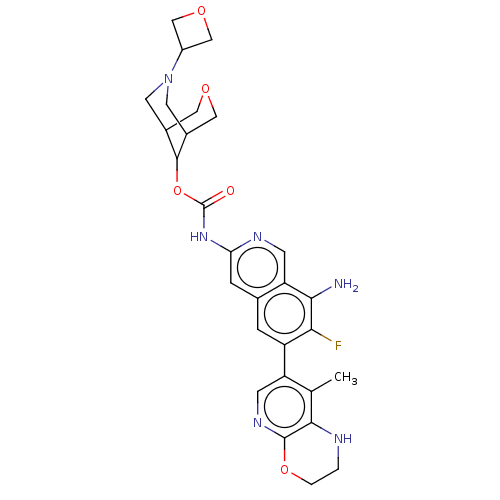
((1R,5S,9s)-7-(Oxetan-3-yl)-3-oxa-7- | US11612606, ...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3C4COCC3CN(C4)C3COC3)ncc2c(N)c1F |THB:29:27:20:22.24.23,19:20:28.27.26:22.24.23| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598258
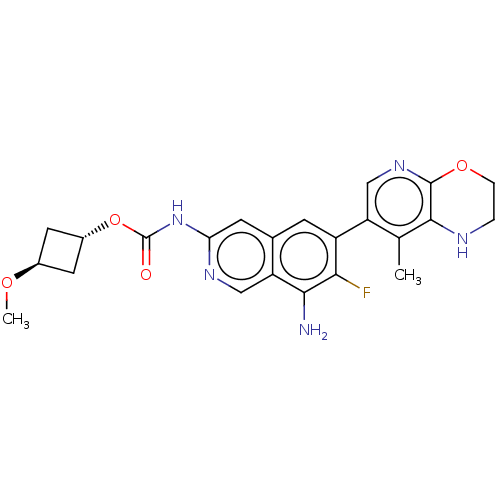
((1r,3r)-3-Methoxycyclobutyl(8-amino-7- | US1161260...)Show SMILES CO[C@H]1C[C@@H](C1)OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r,wU:4.6,wD:2.1,(-8,-5.32,;-6.67,-4.55,;-6.67,-3.01,;-5.58,-1.93,;-6.67,-.84,;-7.76,-1.93,;-6.67,.7,;-5.33,1.47,;-5.33,3.01,;-4,.7,;-2.67,1.47,;-1.33,.7,;,1.47,;1.33,.7,;2.67,1.47,;2.67,3.01,;4,3.78,;1.33,3.78,;1.33,5.32,;,3.01,;-1.33,3.78,;-2.67,3.01,;4,.7,;5.33,1.47,;6.67,.7,;6.67,-.84,;8,-1.61,;8,-3.15,;6.67,-3.92,;5.33,-3.15,;5.33,-1.61,;4,-.84,;2.67,-1.61,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598260

((1r,3r)-3-(Difluoromethyl)cyclobutyl(8- amino-7-fl...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)O[C@H]3C[C@@H](C3)C(F)F)ncc2c(N)c1F |r,wU:20.21,wD:22.26,(2.67,-1.61,;4,-.84,;5.33,-1.61,;5.33,-3.15,;6.67,-3.92,;8,-3.15,;8,-1.61,;6.67,-.84,;6.67,.7,;5.33,1.47,;4,.7,;2.67,1.47,;1.33,.7,;,1.47,;-1.33,.7,;-2.67,1.47,;-4,.7,;-5.33,1.47,;-5.33,3.01,;-6.67,.7,;-6.67,-.84,;-5.58,-1.93,;-6.67,-3.01,;-7.76,-1.93,;-6.67,-4.55,;-8,-5.32,;-5.33,-5.32,;-2.67,3.01,;-1.33,3.78,;,3.01,;1.33,3.78,;1.33,5.32,;2.67,3.01,;4,3.78,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598264
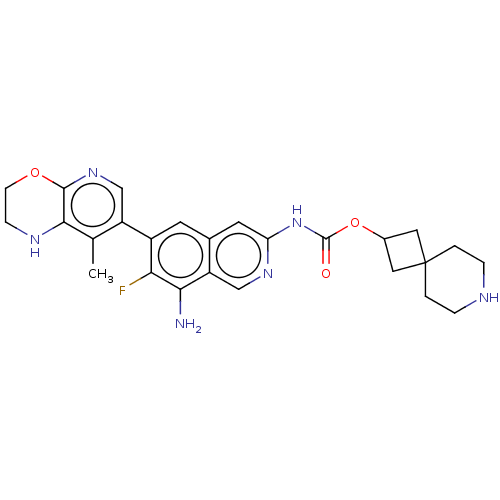
(7-Azaspiro[3.5]nonan-2-yl(8-amino-7- fluoro-6-(8-m...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CC4(C3)CCNCC4)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598274
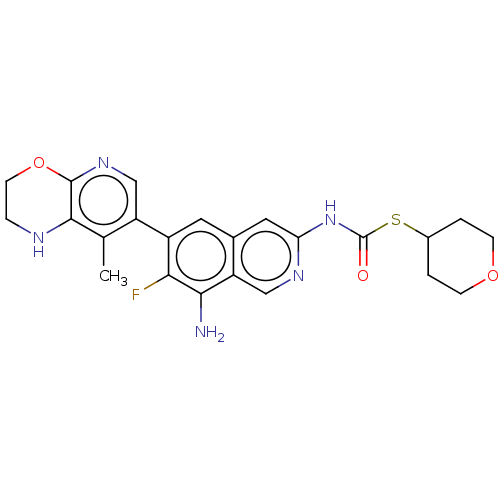
(S-(Tetrahydro-2H-pyran-4-yl)(8-amino- 7-fluoro-6-(...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)SC3CCOCC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598275

(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)NC3CCOCC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598276

(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)NC3CC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598278
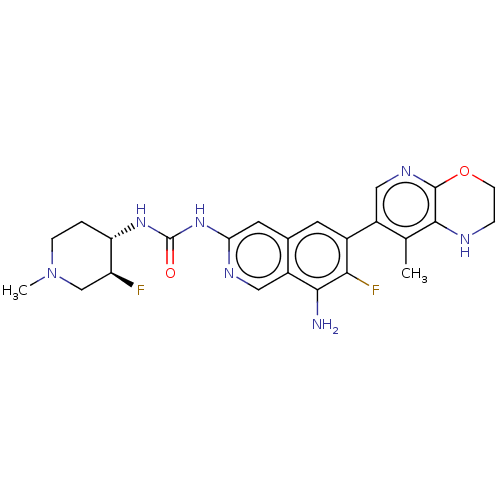
(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES CN1CC[C@H](NC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)[C@@H](F)C1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598280

(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES CNC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598282
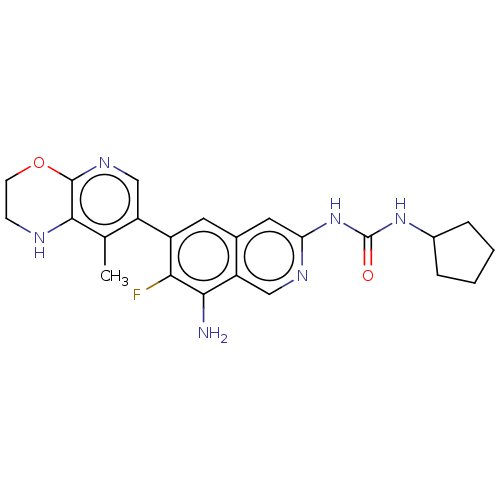
(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)NC3CCCC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598283

(1-(8-Amino-7-fluoro-6-(8-methyl-2,3- dihydro-1H-py...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)NC3CCC3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598071

((1R,5S,6r)-3-Methyl-3- | US11612606, Compound 432a)Show SMILES CN1C[C@H]2[C@@H](C1)[C@@H]2OC(=O)Nc1cc2cc(c(F)c(N)c2cn1)-c1cnc2OCCNc2c1C |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598072
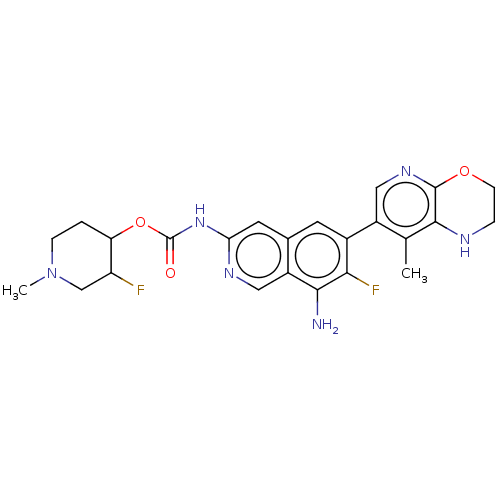
((cis)-3-Fluoro-1-methylpiperidin-4-yl(8- amino-7-f...)Show SMILES CN1CCC(OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C(F)C1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598073

(1-(2,2-Difluoroethypazetidin-3-yl(8- amino-7-fluor...)Show SMILES Cc1c2NCCOc2ncc1-c1cc2cc(NC(=O)OC3CN(CC(F)F)C3)ncc2c(N)c1F | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 1
(Homo sapiens (Human)) | BDBM598076

((3S,4R)-1-Acetyl-3-fluoropiperidin-4-yl | US116126...)Show SMILES CC(=O)N1CC[C@H](OC(=O)Nc2cc3cc(c(F)c(N)c3cn2)-c2cnc3OCCNc3c2C)C(F)C1 |r| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| <0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
TBD |
Citation and Details
BindingDB Entry DOI: 10.7270/Q20Z776H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data