

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044676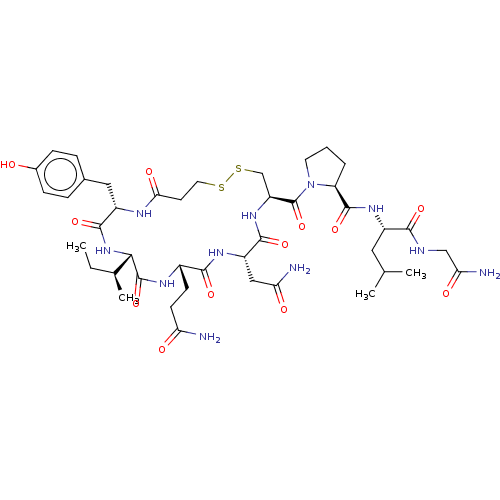 (CHEMBL439044) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180014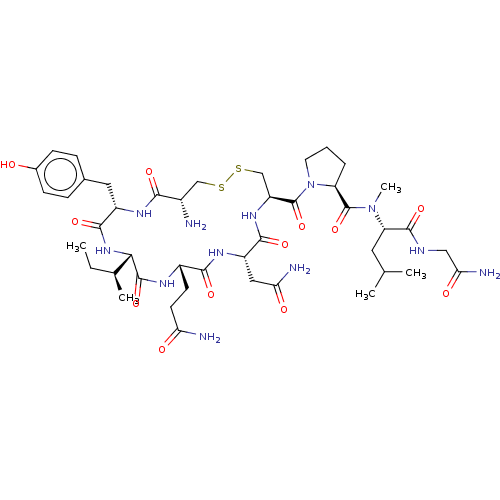 (CHEMBL3814744) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180155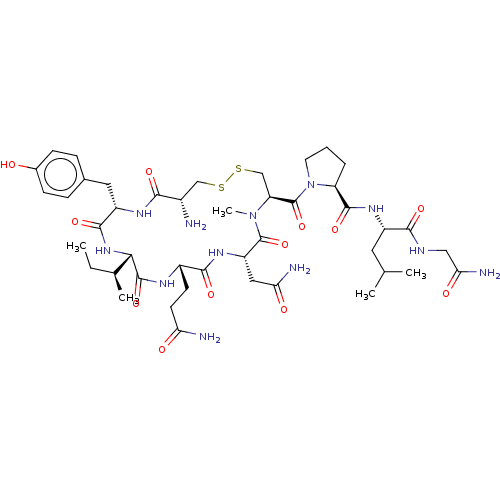 (CHEMBL3813894) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180132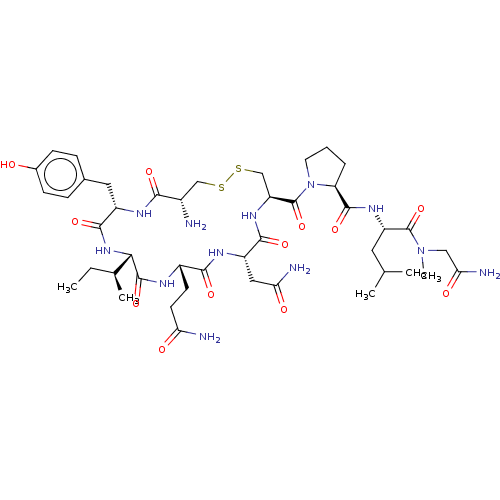 (CHEMBL3814633) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in CD1 mouse brain membranes after 60 mins by liquid scintillation counting method | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470323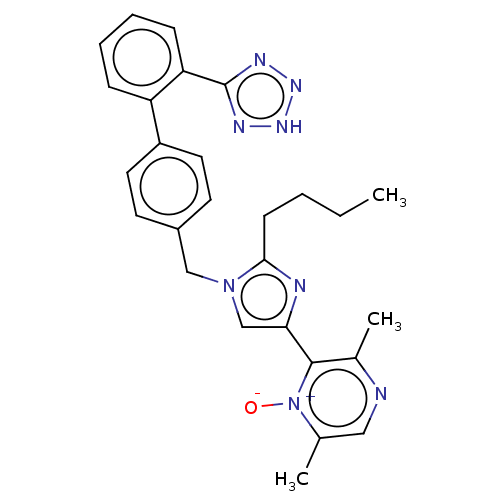 (CHEMBL327505) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50193284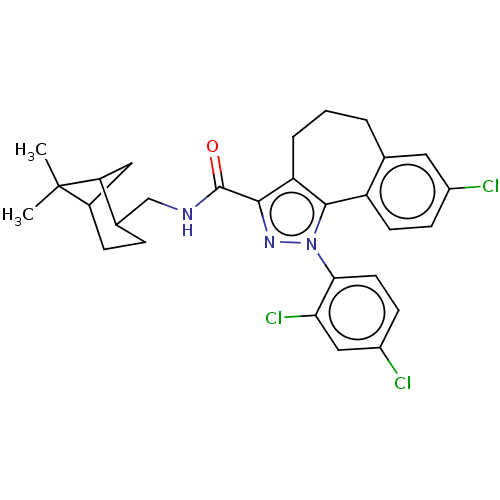 (CHEMBL3978981) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180160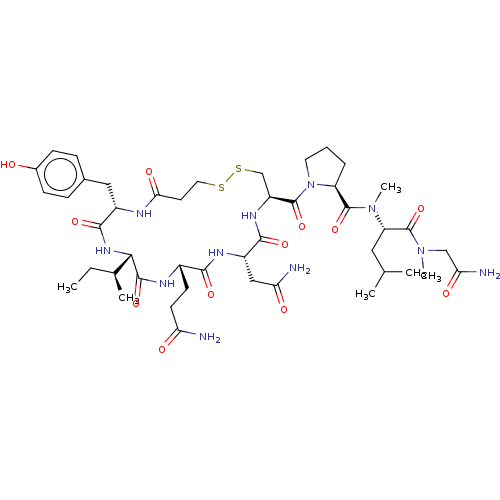 (CHEMBL3815099) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176988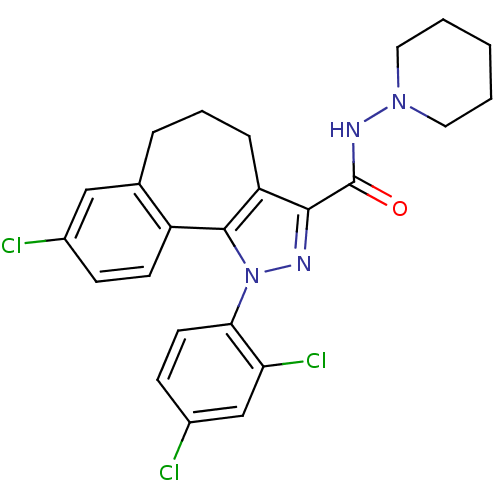 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470328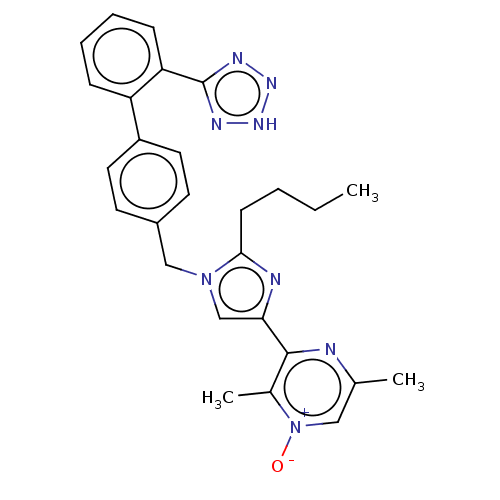 (CHEMBL317300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176980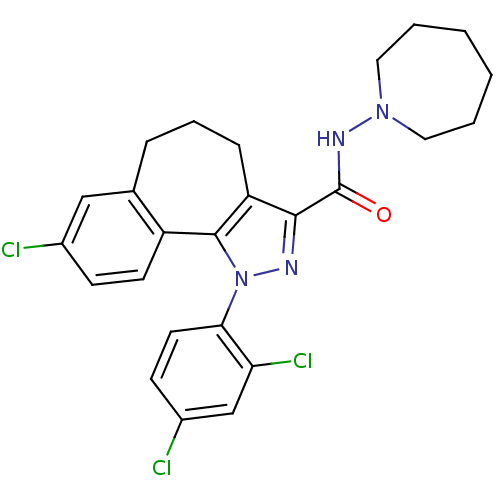 (8-chloro-1-(2',4'-dichlorophenyl)-N-homopiperidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470310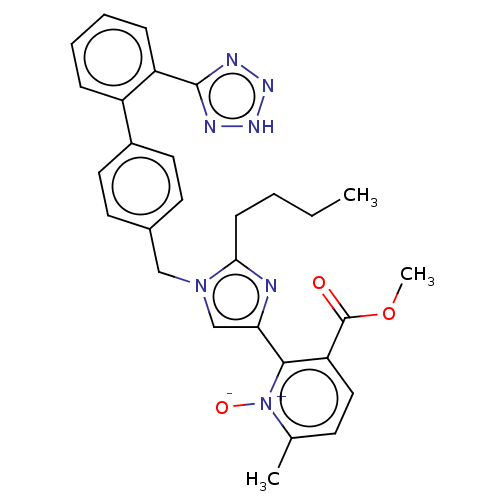 (CHEMBL98592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250387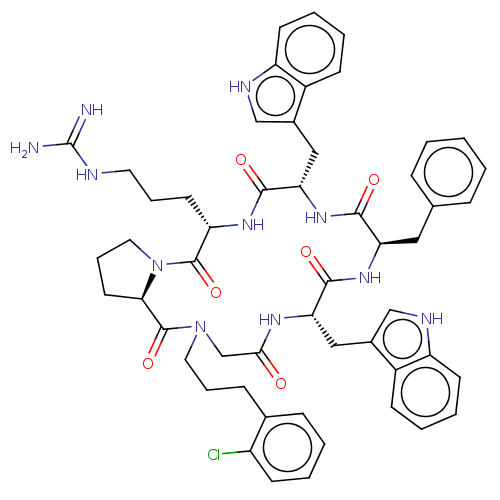 (CHEMBL4102791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250387 (CHEMBL4102791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179992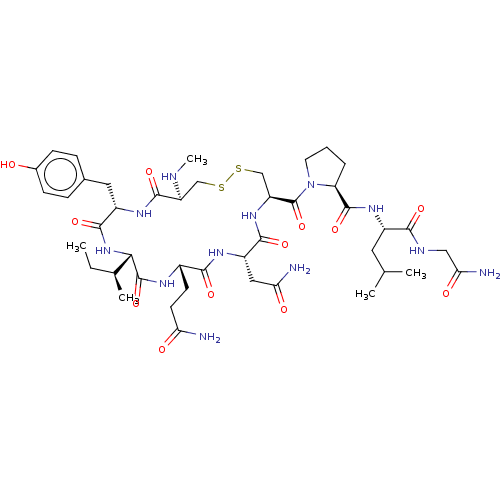 (CHEMBL3814395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50179993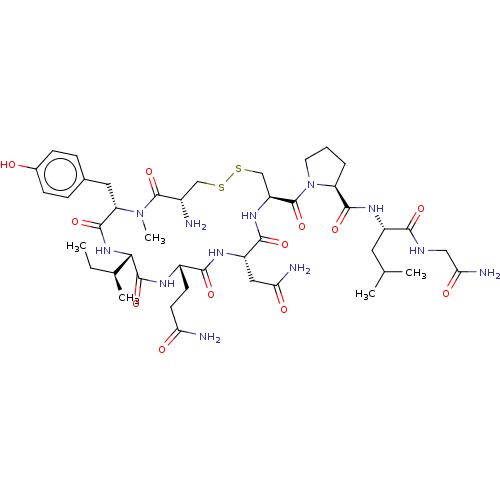 (CHEMBL3814165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470321 (CHEMBL99008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250402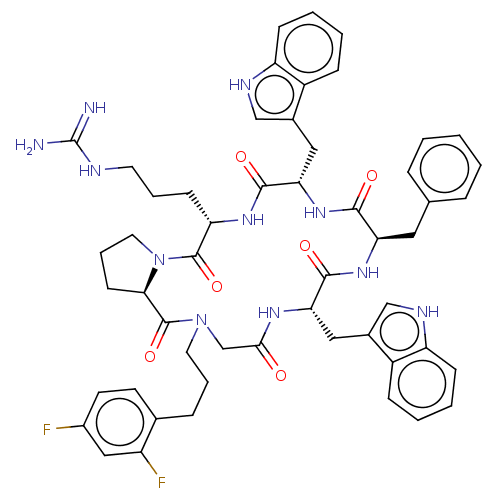 (CHEMBL4084835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250402 (CHEMBL4084835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176988 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176988 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50193280 (CHEMBL3895744) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470340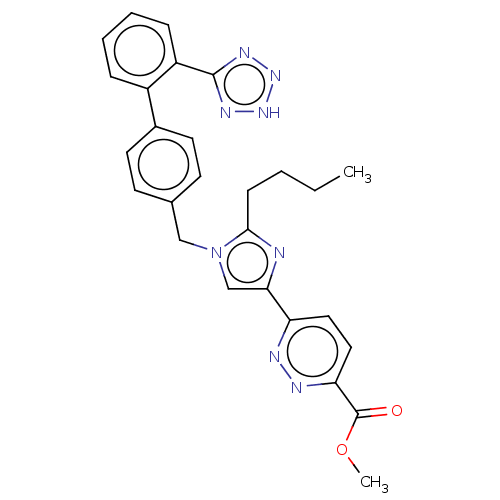 (CHEMBL98370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250375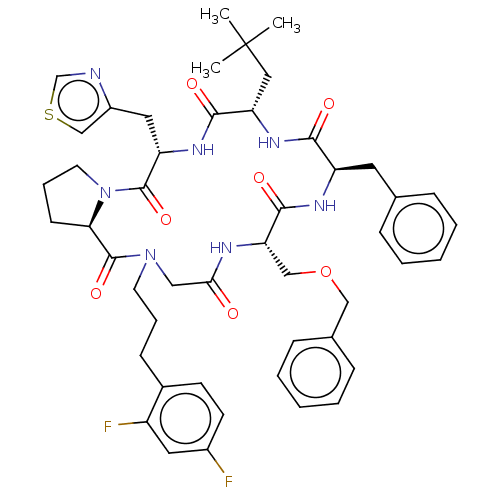 (CHEMBL4077017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250395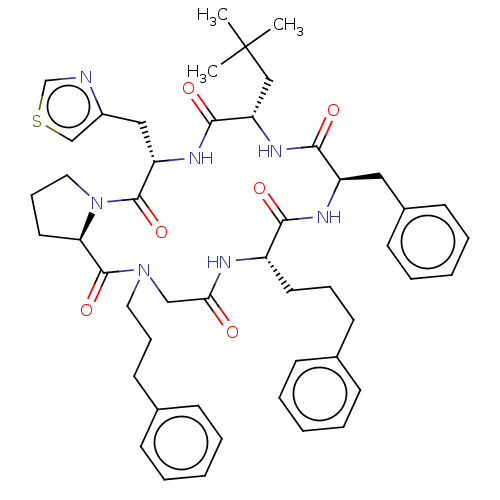 (CHEMBL4103373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250395 (CHEMBL4103373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250375 (CHEMBL4077017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470326 (CHEMBL319990) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50193277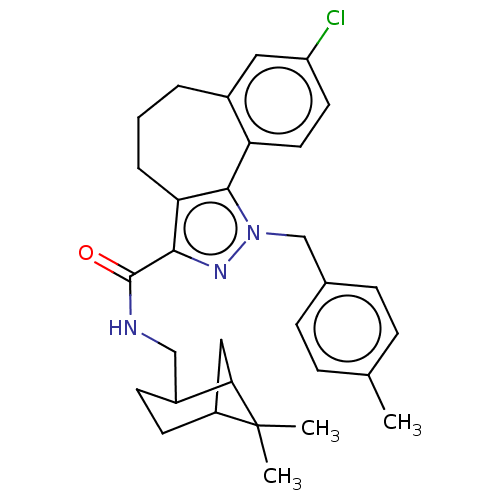 (CHEMBL3984538) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor transfected in CHO cell membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50072775 (2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor transfected in CHO cell membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128873 BindingDB Entry DOI: 10.7270/Q2TT4VX9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176976 (8-chloro-1-(2',4'-dichlorophenyl)-N-cyclohexyl-1,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50180165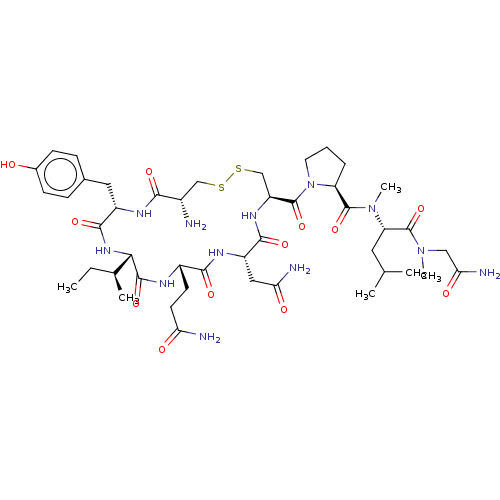 (CHEMBL3813722) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]8-arginine-vasopressin from human oxytocin receptor expressed in CHO cell membrane incubated for 1 hr by liquid scintillation cou... | Bioorg Med Chem 24: 3513-20 (2016) Article DOI: 10.1016/j.bmc.2016.05.062 BindingDB Entry DOI: 10.7270/Q2WH2RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470313 (CHEMBL441640) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470330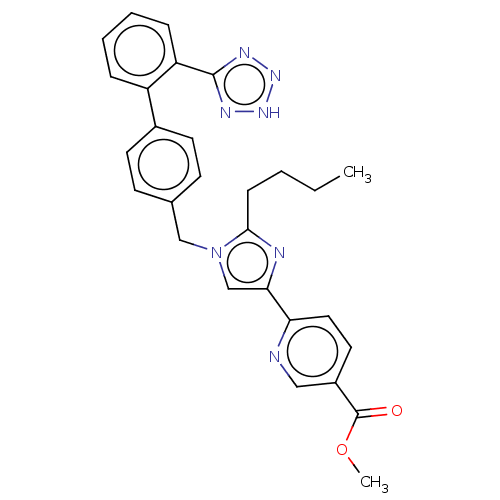 (CHEMBL318194) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50193278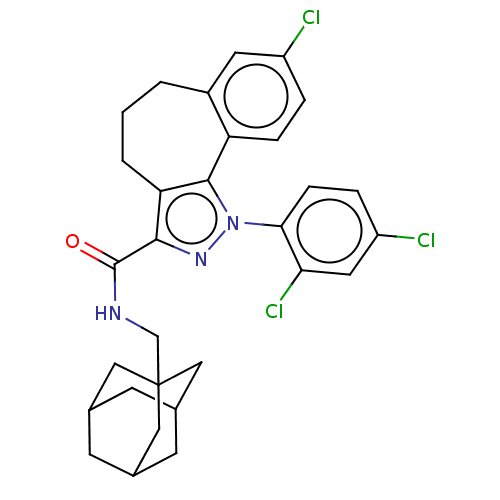 (CHEMBL3889602) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor transfected in CHO cell membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50193279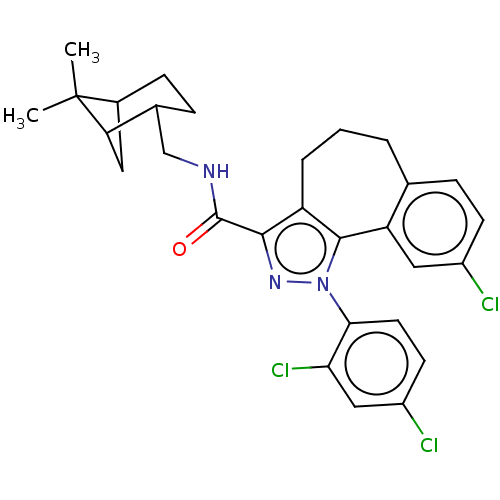 (CHEMBL3914728) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor transfected in CHO cell membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50176989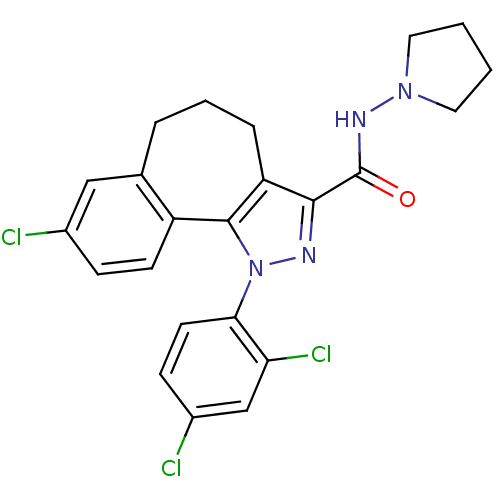 (8-chloro-1-(2',4'-dichlorophenyl)-N-pyrrolidin-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50193279 (CHEMBL3914728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50176976 (8-chloro-1-(2',4'-dichlorophenyl)-N-cyclohexyl-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor transfected in CHO cell membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50193278 (CHEMBL3889602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470317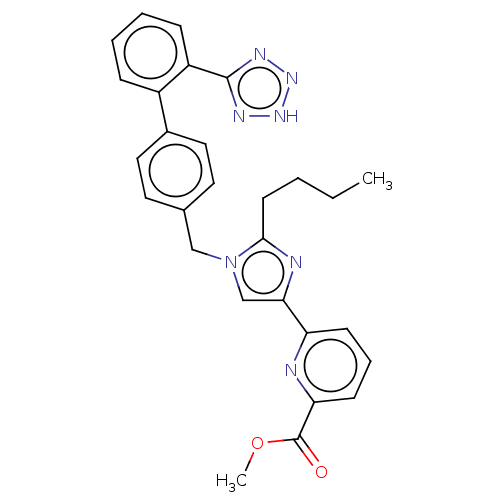 (CHEMBL319370) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description Inhibition of [3H]angiotensin II binding to rat adrenal cortex membrane containing angiotensin II receptor, type 1 | J Med Chem 38: 2925-37 (1995) Article DOI: 10.1021/jm00015a015 BindingDB Entry DOI: 10.7270/Q2697689 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50193285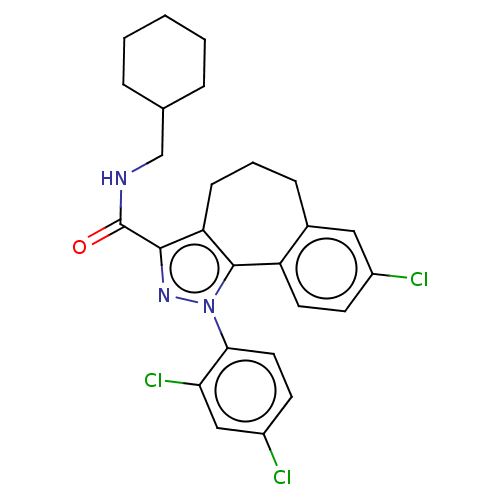 (CHEMBL3917142) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from CB1 receptor in mouse whole brain membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897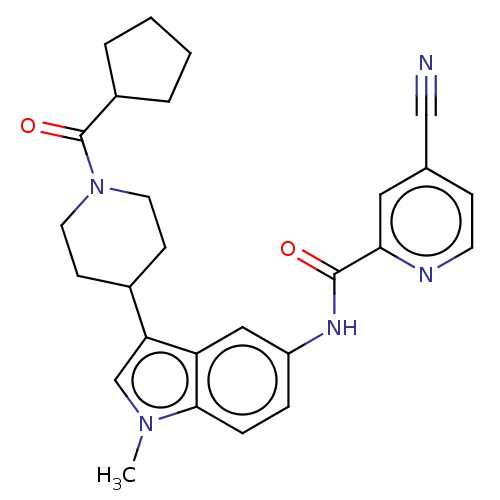 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50193284 (CHEMBL3978981) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscienze PharmaNess S.c.a r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor transfected in CHO cell membranes after 60 mins by liquid scintillation spectrometry | Eur J Med Chem 121: 194-208 (2016) Article DOI: 10.1016/j.ejmech.2016.05.011 BindingDB Entry DOI: 10.7270/Q2PR7XXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250391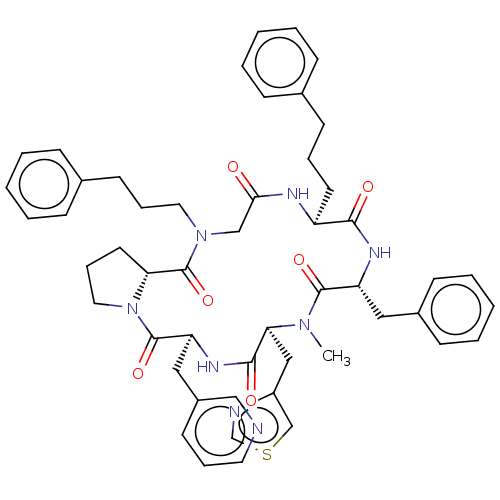 (CHEMBL4064492) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891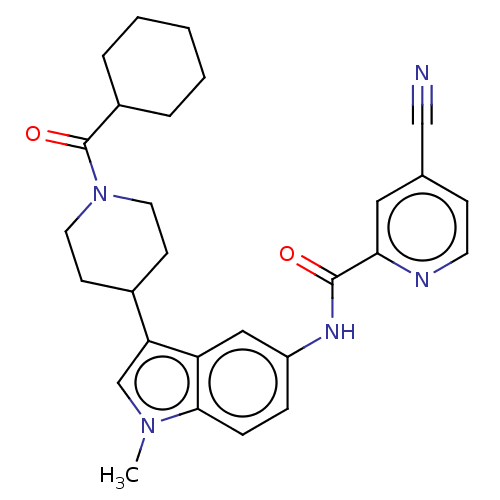 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 3506 total ) | Next | Last >> |