 Found 681 hits with Last Name = 'seward' and Initial = 'em'
Found 681 hits with Last Name = 'seward' and Initial = 'em' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438628
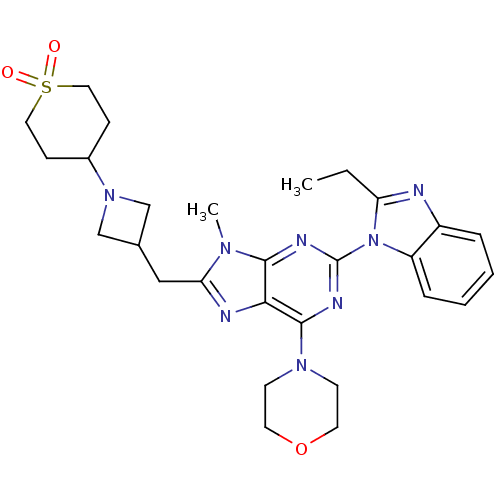
(CHEMBL2414299)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O3S/c1-3-23-29-21-6-4-5-7-22(21)36(23)28-31-26-25(27(32-28)34-10-12-39-13-11-34)30-24(33(26)2)16-19-17-35(18-19)20-8-14-40(37,38)15-9-20/h4-7,19-20H,3,8-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screening |
J Med Chem 57: 10176-91 (2014)
Article DOI: 10.1021/jm501578n
BindingDB Entry DOI: 10.7270/Q2XK8H5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613695
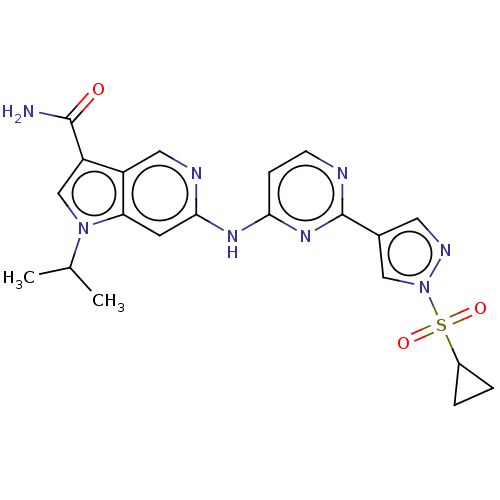
(CHEMBL5274166)Show SMILES CC(C)n1cc(C(N)=O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438634
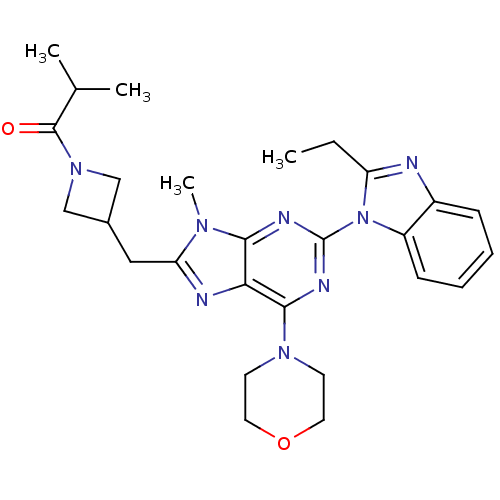
(CHEMBL2414301)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C27H34N8O2/c1-5-21-28-19-8-6-7-9-20(19)35(21)27-30-24-23(25(31-27)33-10-12-37-13-11-33)29-22(32(24)4)14-18-15-34(16-18)26(36)17(2)3/h6-9,17-18H,5,10-16H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403075
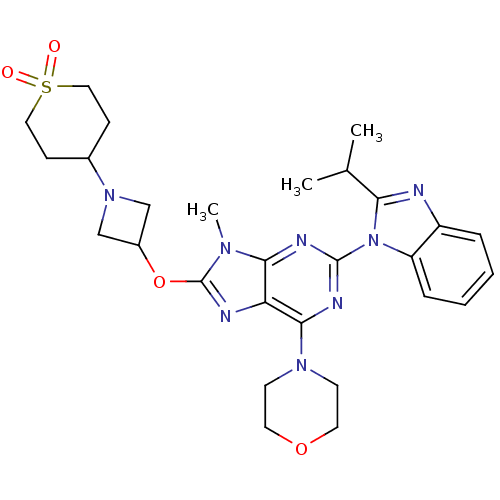
(CHEMBL2216902)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H36N8O4S/c1-18(2)24-29-21-6-4-5-7-22(21)36(24)27-31-25-23(26(32-27)34-10-12-39-13-11-34)30-28(33(25)3)40-20-16-35(17-20)19-8-14-41(37,38)15-9-19/h4-7,18-20H,8-17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172639
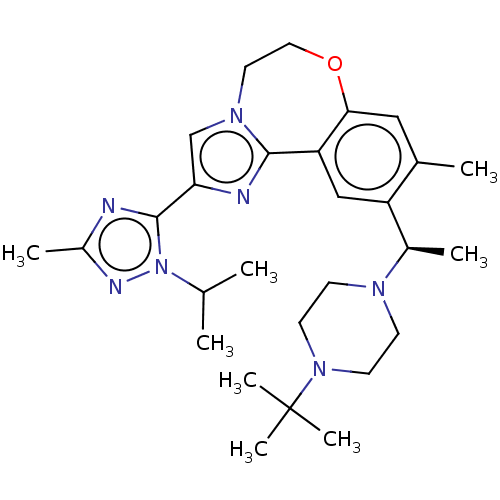
(US9090628, 299)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(C)c(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C28H41N7O/c1-18(2)35-27(29-21(5)31-35)24-17-33-13-14-36-25-15-19(3)22(16-23(25)26(33)30-24)20(4)32-9-11-34(12-10-32)28(6,7)8/h15-18,20H,9-14H2,1-8H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403074
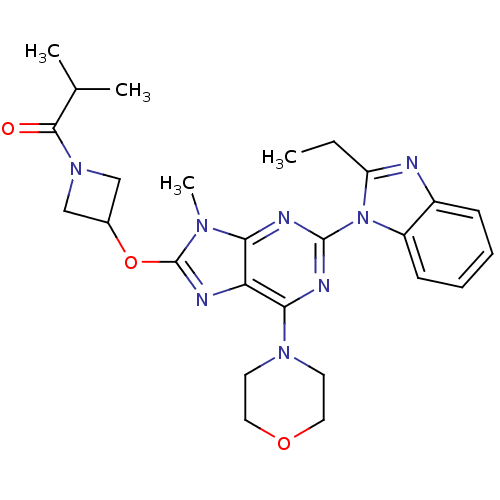
(CHEMBL2216903)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(OC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H32N8O3/c1-5-20-27-18-8-6-7-9-19(18)34(20)25-29-22-21(23(30-25)32-10-12-36-13-11-32)28-26(31(22)4)37-17-14-33(15-17)24(35)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438630
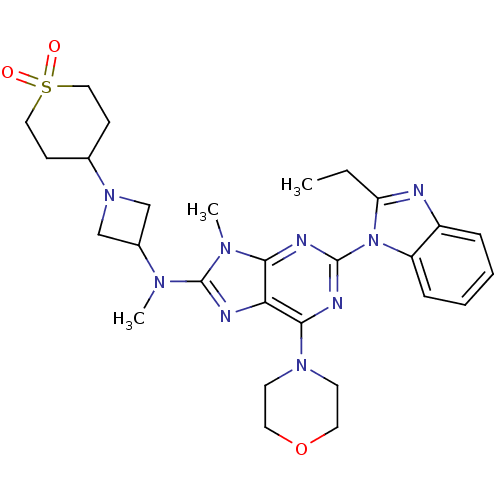
(CHEMBL2414297)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(N(C)C3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C28H37N9O3S/c1-4-23-29-21-7-5-6-8-22(21)37(23)27-31-25-24(26(32-27)35-11-13-40-14-12-35)30-28(34(25)3)33(2)20-17-36(18-20)19-9-15-41(38,39)16-10-19/h5-8,19-20H,4,9-18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613688

(CHEMBL5282716)Show SMILES CC(C)n1nc(N2CC(C2)C(C)(C)O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613687
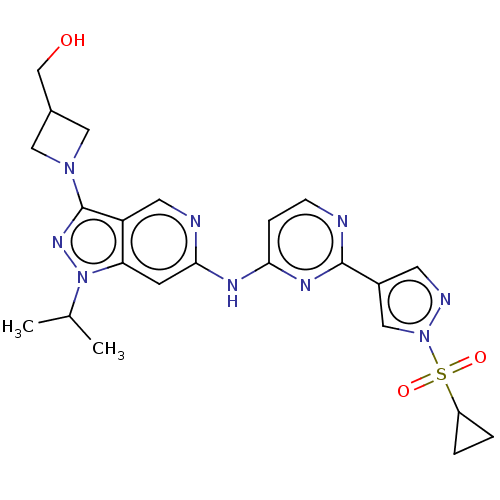
(CHEMBL5285503)Show SMILES CC(C)n1nc(N2CC(CO)C2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | <0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172634
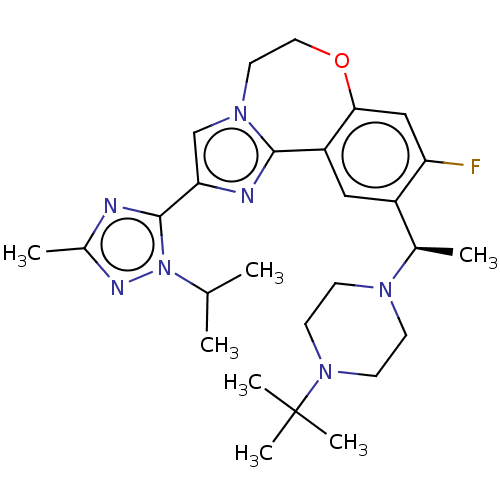
(US9090628, 294)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(F)c(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C27H38FN7O/c1-17(2)35-26(29-19(4)31-35)23-16-33-12-13-36-24-15-22(28)20(14-21(24)25(33)30-23)18(3)32-8-10-34(11-9-32)27(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613684
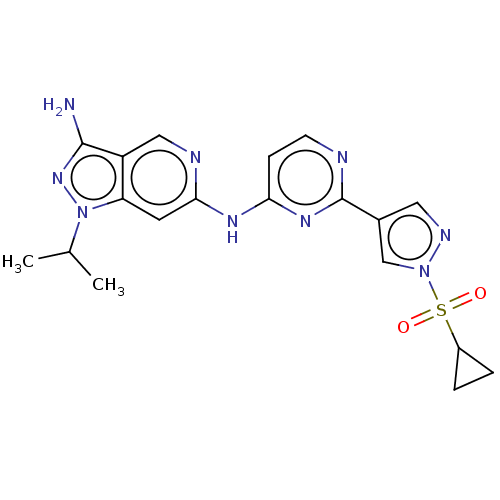
(CHEMBL5280555)Show SMILES CC(C)n1nc(N)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438633
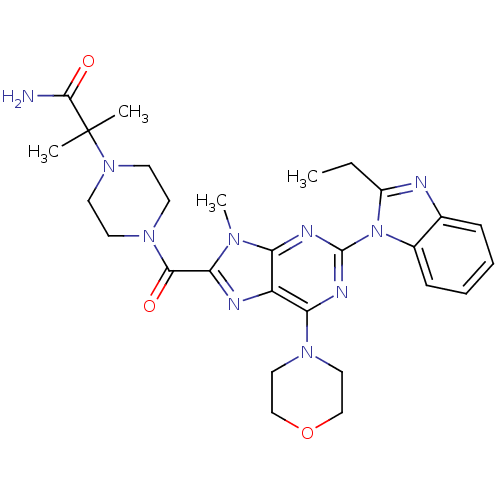
(CHEMBL2414294)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(C(=O)N3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H36N10O3/c1-5-20-30-18-8-6-7-9-19(18)38(20)27-32-22-21(23(33-27)35-14-16-41-17-15-35)31-24(34(22)4)25(39)36-10-12-37(13-11-36)28(2,3)26(29)40/h6-9H,5,10-17H2,1-4H3,(H2,29,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278665
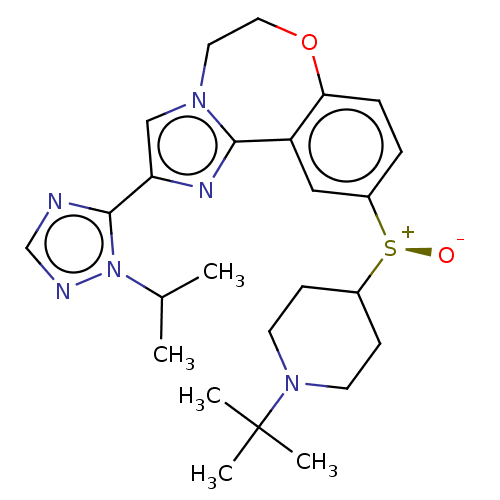
(CHEMBL4175041)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)[S@@+]([O-])C1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C25H34N6O2S/c1-17(2)31-24(26-16-27-31)21-15-29-12-13-33-22-7-6-19(14-20(22)23(29)28-21)34(32)18-8-10-30(11-9-18)25(3,4)5/h6-7,14-18H,8-13H2,1-5H3/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438635
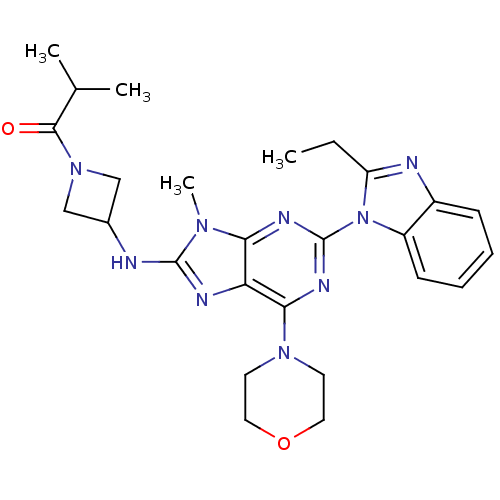
(CHEMBL2414300)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C(=O)C(C)C)n(C)c2n1 Show InChI InChI=1S/C26H33N9O2/c1-5-20-28-18-8-6-7-9-19(18)35(20)26-30-22-21(23(31-26)33-10-12-37-13-11-33)29-25(32(22)4)27-17-14-34(15-17)24(36)16(2)3/h6-9,16-17H,5,10-15H2,1-4H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172640
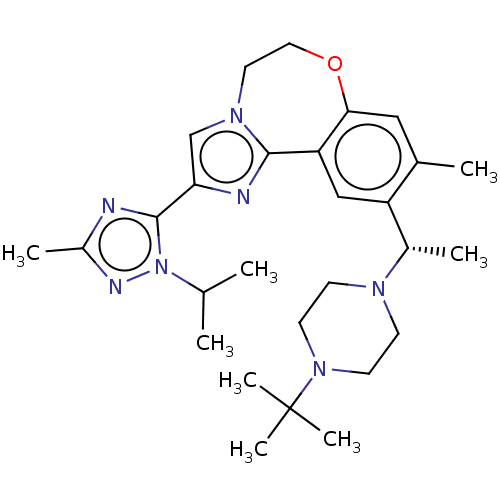
(US9090628, 300)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(C)c(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C28H41N7O/c1-18(2)35-27(29-21(5)31-35)24-17-33-13-14-36-25-15-19(3)22(16-23(25)26(33)30-24)20(4)32-9-11-34(12-10-32)28(6,7)8/h15-18,20H,9-14H2,1-8H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438629
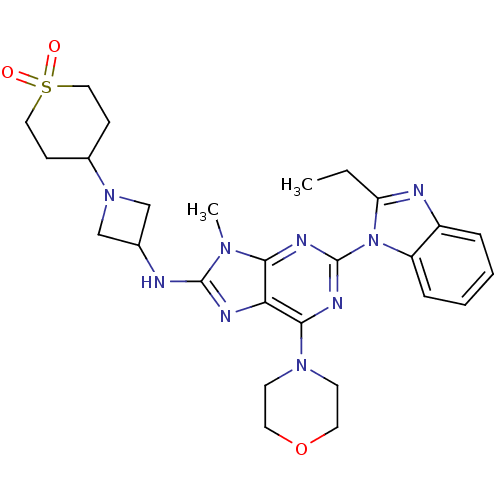
(CHEMBL2414298)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(NC3CN(C3)C3CCS(=O)(=O)CC3)n(C)c2n1 Show InChI InChI=1S/C27H35N9O3S/c1-3-22-29-20-6-4-5-7-21(20)36(22)27-31-24-23(25(32-27)34-10-12-39-13-11-34)30-26(33(24)2)28-18-16-35(17-18)19-8-14-40(37,38)15-9-19/h4-7,18-19H,3,8-17H2,1-2H3,(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438637
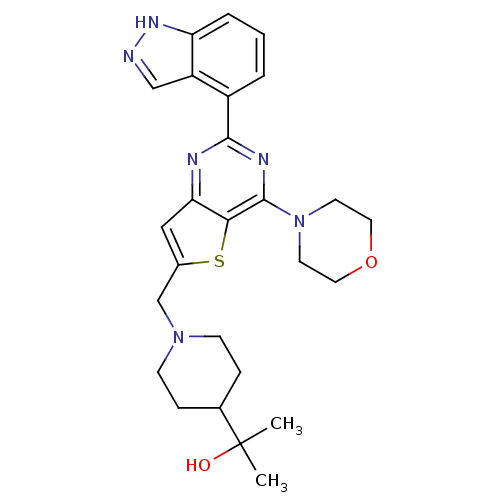
(CHEMBL2414238)Show SMILES CC(C)(O)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C26H32N6O2S/c1-26(2,33)17-6-8-31(9-7-17)16-18-14-22-23(35-18)25(32-10-12-34-13-11-32)29-24(28-22)19-4-3-5-21-20(19)15-27-30-21/h3-5,14-15,17,33H,6-13,16H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613698
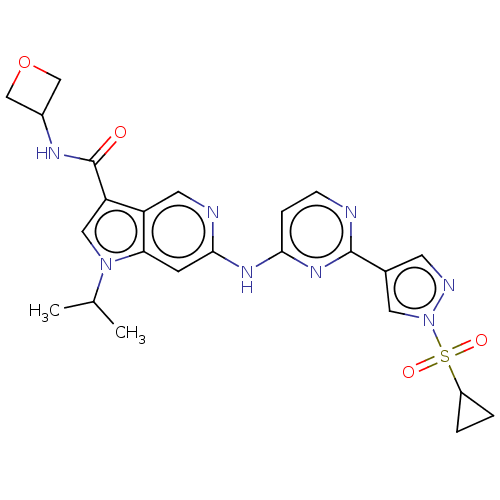
(CHEMBL5282384)Show SMILES CC(C)n1cc(C(=O)NC2COC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613695

(CHEMBL5274166)Show SMILES CC(C)n1cc(C(N)=O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613686

(CHEMBL5276357)Show SMILES CC(C)n1nc(N2CCC(O)CC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613699
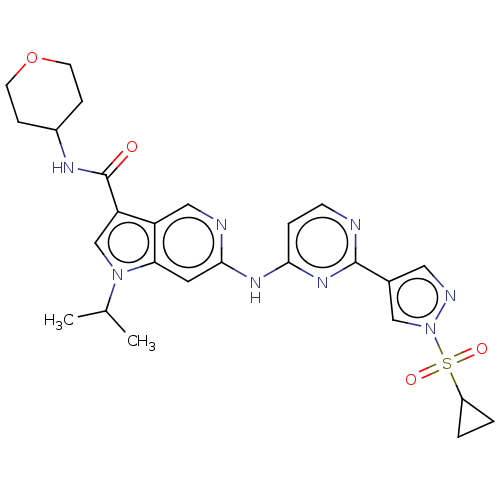
(CHEMBL5279275)Show SMILES CC(C)n1cc(C(=O)NC2CCOCC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278667

(CHEMBL4167171)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)S(=O)(=O)N1CCN(CC1)C(C)(C)C Show InChI InChI=1S/C24H33N7O3S/c1-17(2)31-23(25-16-26-31)20-15-28-12-13-34-21-7-6-18(14-19(21)22(28)27-20)35(32,33)30-10-8-29(9-11-30)24(3,4)5/h6-7,14-17H,8-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613695

(CHEMBL5274166)Show SMILES CC(C)n1cc(C(N)=O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613699

(CHEMBL5279275)Show SMILES CC(C)n1cc(C(=O)NC2CCOCC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613699

(CHEMBL5279275)Show SMILES CC(C)n1cc(C(=O)NC2CCOCC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172467

(US9090628, 110)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)C(=O)N1CCN(CC1)C(C)(C)C Show InChI InChI=1S/C25H33N7O2/c1-17(2)32-23(26-16-27-32)20-15-30-12-13-34-21-7-6-18(14-19(21)22(30)28-20)24(33)29-8-10-31(11-9-29)25(3,4)5/h6-7,14-17H,8-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
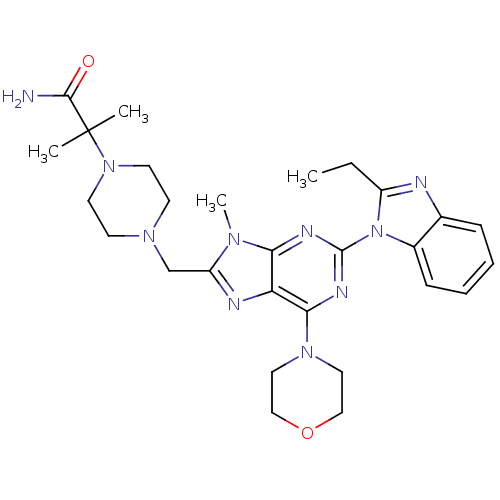
(Homo sapiens (Human)) | BDBM50396642
(CHEMBL2171939)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCN(CC3)C(C)(C)C(N)=O)n(C)c2n1 Show InChI InChI=1S/C28H38N10O2/c1-5-21-30-19-8-6-7-9-20(19)38(21)27-32-24-23(25(33-27)36-14-16-40-17-15-36)31-22(34(24)4)18-35-10-12-37(13-11-35)28(2,3)26(29)39/h6-9H,5,10-18H2,1-4H3,(H2,29,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613693

(CHEMBL5281373)Show SMILES CC(C)n1c(C)nc2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172643
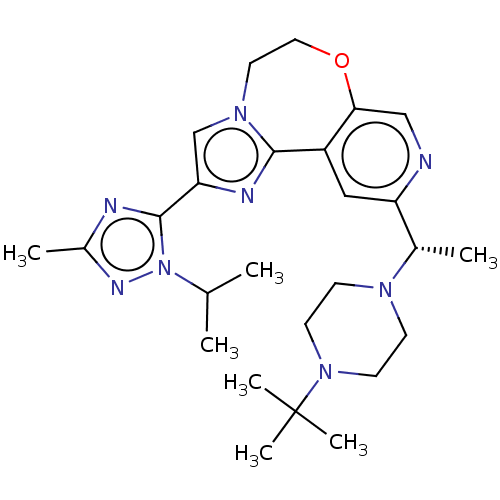
(US9090628, 303)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cnc(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C26H38N8O/c1-17(2)34-25(28-19(4)30-34)22-16-32-12-13-35-23-15-27-21(14-20(23)24(32)29-22)18(3)31-8-10-33(11-9-31)26(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396628
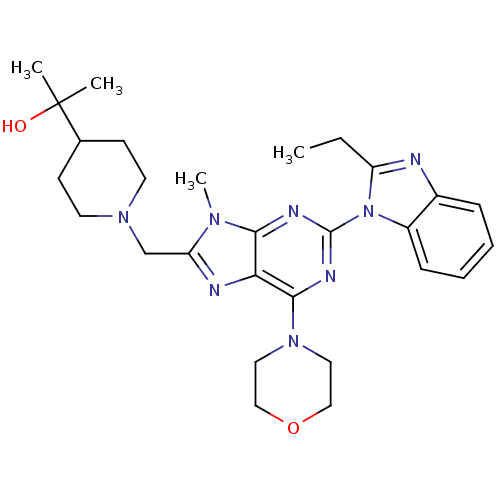
(CHEMBL2171944)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C28H38N8O2/c1-5-22-29-20-8-6-7-9-21(20)36(22)27-31-25-24(26(32-27)35-14-16-38-17-15-35)30-23(33(25)4)18-34-12-10-19(11-13-34)28(2,3)37/h6-9,19,37H,5,10-18H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613698

(CHEMBL5282384)Show SMILES CC(C)n1cc(C(=O)NC2COC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613698

(CHEMBL5282384)Show SMILES CC(C)n1cc(C(=O)NC2COC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613687

(CHEMBL5285503)Show SMILES CC(C)n1nc(N2CC(CO)C2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50396633
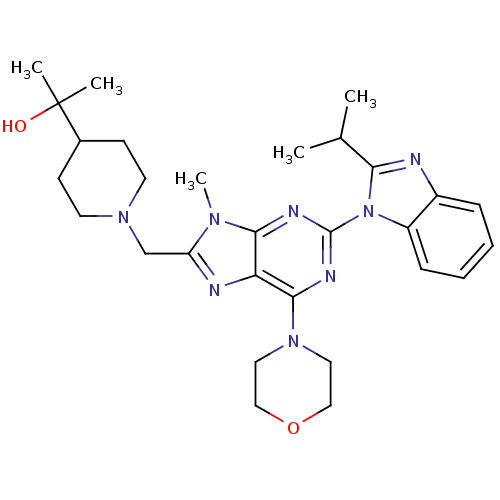
(CHEMBL2171952)Show SMILES CC(C)c1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(CN3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C29H40N8O2/c1-19(2)25-30-21-8-6-7-9-22(21)37(25)28-32-26-24(27(33-28)36-14-16-39-17-15-36)31-23(34(26)5)18-35-12-10-20(11-13-35)29(3,4)38/h6-9,19-20,38H,10-18H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613685
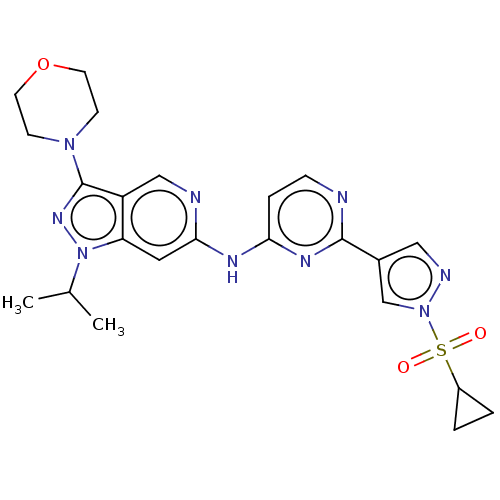
(CHEMBL5273791)Show SMILES CC(C)n1nc(N2CCOCC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438636
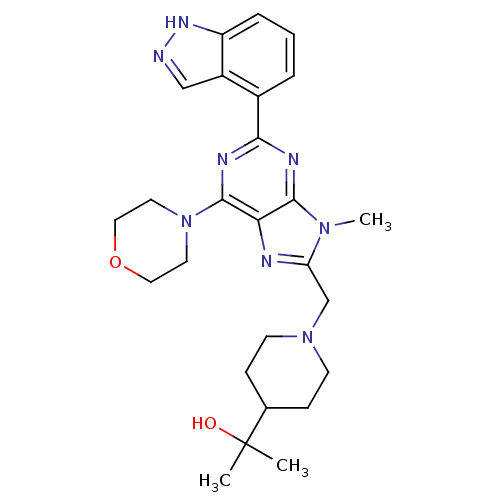
(CHEMBL2414239)Show SMILES Cn1c(CN2CCC(CC2)C(C)(C)O)nc2c(nc(nc12)-c1cccc2[nH]ncc12)N1CCOCC1 Show InChI InChI=1S/C26H34N8O2/c1-26(2,35)17-7-9-33(10-8-17)16-21-28-22-24(32(21)3)29-23(30-25(22)34-11-13-36-14-12-34)18-5-4-6-20-19(18)15-27-31-20/h4-6,15,17,35H,7-14,16H2,1-3H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172637

(US9090628, 297)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(F)c(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C27H38FN7O/c1-17(2)35-26(29-19(4)31-35)23-16-33-12-13-36-24-15-22(28)20(14-21(24)25(33)30-23)18(3)32-8-10-34(11-9-32)27(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172465
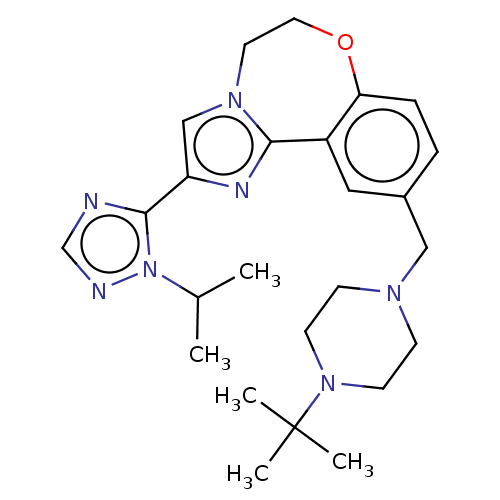
(US9090628, 108)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(CN4CCN(CC4)C(C)(C)C)cc3-c2n1 Show InChI InChI=1S/C25H35N7O/c1-18(2)32-24(26-17-27-32)21-16-30-12-13-33-22-7-6-19(14-20(22)23(30)28-21)15-29-8-10-31(11-9-29)25(3,4)5/h6-7,14,16-18H,8-13,15H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172645
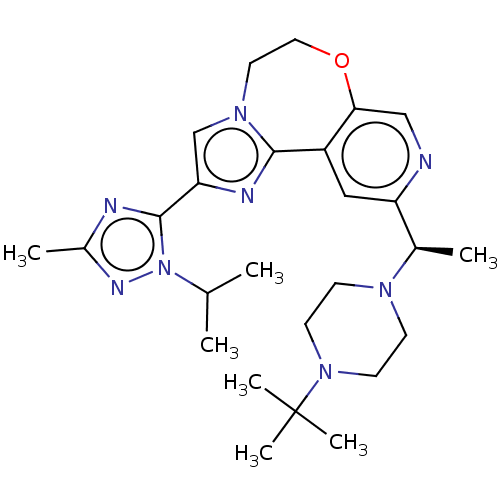
(US9090628, 305)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cnc(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C26H38N8O/c1-17(2)34-25(28-19(4)30-34)22-16-32-12-13-35-23-15-27-21(14-20(23)24(32)29-22)18(3)31-8-10-33(11-9-31)26(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50438631
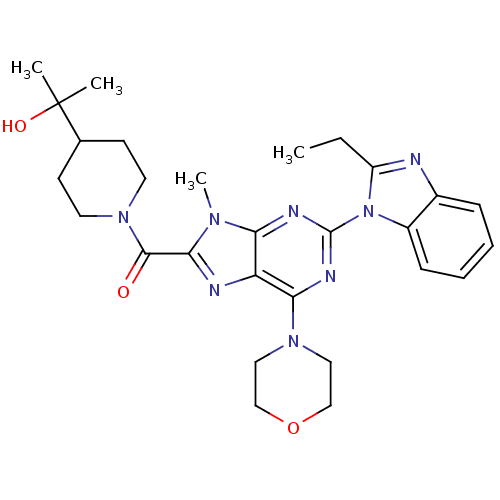
(CHEMBL2414296)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2nc(C(=O)N3CCC(CC3)C(C)(C)O)n(C)c2n1 Show InChI InChI=1S/C28H36N8O3/c1-5-21-29-19-8-6-7-9-20(19)36(21)27-31-23-22(24(32-27)34-14-16-39-17-15-34)30-25(33(23)4)26(37)35-12-10-18(11-13-35)28(2,3)38/h6-9,18,38H,5,10-17H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as formation of PIP3 by competitive fluorescence polarization assay |
Bioorg Med Chem Lett 23: 4953-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.06.052
BindingDB Entry DOI: 10.7270/Q2ZG6TP9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613699

(CHEMBL5279275)Show SMILES CC(C)n1cc(C(=O)NC2CCOCC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613692
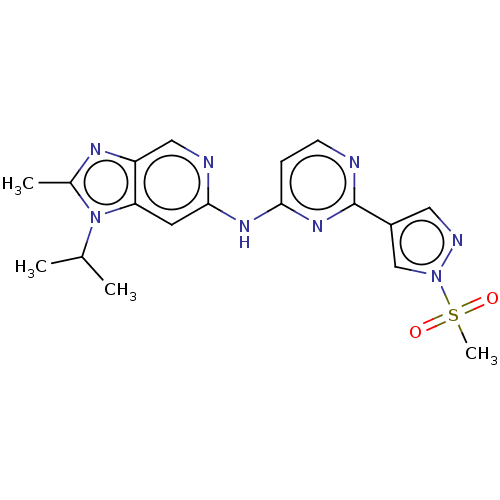
(CHEMBL5277269)Show SMILES CC(C)n1c(C)nc2cnc(Nc3ccnc(n3)-c3cnn(c3)S(C)(=O)=O)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50498976
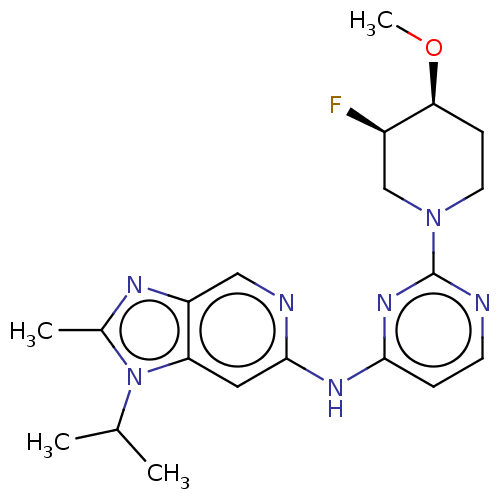
(CHEMBL3736183)Show SMILES CO[C@H]1CCN(C[C@H]1F)c1nccc(Nc2cc3n(C(C)C)c(C)nc3cn2)n1 |r| Show InChI InChI=1S/C20H26FN7O/c1-12(2)28-13(3)24-15-10-23-19(9-16(15)28)25-18-5-7-22-20(26-18)27-8-6-17(29-4)14(21)11-27/h5,7,9-10,12,14,17H,6,8,11H2,1-4H3,(H,22,23,25,26)/t14-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613699

(CHEMBL5279275)Show SMILES CC(C)n1cc(C(=O)NC2CCOCC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613693

(CHEMBL5281373)Show SMILES CC(C)n1c(C)nc2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613695

(CHEMBL5274166)Show SMILES CC(C)n1cc(C(N)=O)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613698

(CHEMBL5282384)Show SMILES CC(C)n1cc(C(=O)NC2COC2)c2cnc(Nc3ccnc(n3)-c3cnn(c3)S(=O)(=O)C3CC3)cc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data