

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098668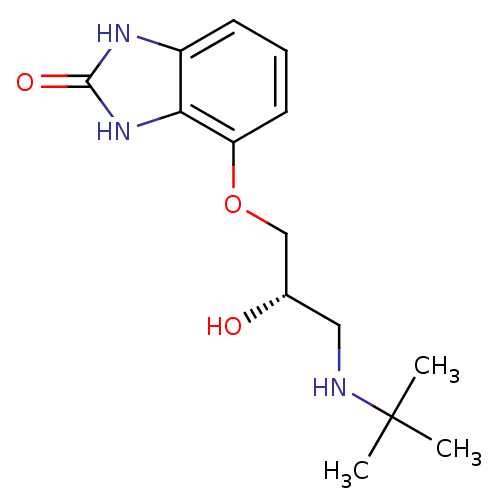 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098668 (4-(3-tert-Butylamino-2-hydroxy-propoxy)-1,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50099151 (CHEMBL177442 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(2-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50099151 (CHEMBL177442 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202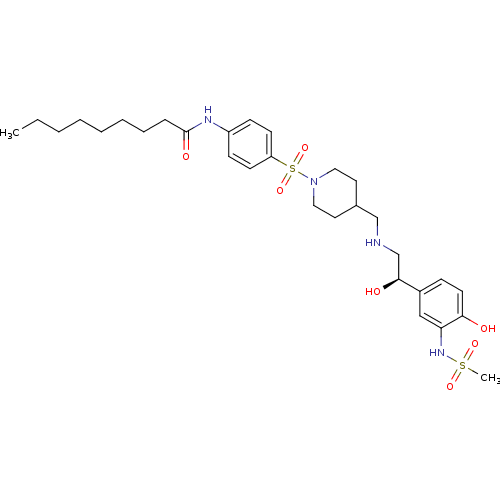 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50070156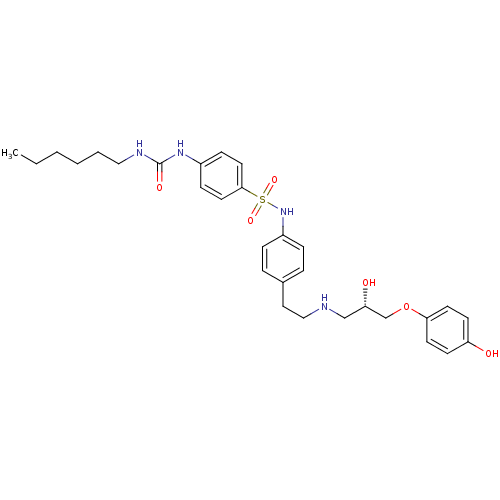 ((S)-4-(3-hexylureido)-N-(4-(2-(1-hydroxy-2-(4-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098662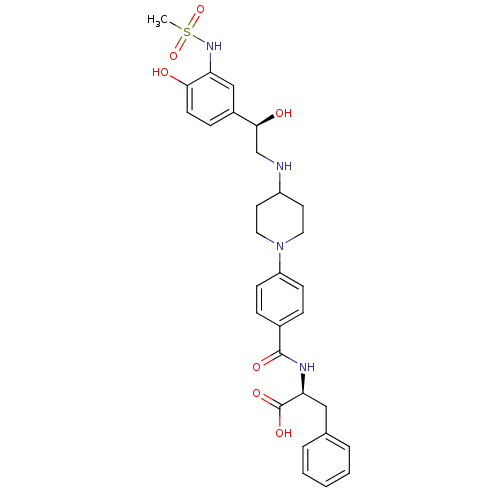 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50070156 ((S)-4-(3-hexylureido)-N-(4-(2-(1-hydroxy-2-(4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50099160 (CHEMBL354906 | [5-(4-{4-[(R)-2-Hydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098659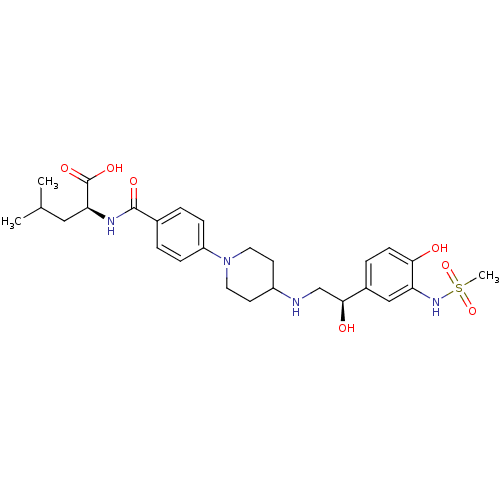 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098661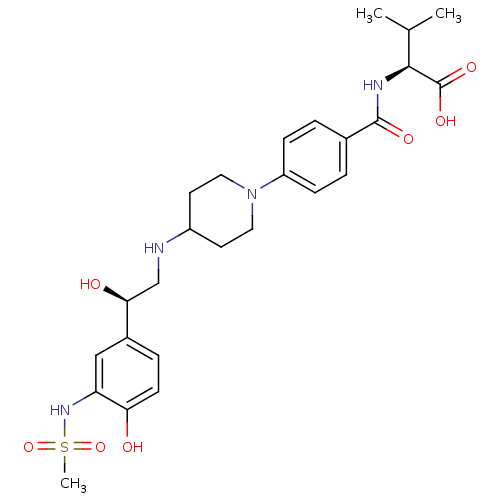 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50099160 (CHEMBL354906 | [5-(4-{4-[(R)-2-Hydroxy-2-(4-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50099150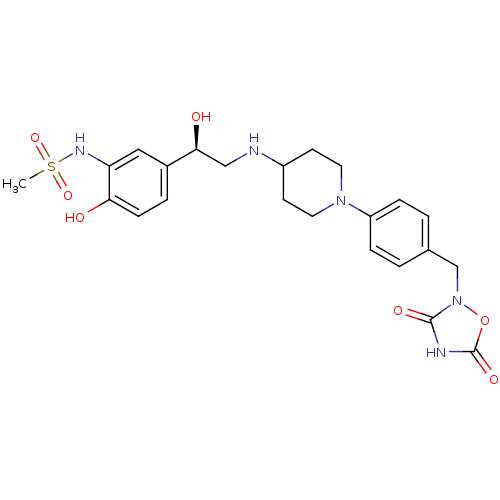 (CHEMBL368584 | N-[5-(2-{1-[4-((R)-3,5-Dioxo-[1,2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098654 ((4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50099150 (CHEMBL368584 | N-[5-(2-{1-[4-((R)-3,5-Dioxo-[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134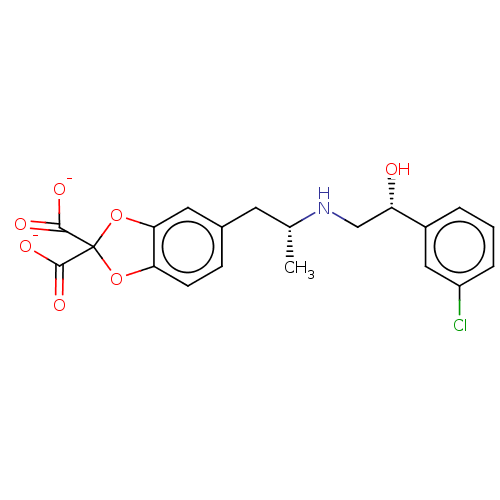 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098659 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098654 ((4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098662 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098661 (2-(4-{4-[2-Hydroxy-2-(4-hydroxy-3-methanesulfonyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50002134 (5-((2R)-2-{[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50098658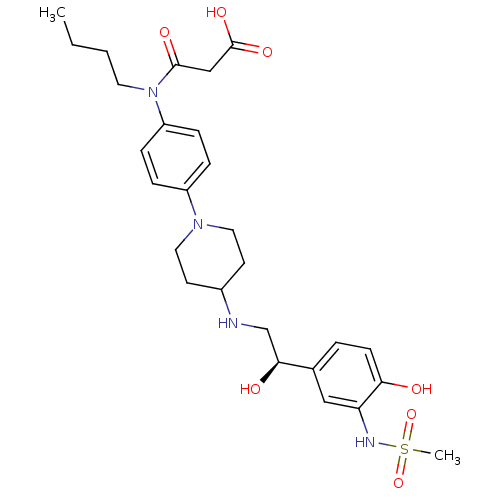 (CHEMBL418600 | N-Butyl-N-(4-{4-[2-hydroxy-2-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 1 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50098658 (CHEMBL418600 | N-Butyl-N-(4-{4-[2-hydroxy-2-(4-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of I-iodocyanopindolol binding to human beta 2 adrenergic receptors | J Med Chem 44: 1456-66 (2001) BindingDB Entry DOI: 10.7270/Q2T72J53 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119183 (1-[4-(4-{[(S)-2-Hydroxy-3-(4-hydroxy-phenoxy)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Compound was tested for the antagonistic activity against Beta-2 adrenergic receptor | Bioorg Med Chem Lett 12: 2957-61 (2002) BindingDB Entry DOI: 10.7270/Q2HT2NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for beta-adrenergic activity against beta-1 adrenergic receptor by the inhibition of insulin stimulated [14C]- glucos... | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119183 (1-[4-(4-{[(S)-2-Hydroxy-3-(4-hydroxy-phenoxy)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Compound was tested for the antagonistic activity against Beta-1 adrenergic receptor | Bioorg Med Chem Lett 12: 2957-61 (2002) BindingDB Entry DOI: 10.7270/Q2HT2NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Rattus norvegicus) | BDBM50002132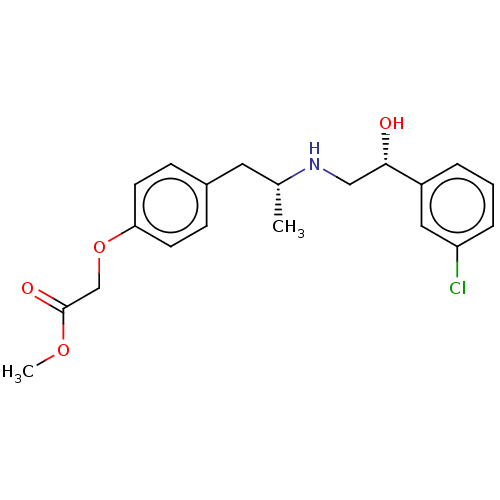 ((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-2 adrenergic receptor in rat soleus membrane by displacing (-)-isoproterenol (50 microM) | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50002132 ((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards Beta-1 adrenergic receptor in rat heart membrane by displacing [125I]- iodocyanopindolol | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279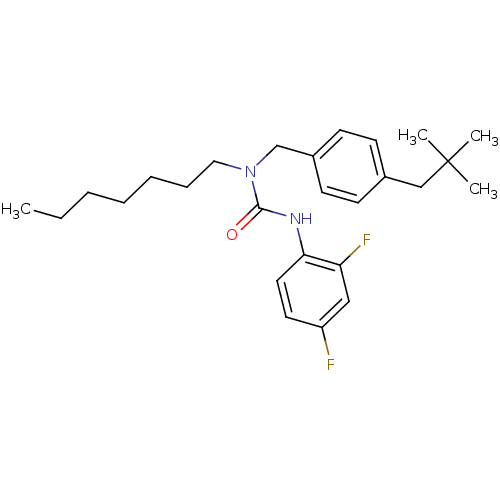 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of smooth muscle cell ACAT activity for cells stimulated by cationized LDL. | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227933 (CHEMBL77761) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Muscarinic acetylcholine receptor derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50002133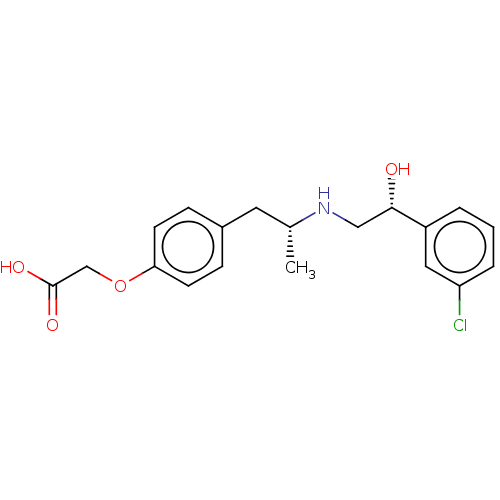 ((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for beta-adrenergic activity against beta-1 adrenergic receptor by the inhibition of insulin stimulated [14C]- glucos... | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119187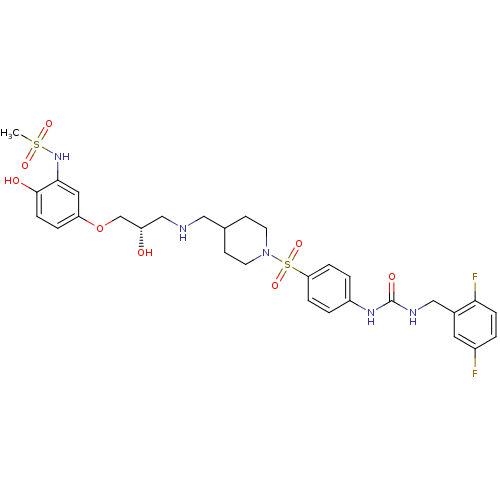 (CHEMBL431048 | N-(5-{(S)-3-[(1-{4-[3-(2,5-Difluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 268 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Compound was tested for the antagonistic activity against Beta-2 adrenergic receptor | Bioorg Med Chem Lett 12: 2957-61 (2002) BindingDB Entry DOI: 10.7270/Q2HT2NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50227908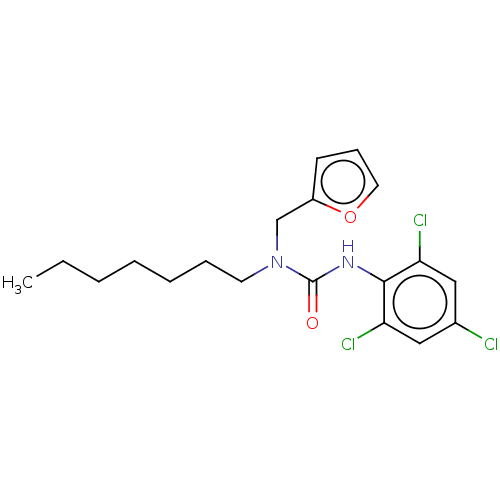 (CHEMBL77988) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227935 (CHEMBL75708) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227908 (CHEMBL77988) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227910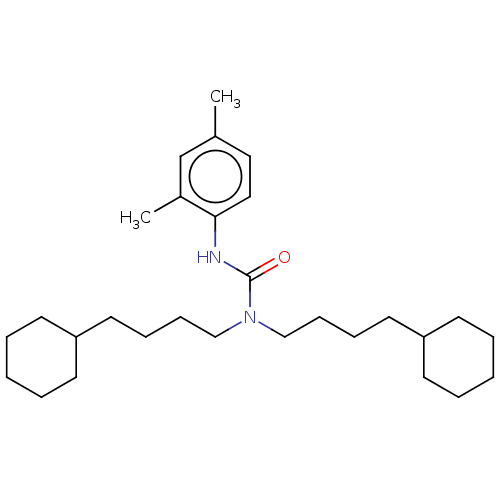 (CHEMBL305937) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from smooth muscle cells from thoracic aorta of monkeys | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227918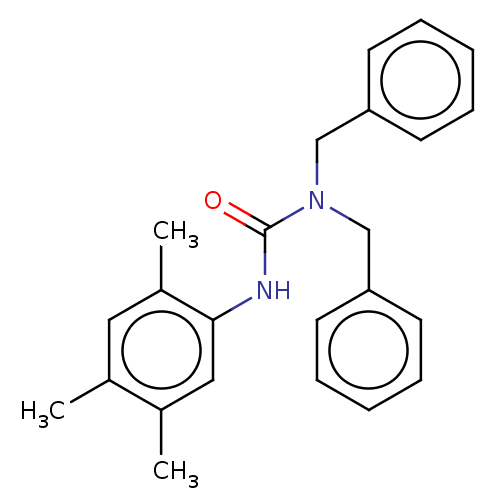 (CHEMBL75224) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 446 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227912 (CHEMBL75204) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50002132 ((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated in vitro for beta-adrenergic activity against beta-1 adrenergic receptor by the inhibition of insulin stimulated [14C]- glucos... | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Rattus norvegicus (Rat)) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards beta-1 adrenergic receptor in rat heart membrane by displacing [125I]- iodocyanopindolol | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227931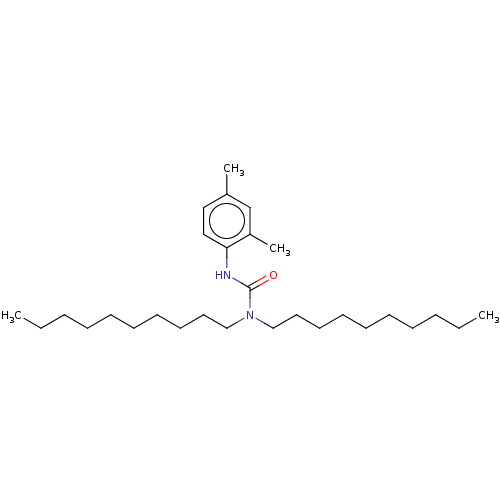 (CHEMBL76938) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 629 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227921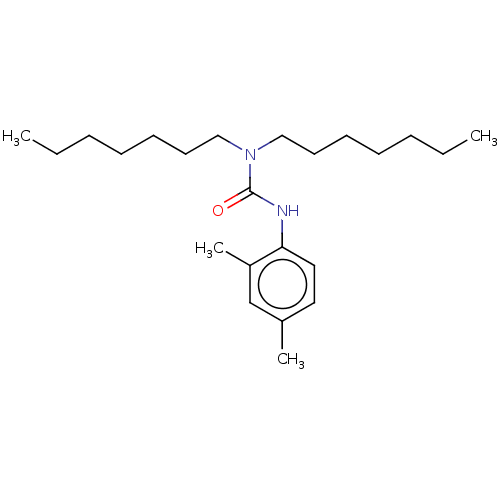 (CHEMBL75709) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in Liver microsomes | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50022279 (3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the effect on Cholesterol O-Acyltransferase (ACAT) in intestinal microsomes | J Med Chem 29: 1131-3 (1987) BindingDB Entry DOI: 10.7270/Q2V69HKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227943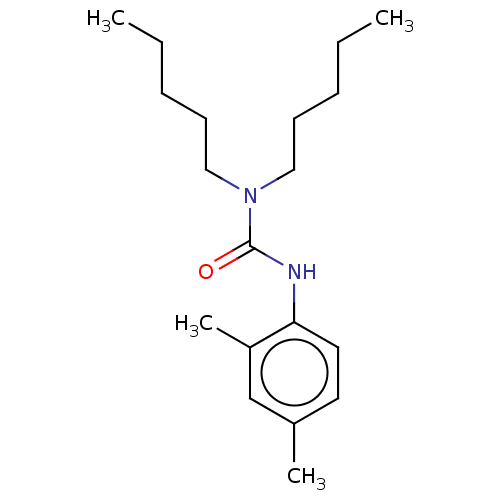 (CHEMBL76935) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 952 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against ACAT derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227909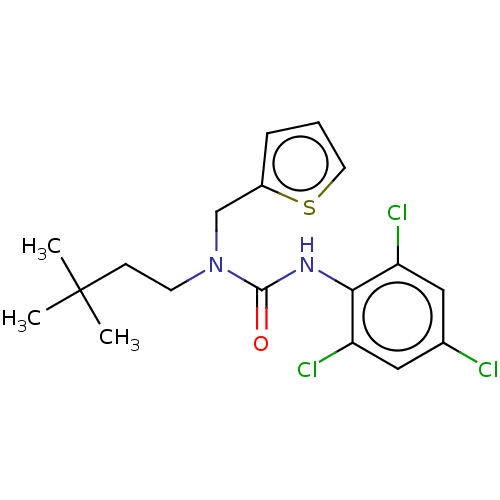 (CHEMBL307505) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 976 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM25392 (4-[1-hydroxy-2-(isopropylamino)ethyl]pyrocatechol;...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards beta-2 adrenergic receptor in rat soleus membrane by displacing(-)-isoproterenol (50 microM) | J Med Chem 35: 3081-4 (1992) BindingDB Entry DOI: 10.7270/Q2PR7TWN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 351 total ) | Next | Last >> |