

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ITK/TSK (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0269 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase TXK (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.545 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.679 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891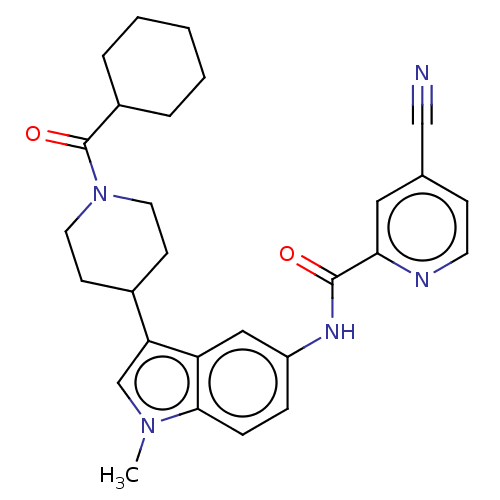 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897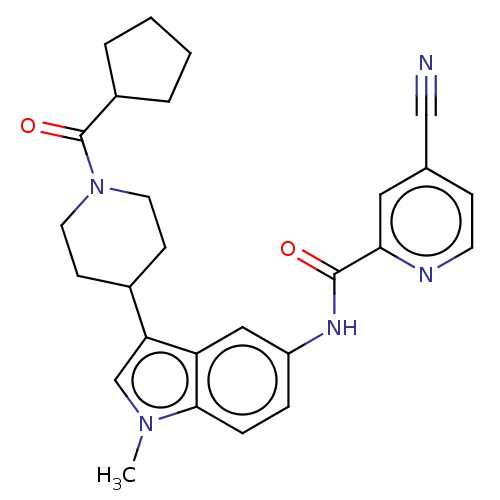 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 32.4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893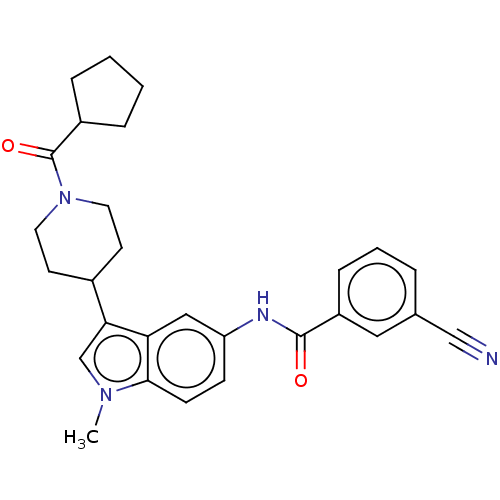 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 62.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Worldwide R&D | Assay Description Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892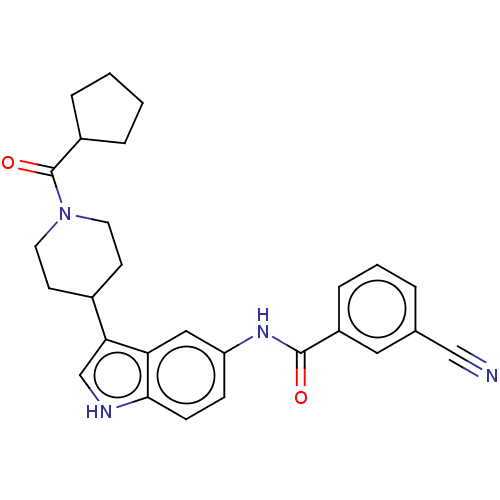 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406141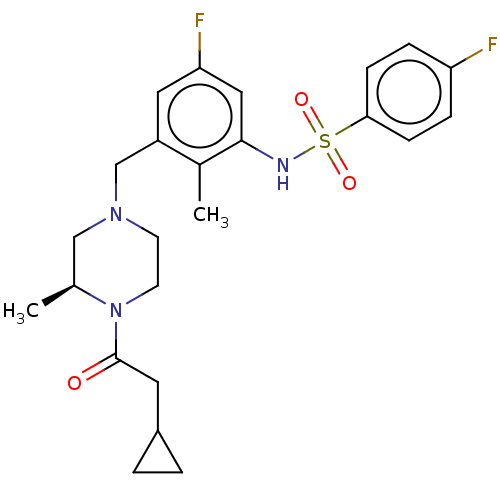 (CHEMBL5286547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406139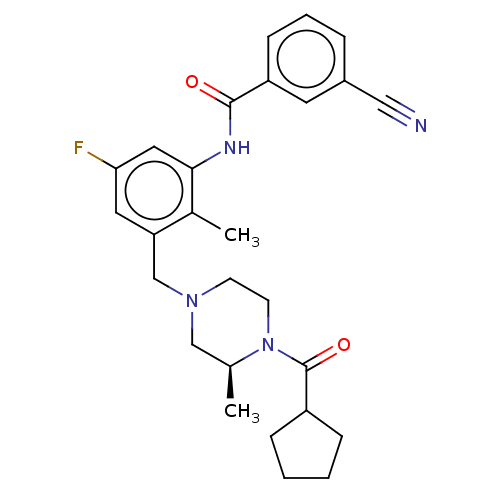 (CHEMBL5281816) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406140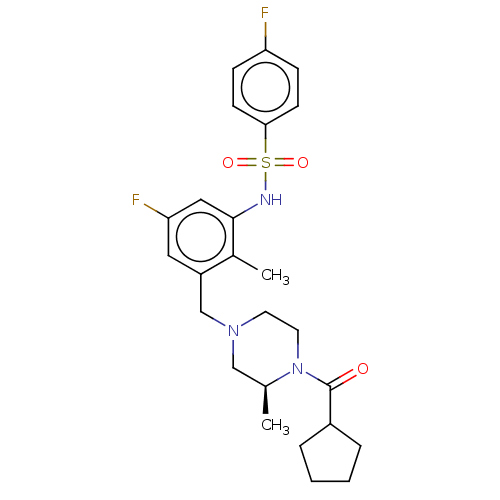 (CHEMBL5284779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406144 (CHEMBL5283506) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406145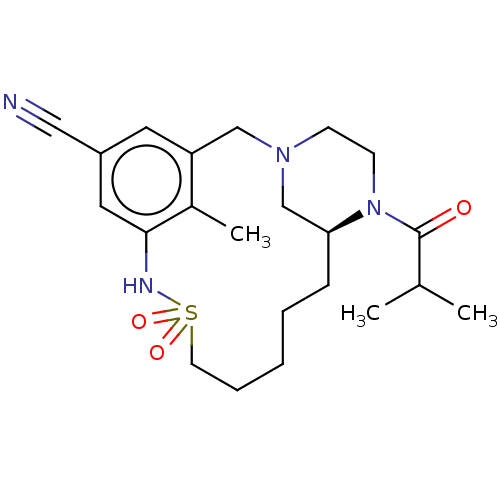 (CHEMBL5287800) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406143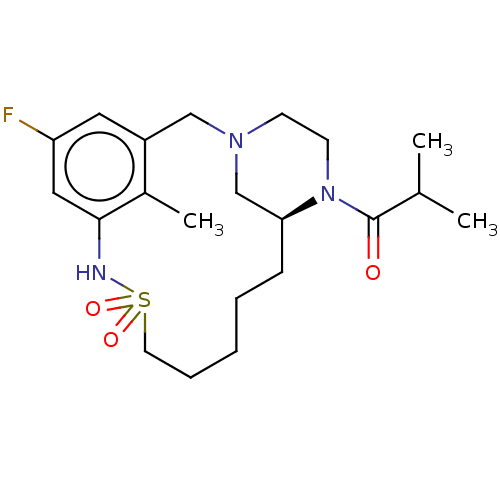 (CHEMBL5287916) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50406142 (CHEMBL5280788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480924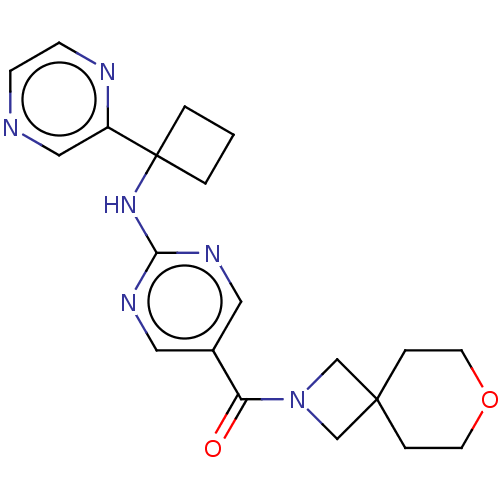 (US10906888, Example 138) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM50585537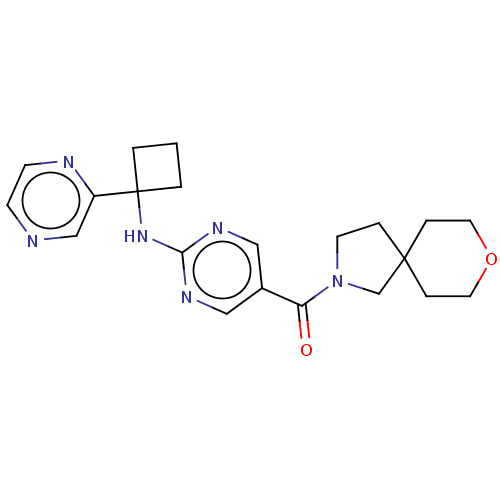 (CHEMBL5084938) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480965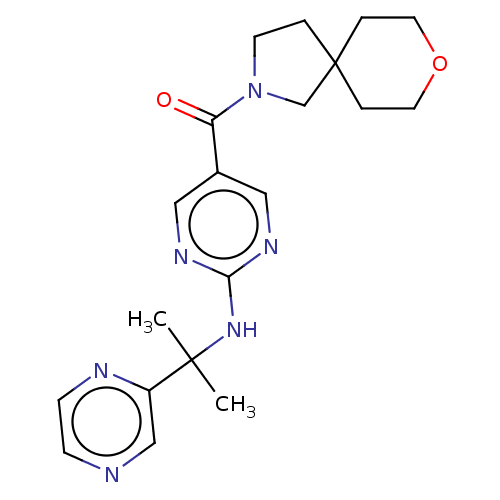 (8-oxa-2-azaspiro[4.5]dec-2-yl(2-{[2-(pyrazin-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480929 (8-oxa-2-azaspiro[4.5]dec-2-yl(2-{[2-(pyrimidin-5-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480917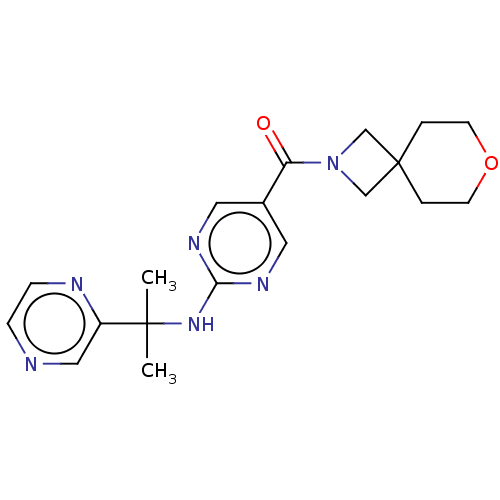 (7-oxa-2-azaspiro[3.5]non-2-yl(2-{[2-(pyrazin-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480956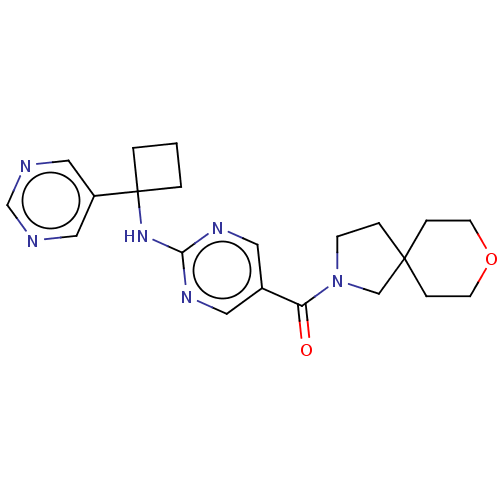 (US10906888, Example 173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480931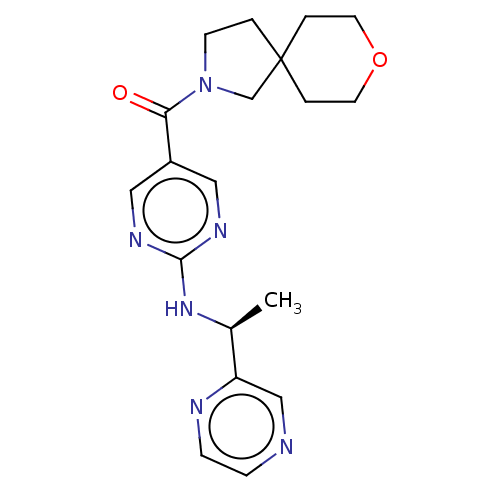 (8-oxa-2-azaspiro[4.5]dec-2-yl(2-{[(1S)-1-(pyrazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480971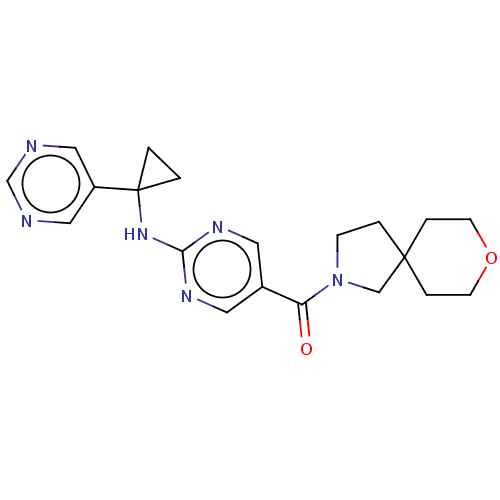 (8-oxa-2-azaspiro[4.5]dec-2-yl(2-{[1-(pyrimidin-5-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM209866 (PF-06651600 | US11111242, Example 5 | US2023034848...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.346 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480918 (US10906888, Example 132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Mus musculus) | BDBM480917 (7-oxa-2-azaspiro[3.5]non-2-yl(2-{[2-(pyrazin-2-yl)...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse vanin-1 | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480919 (US10906888, Example 133) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Mus musculus) | BDBM480931 (8-oxa-2-azaspiro[4.5]dec-2-yl(2-{[(1S)-1-(pyrazin-...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse vanin-1 | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480933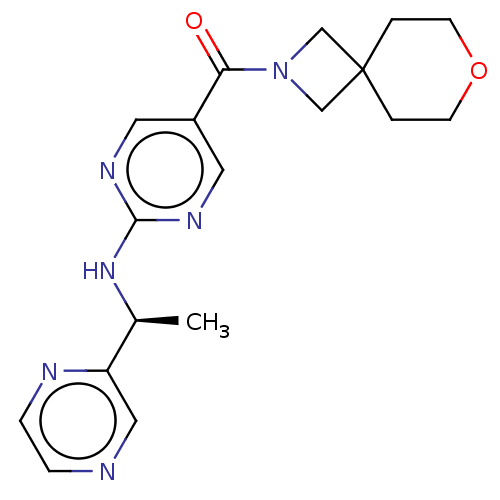 (US10906888, Example 147) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480934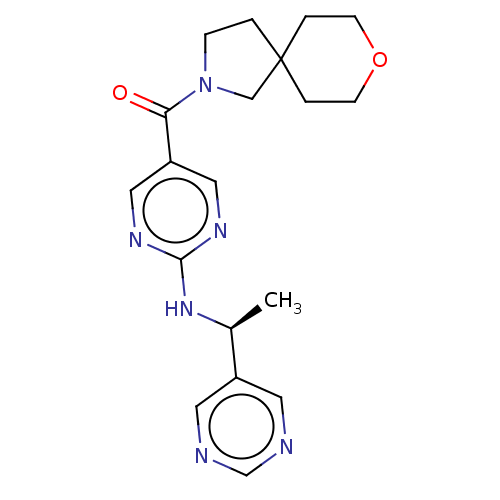 (US10906888, Example 149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480917 (7-oxa-2-azaspiro[3.5]non-2-yl(2-{[2-(pyrazin-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma vanin-1 using pantetheine-7-amino-4-trifluoromethykournarin as substrate preincubated for 30 mins followed by substrate ad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM159756 (PF-02384554 | US10966980, Example 8 | US9035074, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480783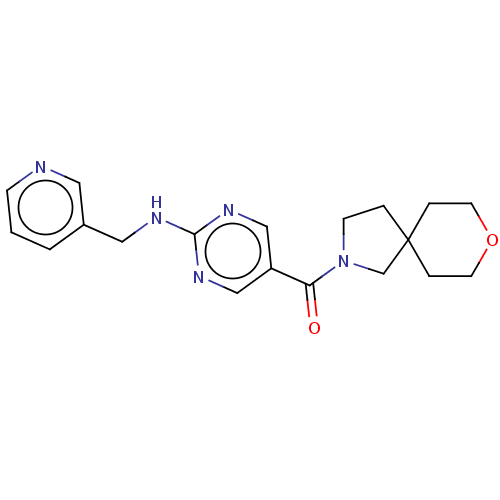 (US10906888, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480931 (8-oxa-2-azaspiro[4.5]dec-2-yl(2-{[(1S)-1-(pyrazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma vanin-1 using pantetheine-7-amino-4-trifluoromethykournarin as substrate preincubated for 30 mins followed by substrate ad... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM159756 (PF-02384554 | US10966980, Example 8 | US9035074, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50193995 (3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Worldwide R&D | Assay Description Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ... | ACS Chem Biol 11: 3442-3451 (2016) Article DOI: 10.1021/acschembio.6b00677 BindingDB Entry DOI: 10.7270/Q2PN94F8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480932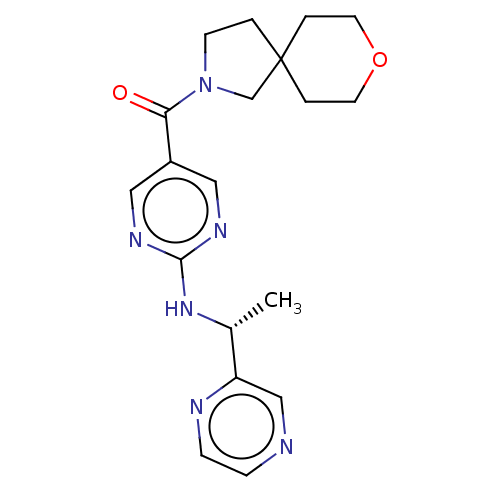 (US10906888, Example 146) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890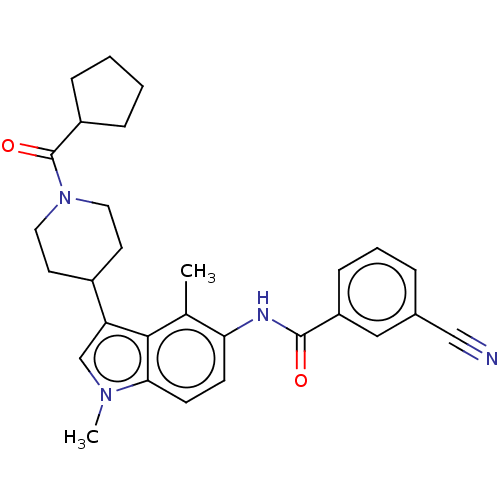 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM480921 (US10906888, Example 135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189877 (US10227346, Example 5 | US10426135, Example 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM394644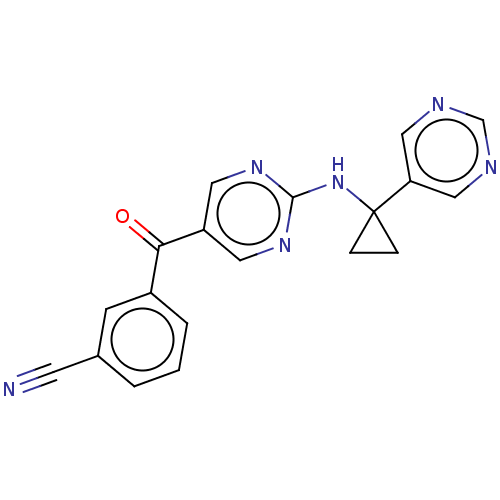 (3-[(2-{[1-(pyrimidin-5-yl)cyclopropyl]amino}pyrimi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Homo sapiens (Human)) | BDBM395107 (3-({2-[(pyridin-3- ylmethyl)amino] pyrimidin-5- yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant N-terminal FLAG/His6-tagged human vanin-1 expressed in CHO cells using Pantetheine-7-amino-4-trifluoromethykournarin as sub... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B (Homo sapiens (Human)) | BDBM50272575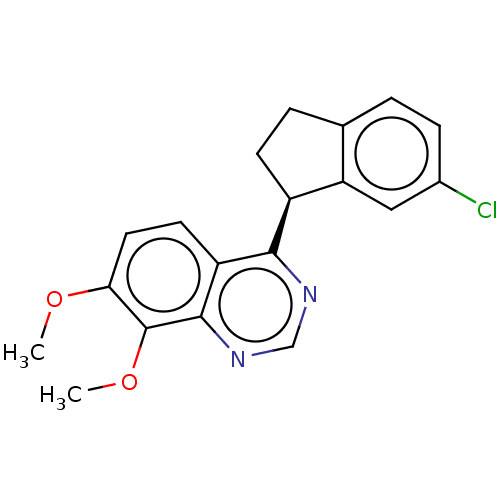 (CHEMBL4126270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of full length recombinant human PDE1B1 using 3',5'-[3H]cAMP as substrate after 30 mins by scintillation proximity assay | J Med Chem 61: 4635-4640 (2018) Article DOI: 10.1021/acs.jmedchem.8b00374 BindingDB Entry DOI: 10.7270/Q2J67KDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantetheinase (Mus musculus) | BDBM394644 (3-[(2-{[1-(pyrimidin-5-yl)cyclopropyl]amino}pyrimi...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of mouse vanin-1 | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 737 total ) | Next | Last >> |