 Found 323 hits with Last Name = 'becker' and Initial = 'f'
Found 323 hits with Last Name = 'becker' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862
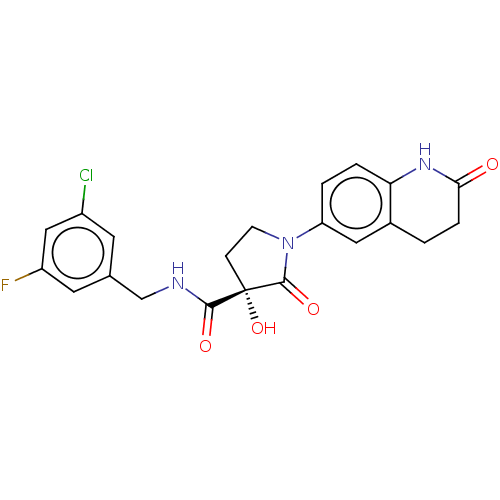
(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862

(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401307

(US10005756, Compound A78)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163
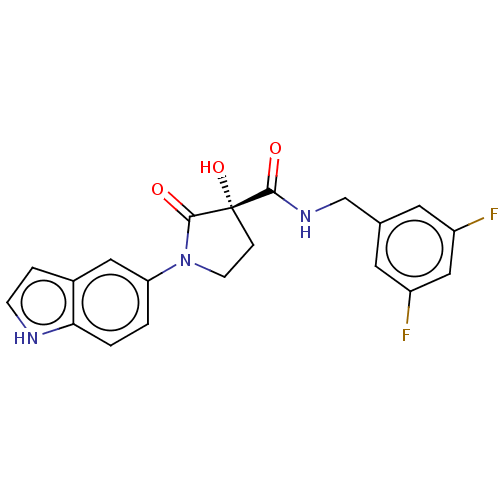
(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163

(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610836

(MSC-4381)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccc(Cl)cc1C#Cc1cnc(cc1OC)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610833

(CHEMBL5276884)Show SMILES COc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(C)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610834
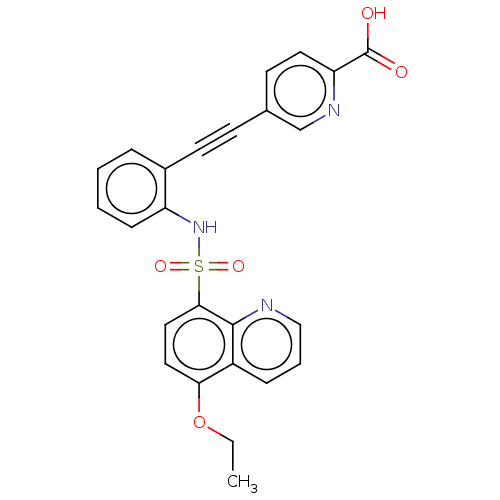
(CHEMBL5279064)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610837
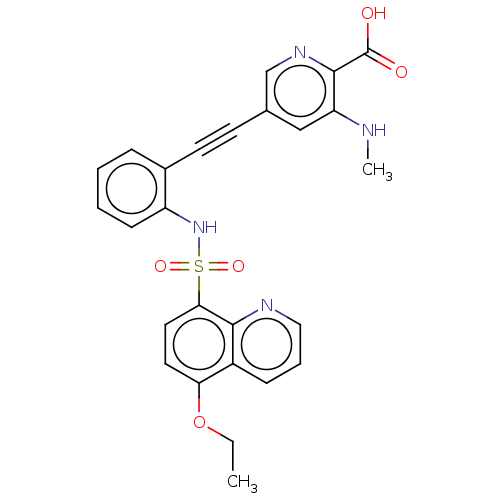
(CHEMBL5281492)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(NC)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610835

(CHEMBL5267349)Show SMILES CCOc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1cnc(cc1OC)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610830
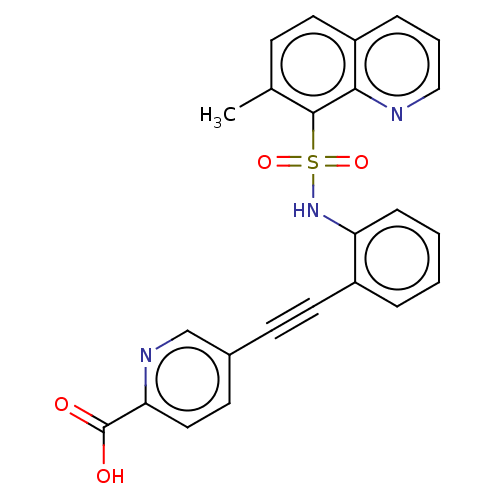
(CHEMBL5267752)Show SMILES Cc1ccc2cccnc2c1S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610826

(CHEMBL5287351)Show SMILES Cc1cnc2c(cccc2c1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610827
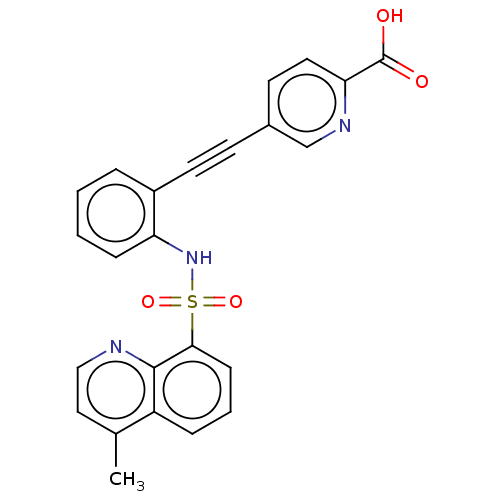
(CHEMBL5287780)Show SMILES Cc1ccnc2c(cccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610824

(CHEMBL5268966)Show SMILES OC(=O)c1ccc(cn1)C#Cc1ccccc1NS(=O)(=O)c1cccc2cccnc12 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610829
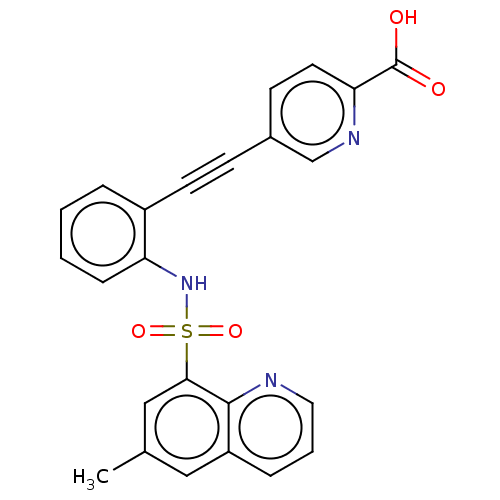
(CHEMBL5288904)Show SMILES Cc1cc(c2ncccc2c1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610831
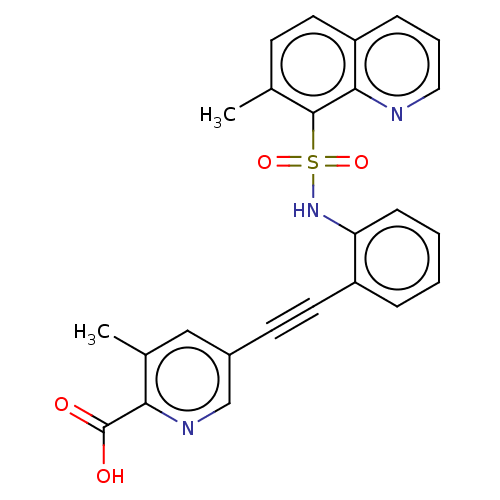
(CHEMBL5265956)Show SMILES Cc1ccc2cccnc2c1S(=O)(=O)Nc1ccccc1C#Cc1cnc(C(O)=O)c(C)c1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610825

(CHEMBL5266104)Show SMILES Cc1ccc2cccc(c2n1)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610828
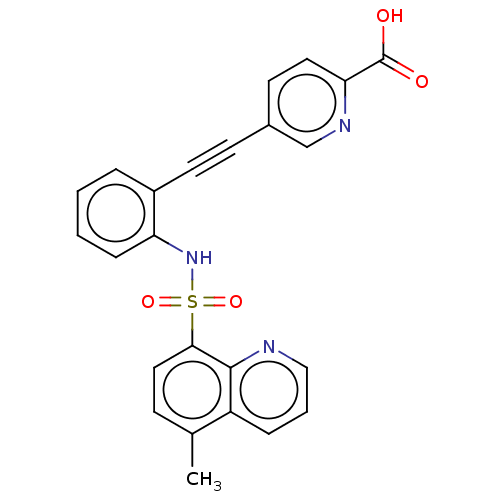
(CHEMBL5271816)Show SMILES Cc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Mus musculus) | BDBM50610868
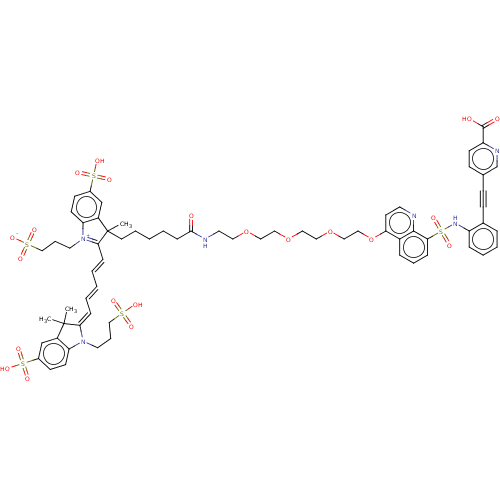
(CHEMBL5282978)Show SMILES CC1(C)\C(=C\C=C\C=C\C2=[N+](CCCS([O-])(=O)=O)c3ccc(cc3C2(C)CCCCCC(=O)NCCOCCOCCOCCOc2ccnc3c(cccc23)S(=O)(=O)Nc2ccccc2C#Cc2ccc(nc2)C(O)=O)S(O)(=O)=O)N(CCCS(O)(=O)=O)c2ccc(cc12)S(O)(=O)=O |c:9| | MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 301 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM21998
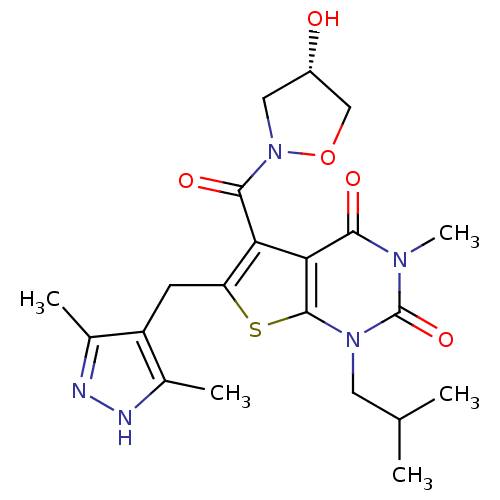
(6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-{[(4S)-...)Show SMILES CC(C)Cn1c2sc(Cc3c(C)n[nH]c3C)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H27N5O5S/c1-10(2)7-25-20-17(18(28)24(5)21(25)30)16(19(29)26-8-13(27)9-31-26)15(32-20)6-14-11(3)22-23-12(14)4/h10,13,27H,6-9H2,1-5H3,(H,22,23)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59229
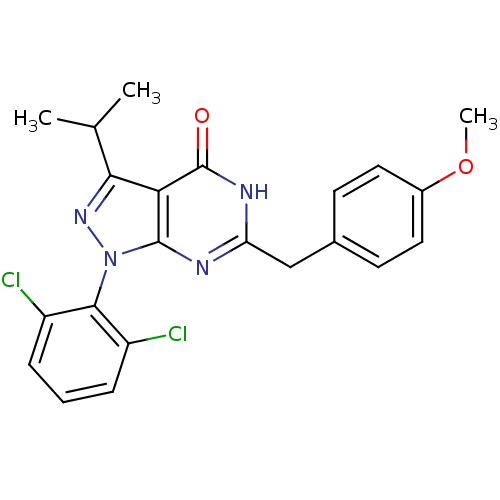
(Pyrazolopyrimidone analog, RGB-286331)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cccc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H20Cl2N4O2/c1-12(2)19-18-21(28(27-19)20-15(23)5-4-6-16(20)24)25-17(26-22(18)29)11-13-7-9-14(30-3)10-8-13/h4-10,12H,11H2,1-3H3,(H,25,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM21998

(6-[(3,5-dimethyl-1H-pyrazol-4-yl)methyl]-5-{[(4S)-...)Show SMILES CC(C)Cn1c2sc(Cc3c(C)n[nH]c3C)c(C(=O)N3C[C@H](O)CO3)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H27N5O5S/c1-10(2)7-25-20-17(18(28)24(5)21(25)30)16(19(29)26-8-13(27)9-31-26)15(32-20)6-14-11(3)22-23-12(14)4/h10,13,27H,6-9H2,1-5H3,(H,22,23)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59228
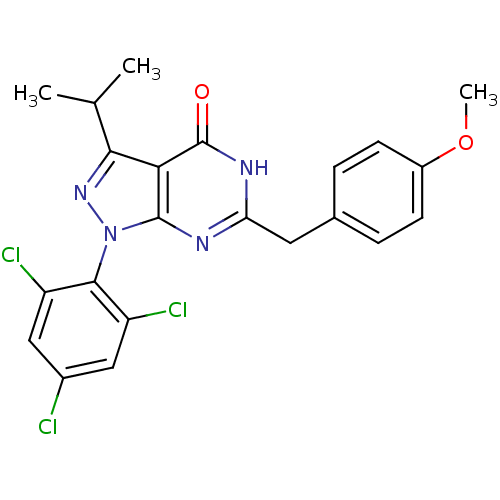
(Pyrazolopyrimidone analog, RGB-285940)Show SMILES COc1ccc(Cc2nc3n(nc(C(C)C)c3c(=O)[nH]2)-c2c(Cl)cc(Cl)cc2Cl)cc1 |(-14.46,2.58,;-13.13,3.35,;-11.8,2.58,;-11.8,1.04,;-10.46,.27,;-9.13,1.04,;-7.8,.27,;-6.46,1.04,;-5.13,.27,;-3.79,1.04,;-2.33,.57,;-1.43,1.81,;-2.33,3.06,;-1.56,4.39,;-2.33,5.73,;-.02,4.39,;-3.79,2.58,;-5.13,3.35,;-5.13,4.89,;-6.46,2.58,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-.74,-5.38,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-9.13,2.58,;-10.46,3.35,)| Show InChI InChI=1S/C22H19Cl3N4O2/c1-11(2)19-18-21(29(28-19)20-15(24)9-13(23)10-16(20)25)26-17(27-22(18)30)8-12-4-6-14(31-3)7-5-12/h4-7,9-11H,8H2,1-3H3,(H,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50103571
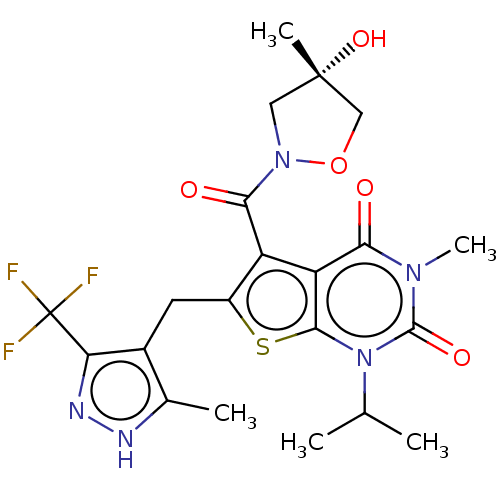
(CHEMBL3335793)Show SMILES CC(C)n1c2sc(Cc3c(C)[nH]nc3C(F)(F)F)c(C(=O)N3C[C@](C)(O)CO3)c2c(=O)n(C)c1=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531149

(CHEMBL4572028)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]cc(Cl)c2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H15Cl2FN4O3/c20-11-3-10(4-12(22)5-11)7-25-17(27)19(29)1-2-26(18(19)28)13-6-14-15(21)9-24-16(14)23-8-13/h3-6,8-9,29H,1-2,7H2,(H,23,24)(H,25,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59230
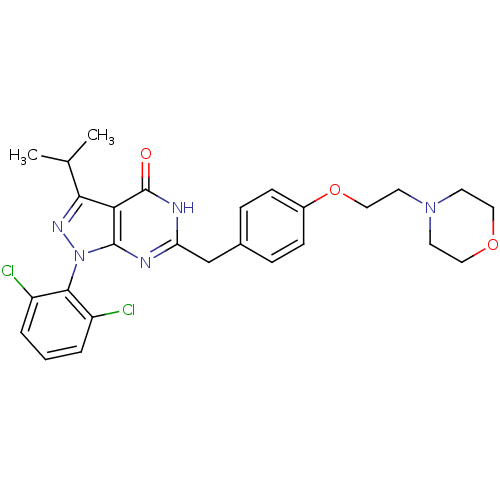
(Pyrazolopyrimidone analog, RGB-285960)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCN4CCOCC4)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-18.47,3.35,;-19.8,2.58,;-19.8,1.04,;-18.47,.27,;-17.13,1.04,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C27H29Cl2N5O3/c1-17(2)24-23-26(34(32-24)25-20(28)4-3-5-21(25)29)30-22(31-27(23)35)16-18-6-8-19(9-7-18)37-15-12-33-10-13-36-14-11-33/h3-9,17H,10-16H2,1-2H3,(H,30,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 3/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59227
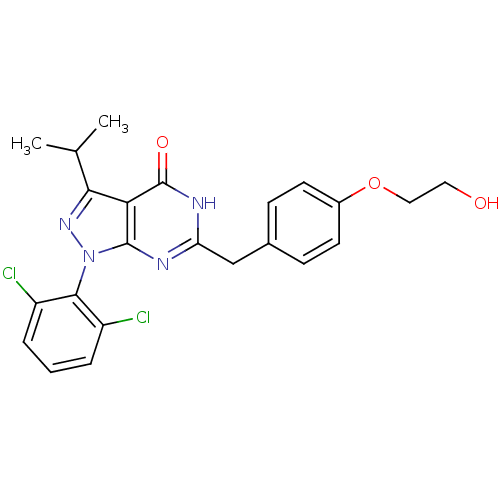
(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531149

(CHEMBL4572028)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]cc(Cl)c2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H15Cl2FN4O3/c20-11-3-10(4-12(22)5-11)7-25-17(27)19(29)1-2-26(18(19)28)13-6-14-15(21)9-24-16(14)23-8-13/h3-6,8-9,29H,1-2,7H2,(H,23,24)(H,25,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531146

(CHEMBL4463138)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C20H17ClFN3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531146

(CHEMBL4463138)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C20H17ClFN3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401308

(US10005756, Compound A79)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]cc(Cl)c2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C19H15ClF2N4O3/c20-15-9-24-16-14(15)6-13(8-23-16)26-2-1-19(29,18(26)28)17(27)25-7-10-3-11(21)5-12(22)4-10/h3-6,8-9,29H,1-2,7H2,(H,23,24)(H,25,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM401308

(US10005756, Compound A79)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]cc(Cl)c2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C19H15ClF2N4O3/c20-15-9-24-16-14(15)6-13(8-23-16)26-2-1-19(29,18(26)28)17(27)25-7-10-3-11(21)5-12(22)4-10/h3-6,8-9,29H,1-2,7H2,(H,23,24)(H,25,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610832
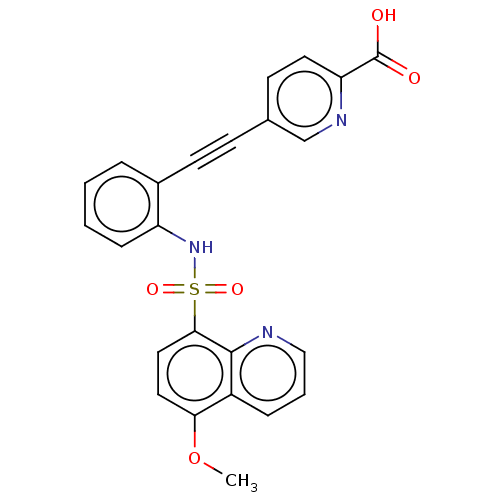
(CHEMBL5282741)Show SMILES COc1ccc(c2ncccc12)S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531155

(CHEMBL4553094)Show SMILES Cc1cc2cc(ccc2[nH]1)N1CC[C@](O)(C(=O)NCc2cc(F)cc(Cl)c2)C1=O |r| Show InChI InChI=1S/C21H19ClFN3O3/c1-12-6-14-9-17(2-3-18(14)25-12)26-5-4-21(29,20(26)28)19(27)24-11-13-7-15(22)10-16(23)8-13/h2-3,6-10,25,29H,4-5,11H2,1H3,(H,24,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531162

(CHEMBL4445117)Show SMILES CCNC(=O)c1cc2cc(ccc2[nH]1)N1CC[C@](O)(C(=O)NCc2cc(F)cc(Cl)c2)C1=O |r| Show InChI InChI=1S/C23H22ClFN4O4/c1-2-26-20(30)19-10-14-9-17(3-4-18(14)28-19)29-6-5-23(33,22(29)32)21(31)27-12-13-7-15(24)11-16(25)8-13/h3-4,7-11,28,33H,2,5-6,12H2,1H3,(H,26,30)(H,27,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531155

(CHEMBL4553094)Show SMILES Cc1cc2cc(ccc2[nH]1)N1CC[C@](O)(C(=O)NCc2cc(F)cc(Cl)c2)C1=O |r| Show InChI InChI=1S/C21H19ClFN3O3/c1-12-6-14-9-17(2-3-18(14)25-12)26-5-4-21(29,20(26)28)19(27)24-11-13-7-15(22)10-16(23)8-13/h2-3,6-10,25,29H,4-5,11H2,1H3,(H,24,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531162

(CHEMBL4445117)Show SMILES CCNC(=O)c1cc2cc(ccc2[nH]1)N1CC[C@](O)(C(=O)NCc2cc(F)cc(Cl)c2)C1=O |r| Show InChI InChI=1S/C23H22ClFN4O4/c1-2-26-20(30)19-10-14-9-17(3-4-18(14)28-19)29-6-5-23(33,22(29)32)21(31)27-12-13-7-15(24)11-16(25)8-13/h3-4,7-11,28,33H,2,5-6,12H2,1H3,(H,26,30)(H,27,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862

(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM59227

(Pyrazolopyrimidone analog, RGB-286147)Show SMILES CC(C)c1nn(-c2c(Cl)cccc2Cl)c2nc(Cc3ccc(OCCO)cc3)[nH]c(=O)c12 |(-2.33,5.73,;-1.56,4.39,;-.02,4.39,;-2.33,3.06,;-1.43,1.81,;-2.33,.57,;-1.93,-.92,;-.44,-1.32,;.64,-.23,;-.05,-2.8,;-1.13,-3.89,;-2.62,-3.49,;-3.02,-2.01,;-4.51,-1.61,;-3.79,1.04,;-5.13,.27,;-6.46,1.04,;-7.8,.27,;-9.13,1.04,;-10.46,.27,;-11.8,1.04,;-11.8,2.58,;-13.13,3.35,;-14.46,2.58,;-15.8,3.35,;-17.13,2.58,;-10.46,3.35,;-9.13,2.58,;-6.46,2.58,;-5.13,3.35,;-5.13,4.89,;-3.79,2.58,)| Show InChI InChI=1S/C23H22Cl2N4O3/c1-13(2)20-19-22(29(28-20)21-16(24)4-3-5-17(21)25)26-18(27-23(19)31)12-14-6-8-15(9-7-14)32-11-10-30/h3-9,13,30H,10-12H2,1-2H3,(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GPC Biotech, Inc.
| Assay Description
Inhibition of in vitro kinase activity of purified CDKs. |
Chem Biol 12: 1103-15 (2005)
Article DOI: 10.1016/j.chembiol.2005.08.008
BindingDB Entry DOI: 10.7270/Q28W3BR6 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50524862

(CHEMBL4475680)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C21H19ClFN3O4/c22-14-7-12(8-15(23)10-14)11-24-19(28)21(30)5-6-26(20(21)29)16-2-3-17-13(9-16)1-4-18(27)25-17/h2-3,7-10,30H,1,4-6,11H2,(H,24,28)(H,25,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531157

(CHEMBL4550096)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1F |r| Show InChI InChI=1S/C21H18ClF2N3O4/c22-15-9-13(23)7-12(18(15)24)10-25-19(29)21(31)5-6-27(20(21)30)14-2-3-16-11(8-14)1-4-17(28)26-16/h2-3,7-9,31H,1,4-6,10H2,(H,25,29)(H,26,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531157

(CHEMBL4550096)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2NC(=O)CCc2c1)C(=O)NCc1cc(F)cc(Cl)c1F |r| Show InChI InChI=1S/C21H18ClF2N3O4/c22-15-9-13(23)7-12(18(15)24)10-25-19(29)21(31)5-6-27(20(21)30)14-2-3-16-11(8-14)1-4-17(28)26-16/h2-3,7-9,31H,1,4-6,10H2,(H,25,29)(H,26,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Monocarboxylate transporter 4
(Homo sapiens (Human)) | BDBM50610830

(CHEMBL5267752)Show SMILES Cc1ccc2cccnc2c1S(=O)(=O)Nc1ccccc1C#Cc1ccc(nc1)C(O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531161

(CHEMBL4448724)Show SMILES O[C@@]1(CCN(C1=O)c1cnc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C19H16ClFN4O3/c20-13-5-11(6-14(21)8-13)9-24-17(26)19(28)2-4-25(18(19)27)15-7-12-1-3-22-16(12)23-10-15/h1,3,5-8,10,28H,2,4,9H2,(H,22,23)(H,24,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM50531163

(CHEMBL4464946)Show SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C20H17F2N3O3/c21-14-7-12(8-15(22)10-14)11-24-18(26)20(28)4-6-25(19(20)27)16-1-2-17-13(9-16)3-5-23-17/h1-3,5,7-10,23,28H,4,6,11H2,(H,24,26)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare
Curated by ChEMBL
| Assay Description
Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay |
J Med Chem 62: 11119-11134 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01070
BindingDB Entry DOI: 10.7270/Q26H4MXQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data