 Found 17 hits with Last Name = 'lo' and Initial = 'ep'
Found 17 hits with Last Name = 'lo' and Initial = 'ep' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293171
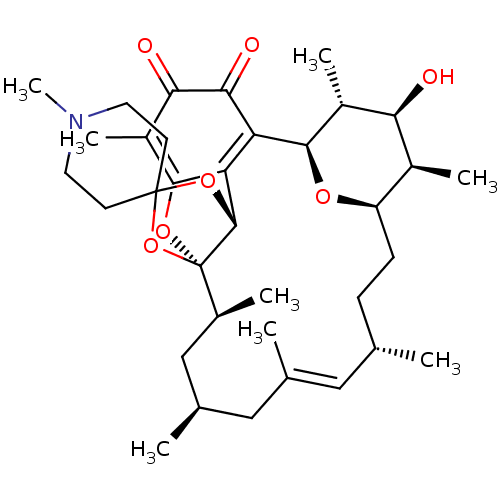
((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]34OC5=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)=C5[C@H]3OC1(CCN(C)CC1)O4 |r,c:7,18,33| Show InChI InChI=1S/C35H51NO7/c1-18-9-10-25-22(5)28(37)23(6)31(40-25)26-27-32(24(7)29(38)30(26)39)41-35(21(4)17-20(3)16-19(2)15-18)33(27)42-34(43-35)11-13-36(8)14-12-34/h15,18,20-23,25,28,31,33,37H,9-14,16-17H2,1-8H3/b19-15+/t18-,20+,21-,22-,23+,25+,28-,31+,33+,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293172
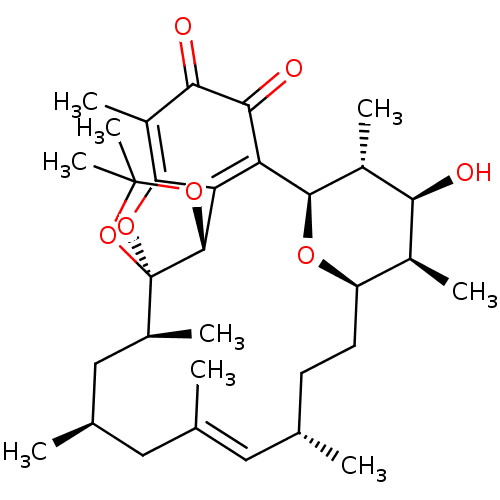
((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]34OC5=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)=C5[C@H]3OC(C)(C)O4 |r,c:7,18,33| Show InChI InChI=1S/C32H46O7/c1-15-10-11-22-19(5)25(33)20(6)28(36-22)23-24-29(21(7)26(34)27(23)35)37-32(30(24)38-31(8,9)39-32)18(4)14-17(3)13-16(2)12-15/h12,15,17-20,22,25,28,30,33H,10-11,13-14H2,1-9H3/b16-12+/t15-,17+,18-,19-,20+,22+,25-,28+,30+,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293169
(CHEMBL523927 | kendomycin)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]3(O)OC4=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)C4=C3 |r,c:7,19,36| Show InChI InChI=1S/C29H42O6/c1-14-8-9-22-18(5)24(30)19(6)28(34-22)23-21-13-29(33,17(4)12-16(3)11-15(2)10-14)35-27(21)20(7)25(31)26(23)32/h10,13-14,16-19,22-24,28,30,33H,8-9,11-12H2,1-7H3/b15-10+/t14-,16+,17-,18-,19+,22+,23?,24-,28+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50250362

(3-Oximo-olean-12-en-29-oic acid | CHEMBL490347)Show SMILES CC1(C)C(CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2CC=C2[C@@H]3C[C@@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O)N=O |r,t:17| Show InChI InChI=1S/C30H47NO3/c1-25(2)21-10-13-30(7)22(28(21,5)12-11-23(25)31-34)9-8-19-20-18-27(4,24(32)33)15-14-26(20,3)16-17-29(19,30)6/h8,20-23H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23?,26+,27+,28-,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Charlottesville
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta |
J Nat Prod 62: 1110-3 (1999)
Article DOI: 10.1021/np990104r
BindingDB Entry DOI: 10.7270/Q2C8293M |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50250352
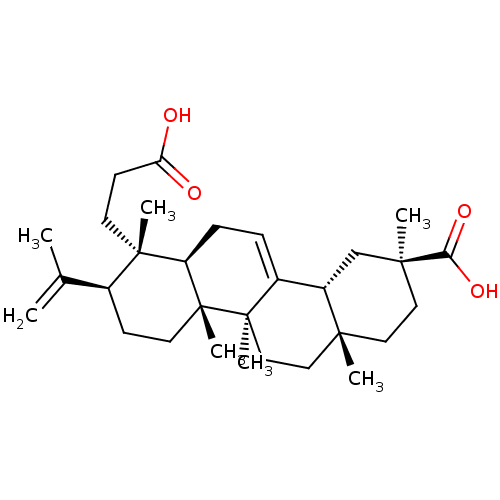
(3,4-seco-olean-4(23),12-diene-3,30-dioic acid | CH...)Show SMILES CC(=C)[C@@H]1CC[C@]2(C)[C@H](CC=C3[C@@H]4C[C@](C)(CC[C@]4(C)CC[C@@]23C)C(O)=O)[C@@]1(C)CCC(O)=O |r,t:10| Show InChI InChI=1S/C30H46O4/c1-19(2)20-10-13-30(7)23(28(20,5)12-11-24(31)32)9-8-21-22-18-27(4,25(33)34)15-14-26(22,3)16-17-29(21,30)6/h8,20,22-23H,1,9-18H2,2-7H3,(H,31,32)(H,33,34)/t20-,22-,23+,26+,27-,28-,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Charlottesville
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta |
J Nat Prod 62: 1110-3 (1999)
Article DOI: 10.1021/np990104r
BindingDB Entry DOI: 10.7270/Q2C8293M |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50250348

(3-oxo-olean-12-en-29-oic acid | 3-oxoolean-12-en-2...)Show SMILES CC1(C)[C@@H]2CC[C@]3(C)[C@H](CC=C4[C@@H]5C[C@@](C)(CC[C@]5(C)CC[C@@]34C)C(O)=O)[C@@]2(C)CCC1=O |r,t:10| Show InChI InChI=1S/C30H46O3/c1-25(2)21-10-13-30(7)22(28(21,5)12-11-23(25)31)9-8-19-20-18-27(4,24(32)33)15-14-26(20,3)16-17-29(19,30)6/h8,20-22H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,26+,27+,28-,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Charlottesville
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta |
J Nat Prod 62: 1110-3 (1999)
Article DOI: 10.1021/np990104r
BindingDB Entry DOI: 10.7270/Q2C8293M |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50250360
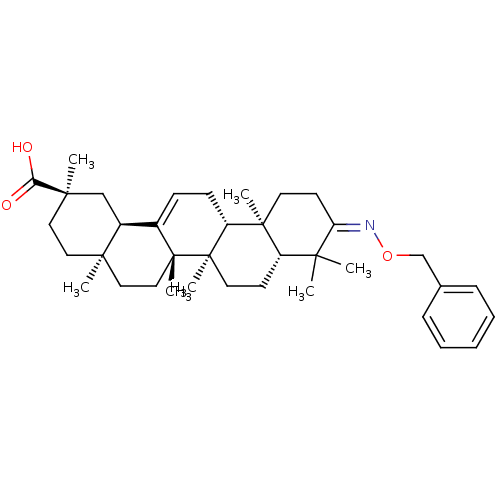
(3-benzyloximo-olean-12-en-29-oic acid | CHEMBL4557...)Show SMILES C[C@]12CC[C@](C)(C[C@H]1C1=CC[C@@H]3[C@@]4(C)CC\C(=N\OCc5ccccc5)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2)C(O)=O |r,t:9| Show InChI InChI=1S/C37H53NO3/c1-32(2)28-15-18-37(7)29(35(28,5)17-16-30(32)38-41-24-25-11-9-8-10-12-25)14-13-26-27-23-34(4,31(39)40)20-19-33(27,3)21-22-36(26,37)6/h8-13,27-29H,14-24H2,1-7H3,(H,39,40)/b38-30-/t27-,28-,29+,33+,34+,35-,36+,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Charlottesville
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta |
J Nat Prod 62: 1110-3 (1999)
Article DOI: 10.1021/np990104r
BindingDB Entry DOI: 10.7270/Q2C8293M |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50250361
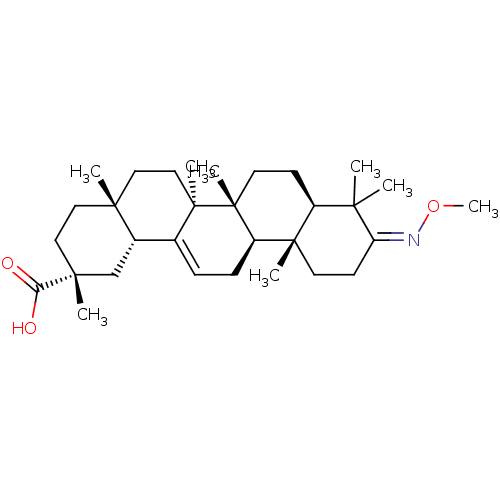
(3-methyloximo-olean-12-en-29-oic acid | CHEMBL5241...)Show SMILES CO\N=C1\CC[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC=C2[C@@H]4C[C@@](C)(CC[C@]4(C)CC[C@@]32C)C(O)=O)C1(C)C |r,t:16| Show InChI InChI=1S/C31H49NO3/c1-26(2)22-11-14-31(7)23(29(22,5)13-12-24(26)32-35-8)10-9-20-21-19-28(4,25(33)34)16-15-27(21,3)17-18-30(20,31)6/h9,21-23H,10-19H2,1-8H3,(H,33,34)/b32-24-/t21-,22-,23+,27+,28+,29-,30+,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Charlottesville
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta |
J Nat Prod 62: 1110-3 (1999)
Article DOI: 10.1021/np990104r
BindingDB Entry DOI: 10.7270/Q2C8293M |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50250349
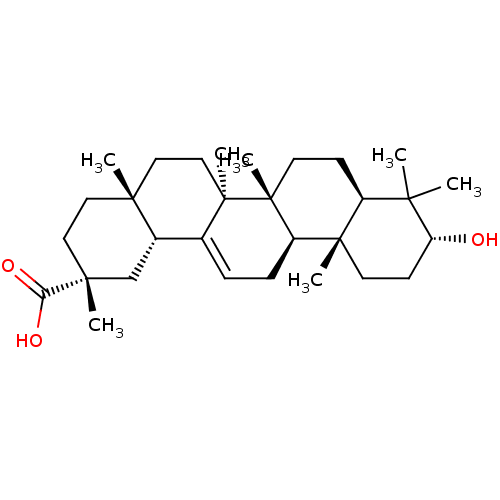
(3alpha-hydroxyolean-12-en-29-oic acid | CHEMBL4905...)Show SMILES CC1(C)[C@H](O)CC[C@@]2(C)[C@H]1CC[C@]1(C)[C@@H]2CC=C2[C@@H]3C[C@@](C)(CC[C@]3(C)CC[C@@]12C)C(O)=O |r,t:18| Show InChI InChI=1S/C30H48O3/c1-25(2)21-10-13-30(7)22(28(21,5)12-11-23(25)31)9-8-19-20-18-27(4,24(32)33)15-14-26(20,3)16-17-29(19,30)6/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23+,26+,27+,28-,29+,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia Charlottesville
Curated by ChEMBL
| Assay Description
Inhibition of rat DNA polymerase beta |
J Nat Prod 62: 1110-3 (1999)
Article DOI: 10.1021/np990104r
BindingDB Entry DOI: 10.7270/Q2C8293M |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293170
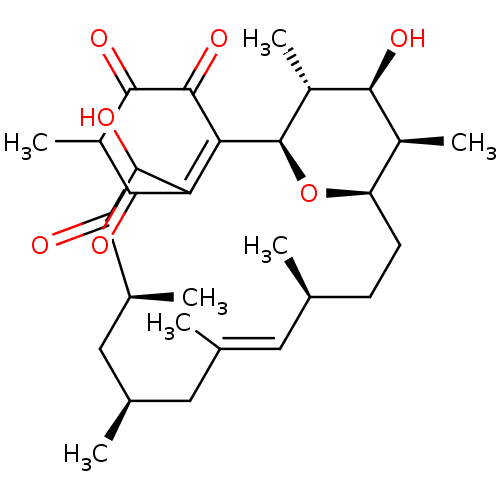
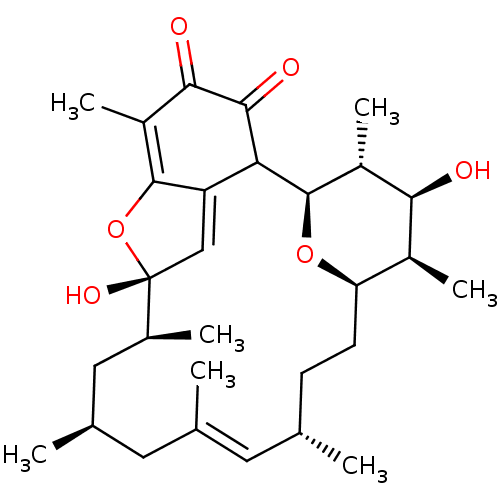
((1R,12S,16S,19R,20R,21S,22R)-4,8,21-Trihydroxy-10-...)Show SMILES C[C@@H]1[C@H](O)[C@@H](C)[C@H]2O[C@@H]1CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)C(=O)C(O)C1=C2C(=O)C(=O)C(C)C1=O |r,c:14,27| Show InChI InChI=1S/C29H42O7/c1-13-8-9-20-17(5)24(31)19(7)29(36-20)22-21(25(32)18(6)26(33)28(22)35)27(34)23(30)16(4)12-15(3)11-14(2)10-13/h10,13,15-20,24,27,29,31,34H,8-9,11-12H2,1-7H3/b14-10+/t13-,15+,16-,17-,18?,19+,20+,24-,27?,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM23223
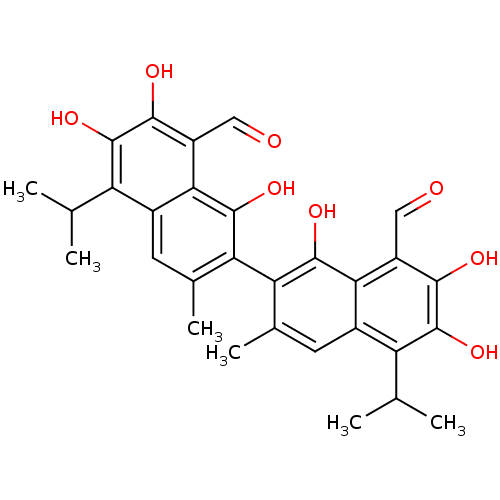
(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by surface plasmon resonance assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293169

(CHEMBL523927 | kendomycin)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]3(O)OC4=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)C4=C3 |r,c:7,19,36| Show InChI InChI=1S/C29H42O6/c1-14-8-9-22-18(5)24(30)19(6)28(34-22)23-21-13-29(33,17(4)12-16(3)11-15(2)10-14)35-27(21)20(7)25(31)26(23)32/h10,13-14,16-19,22-24,28,30,33H,8-9,11-12H2,1-7H3/b15-10+/t14-,16+,17-,18-,19+,22+,23?,24-,28+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.83E+6 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between Bcl-xl and Bak by surface plasmon resonance assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293171

((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]34OC5=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)=C5[C@H]3OC1(CCN(C)CC1)O4 |r,c:7,18,33| Show InChI InChI=1S/C35H51NO7/c1-18-9-10-25-22(5)28(37)23(6)31(40-25)26-27-32(24(7)29(38)30(26)39)41-35(21(4)17-20(3)16-19(2)15-18)33(27)42-34(43-35)11-13-36(8)14-12-34/h15,18,20-23,25,28,31,33,37H,9-14,16-17H2,1-8H3/b19-15+/t18-,20+,21-,22-,23+,25+,28-,31+,33+,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.99E+5 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between Bcl-xl and Bak by surface plasmon resonance assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM23223

(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.38E+5 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between Bcl-xl and Bak by surface plasmon resonance assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293170

((1R,12S,16S,19R,20R,21S,22R)-4,8,21-Trihydroxy-10-...)Show SMILES C[C@@H]1[C@H](O)[C@@H](C)[C@H]2O[C@@H]1CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)C(=O)C(O)C1=C2C(=O)C(=O)C(C)C1=O |r,c:14,27| Show InChI InChI=1S/C29H42O7/c1-13-8-9-20-17(5)24(31)19(7)29(36-20)22-21(25(32)18(6)26(33)28(22)35)27(34)23(30)16(4)12-15(3)11-14(2)10-13/h10,13,15-20,24,27,29,31,34H,8-9,11-12H2,1-7H3/b14-10+/t13-,15+,16-,17-,18?,19+,20+,24-,27?,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.08E+7 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by surface plasmon resonance assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293169

(CHEMBL523927 | kendomycin)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]3(O)OC4=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)C4=C3 |r,c:7,19,36| Show InChI InChI=1S/C29H42O6/c1-14-8-9-22-18(5)24(30)19(6)28(34-22)23-21-13-29(33,17(4)12-16(3)11-15(2)10-14)35-27(21)20(7)25(31)26(23)32/h10,13-14,16-19,22-24,28,30,33H,8-9,11-12H2,1-7H3/b15-10+/t14-,16+,17-,18-,19+,22+,23?,24-,28+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.28E+5 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by surface plasmon resonance assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50293171

((1R,2S,4S,6E,8S,11R,12R,13S,14R,15R,22R)-13-hydrox...)Show SMILES C[C@H]1[C@H]2CC[C@H](C)\C=C(C)\C[C@@H](C)C[C@H](C)[C@]34OC5=C(C)C(=O)C(=O)C([C@H](O2)[C@H](C)[C@H]1O)=C5[C@H]3OC1(CCN(C)CC1)O4 |r,c:7,18,33| Show InChI InChI=1S/C35H51NO7/c1-18-9-10-25-22(5)28(37)23(6)31(40-25)26-27-32(24(7)29(38)30(26)39)41-35(21(4)17-20(3)16-19(2)15-18)33(27)42-34(43-35)11-13-36(8)14-12-34/h15,18,20-23,25,28,31,33,37H,9-14,16-17H2,1-8H3/b19-15+/t18-,20+,21-,22-,23+,25+,28-,31+,33+,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.77E+5 | n/a | n/a | n/a | n/a | n/a |
MerLion Pharmaceuticals Pte Ltd
Curated by ChEMBL
| Assay Description
Inhibition of interaction between GST-tagged Bcl-xl and Bak by surface plasmon resonance assay |
Bioorg Med Chem Lett 18: 5771-3 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.071
BindingDB Entry DOI: 10.7270/Q2DF6R7B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data