 Found 21 hits with Last Name = 'iram' and Initial = 'f'
Found 21 hits with Last Name = 'iram' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50218973
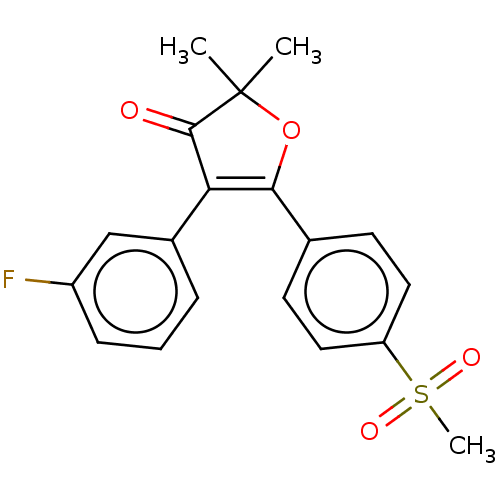
(CHEMBL44001)Show SMILES CC1(C)OC(=C(C1=O)c1cccc(F)c1)c1ccc(cc1)S(C)(=O)=O |c:4| Show InChI InChI=1S/C19H17FO4S/c1-19(2)18(21)16(13-5-4-6-14(20)11-13)17(24-19)12-7-9-15(10-8-12)25(3,22)23/h4-11H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of mouse COX-2 in LPS-induced mouse peritoneal macrophage assessed as reduction in PGE2 release incubated for 2 hrs by ELISA |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Apis mellifera) | BDBM50524139
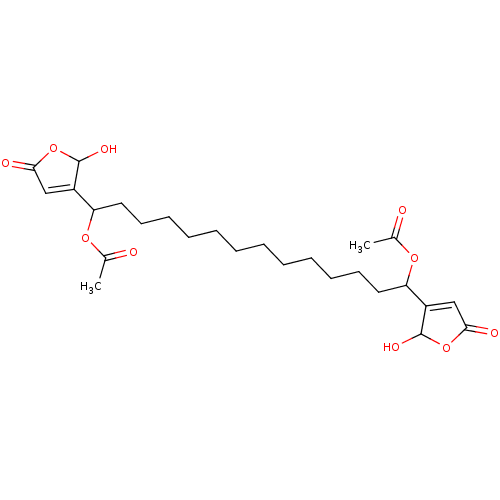
(CHEMBL4467602)Show SMILES CC(=O)OC(CCCCCCCCCCCCC(OC(C)=O)C1=CC(=O)OC1O)C1=CC(=O)OC1O |t:22,30| Show InChI InChI=1S/C26H38O10/c1-17(27)33-21(19-15-23(29)35-25(19)31)13-11-9-7-5-3-4-6-8-10-12-14-22(34-18(2)28)20-16-24(30)36-26(20)32/h15-16,21-22,25-26,31-32H,3-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of bee venom phospholipase A2 preincubated for 1 hr followed by addition of phosphotidylcholine and 1-palmitoyl-2-(1-14C) palmitoyl furthe... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Apis mellifera) | BDBM50524135

(CHEMBL4570349)Show SMILES CC(C)(C)C(=O)OC(CCCCCCCCCCCCC(OC(=O)C(C)(C)C)C1=CC(=O)OC1O)C1=CC(=O)OC1O |t:28,36| Show InChI InChI=1S/C32H50O10/c1-31(2,3)29(37)39-23(21-19-25(33)41-27(21)35)17-15-13-11-9-7-8-10-12-14-16-18-24(40-30(38)32(4,5)6)22-20-26(34)42-28(22)36/h19-20,23-24,27-28,35-36H,7-18H2,1-6H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of bee venom phospholipase A2 preincubated for 1 hr followed by addition of phosphotidylcholine and 1-palmitoyl-2-(1-14C) palmitoyl furthe... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Apis mellifera) | BDBM50524140
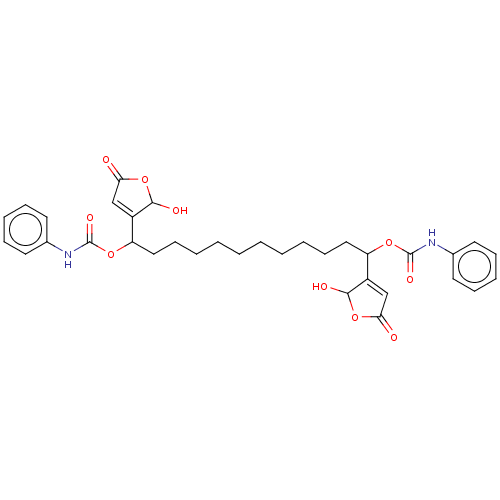
(CHEMBL4447483)Show SMILES OC1OC(=O)C=C1C(CCCCCCCCCCC(OC(=O)Nc1ccccc1)C1=CC(=O)OC1O)OC(=O)Nc1ccccc1 |c:5,t:31| Show InChI InChI=1S/C34H40N2O10/c37-29-21-25(31(39)45-29)27(43-33(41)35-23-15-9-7-10-16-23)19-13-5-3-1-2-4-6-14-20-28(26-22-30(38)46-32(26)40)44-34(42)36-24-17-11-8-12-18-24/h7-12,15-18,21-22,27-28,31-32,39-40H,1-6,13-14,19-20H2,(H,35,41)(H,36,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of bee venom phospholipase A2 preincubated for 1 hr followed by addition of phosphotidylcholine and 1-palmitoyl-2-(1-14C) palmitoyl furthe... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50143486
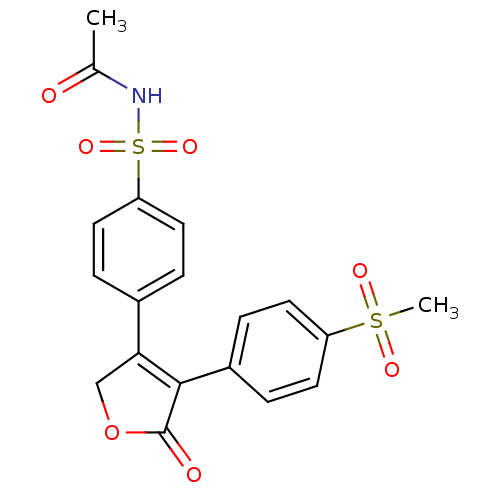
(CHEMBL366422 | N-Acetyl-4-[4-(4-methanesulfonyl-ph...)Show SMILES CC(=O)NS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccc(cc1)S(C)(=O)=O |t:14| Show InChI InChI=1S/C19H17NO7S2/c1-12(21)20-29(25,26)16-9-3-13(4-10-16)17-11-27-19(22)18(17)14-5-7-15(8-6-14)28(2,23)24/h3-10H,11H2,1-2H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 assessed as reduction in PGF2alpha production incubated for 15 mins by ELISA method |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Mus musculus (Mouse)) | BDBM50218964
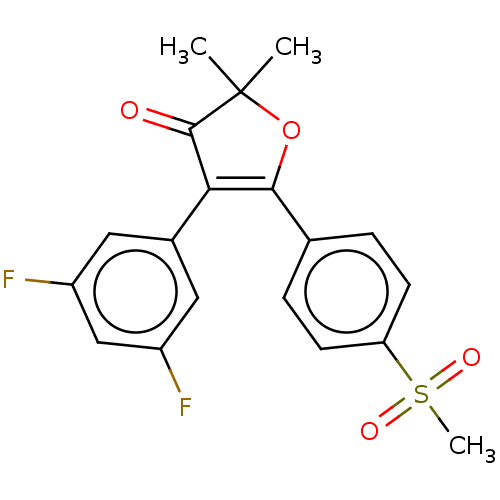
(CHEMBL44223)Show SMILES CC1(C)OC(=C(C1=O)c1cc(F)cc(F)c1)c1ccc(cc1)S(C)(=O)=O |c:4| Show InChI InChI=1S/C19H16F2O4S/c1-19(2)18(22)16(12-8-13(20)10-14(21)9-12)17(25-19)11-4-6-15(7-5-11)26(3,23)24/h4-10H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of mouse COX-2 in LPS-induced mouse peritoneal macrophage assessed as reduction in PGE2 release incubated for 2 hrs by ELISA |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50127653

(1-(4-Methanesulfonyl-phenyl)-4,5-dihydro-3aH-napht...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C2C(CCc3ccccc23)OC1=O |t:11| Show InChI InChI=1S/C19H16O4S/c1-24(21,22)14-9-6-13(7-10-14)17-18-15-5-3-2-4-12(15)8-11-16(18)23-19(17)20/h2-7,9-10,16H,8,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf9 cells |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50127652

(1-(4-Methanesulfonyl-phenyl)-8-methyl-4,5-dihydro-...)Show SMILES Cc1ccc2CCC3OC(=O)C(=C3c2c1)c1ccc(cc1)S(C)(=O)=O |c:11| Show InChI InChI=1S/C20H18O4S/c1-12-3-4-13-7-10-17-19(16(13)11-12)18(20(21)24-17)14-5-8-15(9-6-14)25(2,22)23/h3-6,8-9,11,17H,7,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human COX2 expressed in baculovirus infected Sf9 cells |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Cavia porcellus) | BDBM50078688
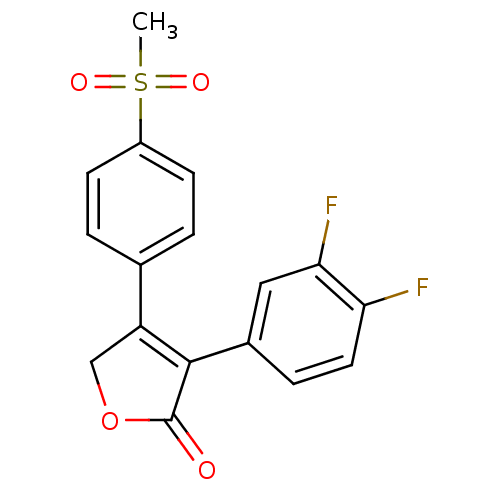
(3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C17H12F2O4S/c1-24(21,22)12-5-2-10(3-6-12)13-9-23-17(20)16(13)11-4-7-14(18)15(19)8-11/h2-8H,9H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in guinea pig blood assessed as reduction of LPS-induced PGE2 accumulation at 10 to 10000 nM preincubated with LPS followed by com... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Apis mellifera) | BDBM50524136

(CHEMBL4553594)Show SMILES CC(C)(C)C(=O)OC(CCCCCCCCCCCCC(OC(=O)C(C)(C)C)C1=CC(=O)OC1OC(=O)Nc1ccccc1)C1=CC(=O)OC1OC(=O)Nc1ccccc1 |t:28,46| Show InChI InChI=1S/C46H60N2O12/c1-45(2,3)41(51)55-35(33-29-37(49)57-39(33)59-43(53)47-31-23-17-15-18-24-31)27-21-13-11-9-7-8-10-12-14-22-28-36(56-42(52)46(4,5)6)34-30-38(50)58-40(34)60-44(54)48-32-25-19-16-20-26-32/h15-20,23-26,29-30,35-36,39-40H,7-14,21-22,27-28H2,1-6H3,(H,47,53)(H,48,54) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of bee venom phospholipase A2 preincubated for 1 hr followed by addition of phosphotidylcholine and 1-palmitoyl-2-(1-14C) palmitoyl furthe... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50125626
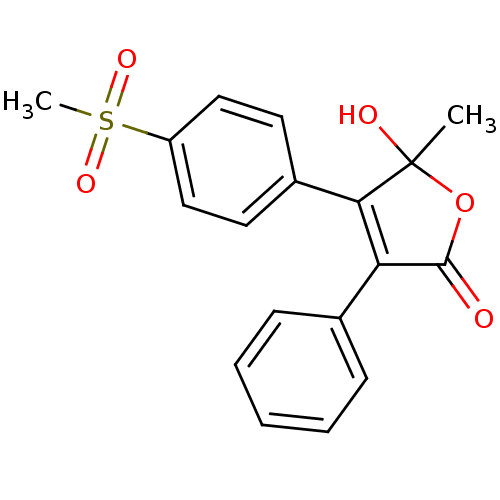
(5-Hydroxy-4-(4-methanesulfonyl-phenyl)-5-methyl-3-...)Show SMILES CC1(O)OC(=O)C(=C1c1ccc(cc1)S(C)(=O)=O)c1ccccc1 |c:6| Show InChI InChI=1S/C18H16O5S/c1-18(20)16(13-8-10-14(11-9-13)24(2,21)22)15(17(19)23-18)12-6-4-3-5-7-12/h3-11,20H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as reduction in PGE2 formation preincubated for 15 mins followed by addition of LPS and measured aft... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Tyrosinase
(Homo sapiens (Human)) | BDBM50219502
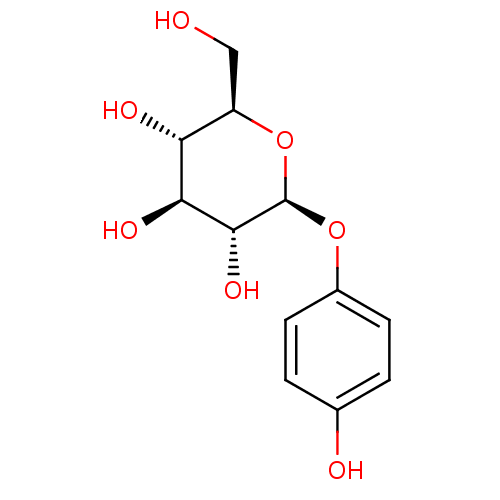
(4-hydroxyphenyl beta-D-glucopyranoside | Arbutin |...)Show SMILES OC[C@H]1O[C@@H](Oc2ccc(O)cc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C12H16O7/c13-5-8-9(15)10(16)11(17)12(19-8)18-7-3-1-6(14)2-4-7/h1-4,8-17H,5H2/t8-,9-,10+,11-,12-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) assessed as reduction in melanin formation |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50125624

(3-(4-Chloro-phenyl)-5-hydroxy-4-(4-methanesulfonyl...)Show SMILES CC1(O)OC(=O)C(=C1c1ccc(cc1)S(C)(=O)=O)c1ccc(Cl)cc1 |c:6| Show InChI InChI=1S/C18H15ClO5S/c1-18(21)16(12-5-9-14(10-6-12)25(2,22)23)15(17(20)24-18)11-3-7-13(19)8-4-11/h3-10,21H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in human whole blood assessed as reduction in PGE2 formation preincubated for 15 mins followed by addition of LPS and measured aft... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Oryctolagus cuniculus) | BDBM50078688

(3-(3,4-Difluoro-phenyl)-4-(4-methanesulfonyl-pheny...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccc(F)c(F)c1 |t:11| Show InChI InChI=1S/C17H12F2O4S/c1-24(21,22)12-5-2-10(3-6-12)13-9-23-17(20)16(13)11-4-7-14(18)15(19)8-11/h2-8H,9H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of COX2 in rabbit whole blood assessed as reduction of LPS-induced PGE2 accumulation at 10 to 10000 nM preincubated with LPS followed by c... |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Apis mellifera) | BDBM50524137
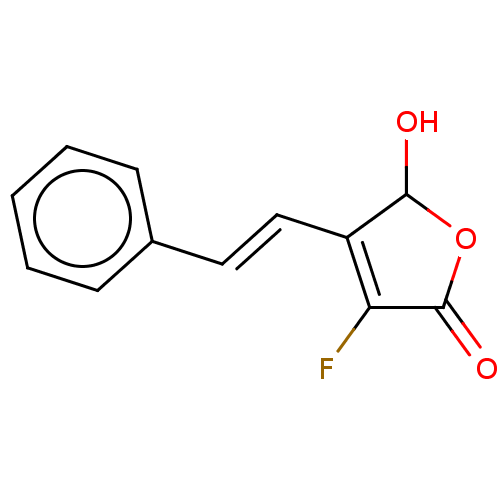
(CHEMBL4452147)Show InChI InChI=1S/C12H9FO3/c13-10-9(11(14)16-12(10)15)7-6-8-4-2-1-3-5-8/h1-7,11,14H/b7-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of bee venom phospholipase A2 |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50487746

(CHEMBL2262593)Show InChI InChI=1S/C12H11IO5/c1-16-7-4-3-6(5-8(7)17-2)11-9(13)10(14)12(15)18-11/h3-5,11,14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of GSK3alpha/beta (unknown origin) |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50487746

(CHEMBL2262593)Show InChI InChI=1S/C12H11IO5/c1-16-7-4-3-6(5-8(7)17-2)11-9(13)10(14)12(15)18-11/h3-5,11,14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p25 (unknown origin) |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50487746

(CHEMBL2262593)Show InChI InChI=1S/C12H11IO5/c1-16-7-4-3-6(5-8(7)17-2)11-9(13)10(14)12(15)18-11/h3-5,11,14H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/cyclinB (unknown origin) |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in CHO cells cells assessed as reduction in PGE2 release |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of human COX2 expressed in human osteosarcoma cells assessed as reduction in PGE2 release |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosinase
(Homo sapiens (Human)) | BDBM50524138
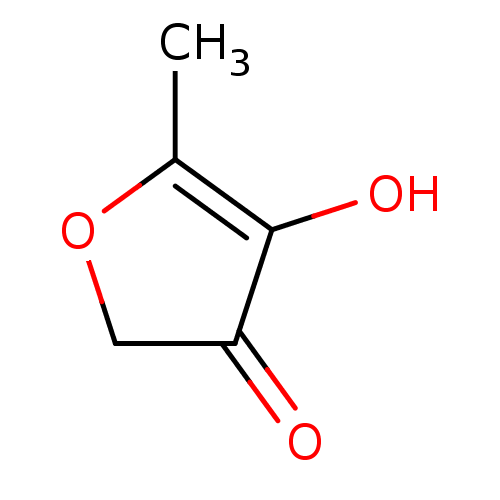
(CHEBI:74456 | CHEMBL3182150)Show InChI InChI=1S/C5H6O3/c1-3-5(7)4(6)2-8-3/h7H,2H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Hamdard
Curated by ChEMBL
| Assay Description
Inhibition of tyrosinase (unknown origin) assessed as reduction in melanin formation |
Eur J Med Chem 171: 66-92 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.021
BindingDB Entry DOI: 10.7270/Q2X92FQN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data