 Found 546 hits with Last Name = 'bifulco' and Initial = 'g'
Found 546 hits with Last Name = 'bifulco' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assay |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544523
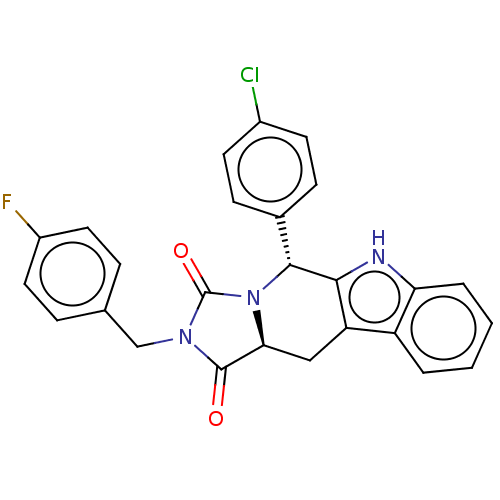
(CHEMBL4647674)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)N(Cc1ccc(F)cc1)C2=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H19ClFN3O2/c27-17-9-7-16(8-10-17)24-23-20(19-3-1-2-4-21(19)29-23)13-22-25(32)30(26(33)31(22)24)14-15-5-11-18(28)12-6-15/h1-12,22,24,29H,13-14H2/t22-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544513
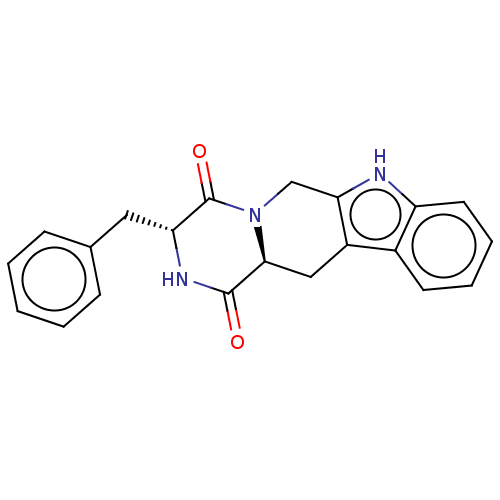
(CHEMBL4649088)Show SMILES [H][C@@]12Cc3c(CN1C(=O)[C@@H](Cc1ccccc1)NC2=O)[nH]c1ccccc31 |r| Show InChI InChI=1S/C21H19N3O2/c25-20-19-11-15-14-8-4-5-9-16(14)22-18(15)12-24(19)21(26)17(23-20)10-13-6-2-1-3-7-13/h1-9,17,19,22H,10-12H2,(H,23,25)/t17-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544531
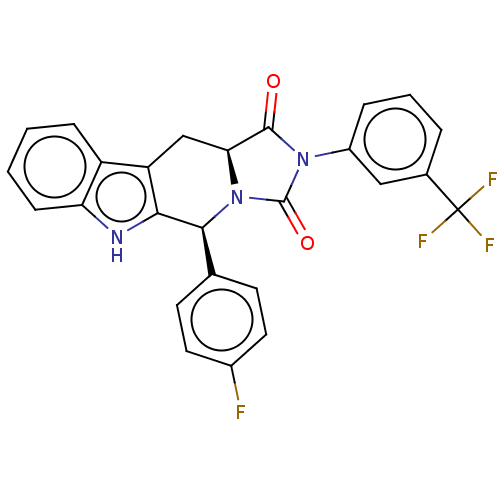
(CHEMBL4648953)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@@H](N1C(=O)N(C2=O)c1cccc(c1)C(F)(F)F)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H17F4N3O2/c27-16-10-8-14(9-11-16)23-22-19(18-6-1-2-7-20(18)31-22)13-21-24(34)32(25(35)33(21)23)17-5-3-4-15(12-17)26(28,29)30/h1-12,21,23,31H,13H2/t21-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544501

(CHEMBL4647444)Show SMILES COC(=O)[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H24N2O2/c1-30-26(29)23-16-21-20-14-8-9-15-22(20)27-24(21)25(19-12-6-3-7-13-19)28(23)17-18-10-4-2-5-11-18/h2-15,23,25,27H,16-17H2,1H3/t23-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544506

(CHEMBL4647464)Show SMILES COC(=O)CC[C@H]1N[C@@H](Cc2c1[nH]c1ccccc21)C(=O)NCc1ccc(F)cc1 |r| Show InChI InChI=1S/C23H24FN3O3/c1-30-21(28)11-10-19-22-17(16-4-2-3-5-18(16)27-22)12-20(26-19)23(29)25-13-14-6-8-15(24)9-7-14/h2-9,19-20,26-27H,10-13H2,1H3,(H,25,29)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50317647
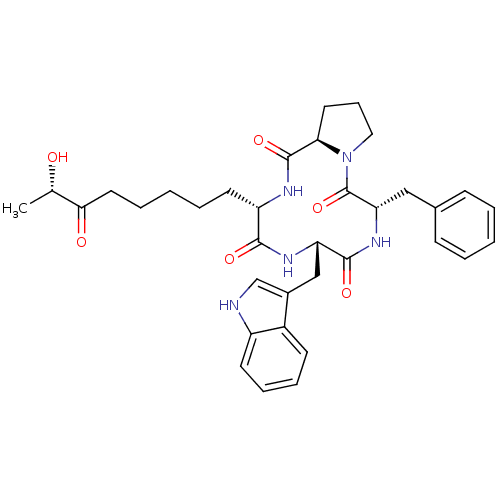
((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...)Show SMILES C[C@H](O)C(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C35H43N5O6/c1-22(41)31(42)17-7-3-6-15-27-32(43)38-28(20-24-21-36-26-14-9-8-13-25(24)26)33(44)39-29(19-23-11-4-2-5-12-23)35(46)40-18-10-16-30(40)34(45)37-27/h2,4-5,8-9,11-14,21-22,27-30,36,41H,3,6-7,10,15-20H2,1H3,(H,37,45)(H,38,43)(H,39,44)/t22-,27-,28-,29-,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of full length HDAC3 expressed in HEK293 cells after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 3252-60 (2010)
Article DOI: 10.1016/j.bmc.2010.03.022
BindingDB Entry DOI: 10.7270/Q2BC3ZP6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544503
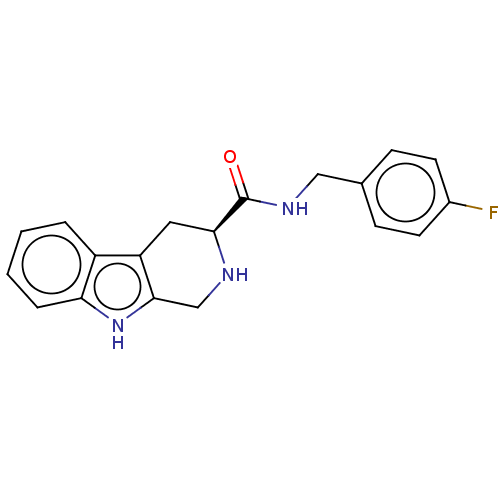
(CHEMBL4644158)Show SMILES Fc1ccc(CNC(=O)[C@@H]2Cc3c(CN2)[nH]c2ccccc32)cc1 |r| Show InChI InChI=1S/C19H18FN3O/c20-13-7-5-12(6-8-13)10-22-19(24)17-9-15-14-3-1-2-4-16(14)23-18(15)11-21-17/h1-8,17,21,23H,9-11H2,(H,22,24)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592161
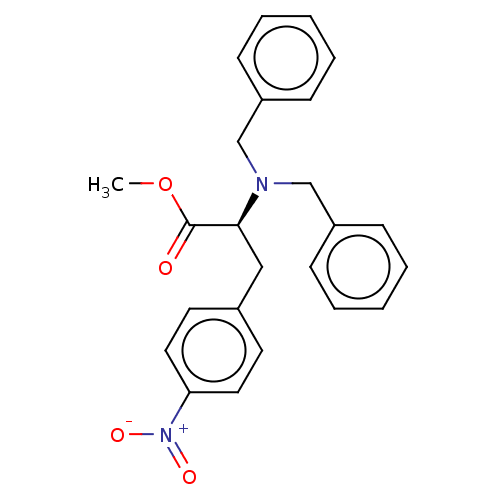
(CHEMBL5198220)Show SMILES COC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591532

(CHEMBL4590950) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50317647

((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...)Show SMILES C[C@H](O)C(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C35H43N5O6/c1-22(41)31(42)17-7-3-6-15-27-32(43)38-28(20-24-21-36-26-14-9-8-13-25(24)26)33(44)39-29(19-23-11-4-2-5-12-23)35(46)40-18-10-16-30(40)34(45)37-27/h2,4-5,8-9,11-14,21-22,27-30,36,41H,3,6-7,10,15-20H2,1H3,(H,37,45)(H,38,43)(H,39,44)/t22-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of full length HDAC1 expressed in HEK293 cells after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 3252-60 (2010)
Article DOI: 10.1016/j.bmc.2010.03.022
BindingDB Entry DOI: 10.7270/Q2BC3ZP6 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544500
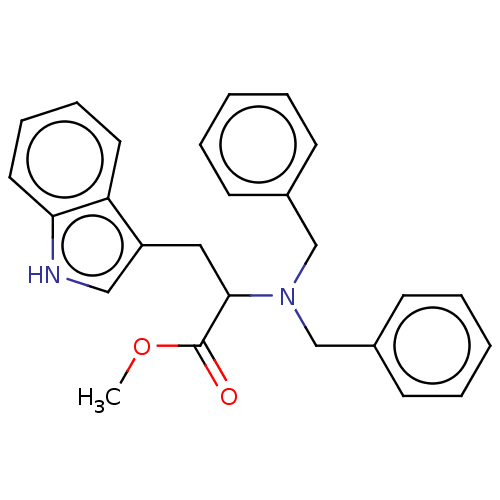
(CHEMBL4649195)Show SMILES COC(=O)C(Cc1c[nH]c2ccccc12)N(Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C26H26N2O2/c1-30-26(29)25(16-22-17-27-24-15-9-8-14-23(22)24)28(18-20-10-4-2-5-11-20)19-21-12-6-3-7-13-21/h2-15,17,25,27H,16,18-19H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592164
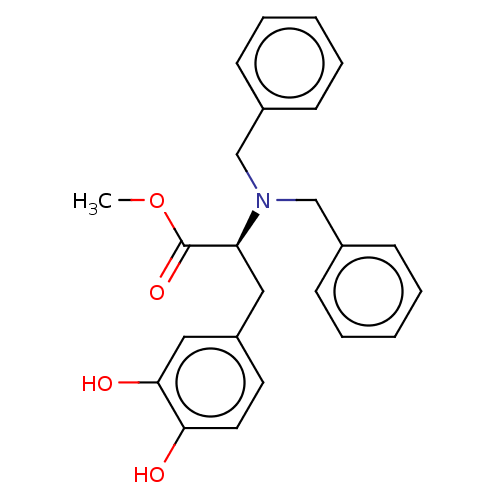
(CHEMBL5208212)Show SMILES COC(=O)[C@H](Cc1ccc(O)c(O)c1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591533
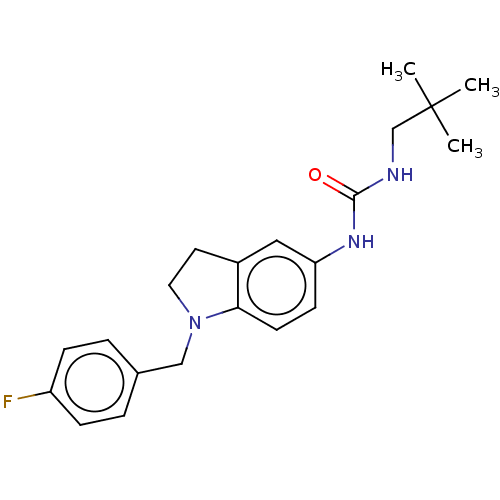
(CHEMBL4449485)Show SMILES CC(C)(C)CNC(=O)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592165
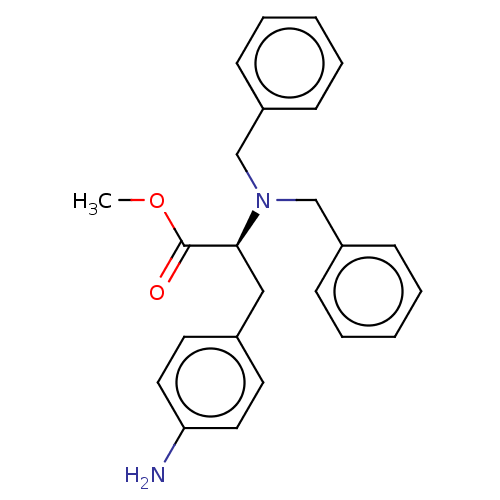
(CHEMBL5198707)Show SMILES COC(=O)[C@H](Cc1ccc(N)cc1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592161

(CHEMBL5198220)Show SMILES COC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50405790
(CHEMBL5284121)Show InChI InChI=1S/C14H18N6O2/c1-3-9-12(13(15)19-14(16)18-9)8-5-6-10(17-4-2)11(7-8)20(21)22/h5-7,17H,3-4H2,1-2H3,(H4,15,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against carbachol induced inhibition of electrically stimulated guinea pig atria Muscarinic acetylcholine receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591535
(CHEMBL5185907) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50317647

((3S,6S,9S,14aR)-6-((1H-indol-3-yl)methyl)-9-benzyl...)Show SMILES C[C@H](O)C(=O)CCCCC[C@@H]1NC(=O)[C@H]2CCCN2C(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C35H43N5O6/c1-22(41)31(42)17-7-3-6-15-27-32(43)38-28(20-24-21-36-26-14-9-8-13-25(24)26)33(44)39-29(19-23-11-4-2-5-12-23)35(46)40-18-10-16-30(40)34(45)37-27/h2,4-5,8-9,11-14,21-22,27-30,36,41H,3,6-7,10,15-20H2,1H3,(H,37,45)(H,38,43)(H,39,44)/t22-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Salerno
Curated by ChEMBL
| Assay Description
Inhibition of full length HDAC2 expressed in HEK293 cells after 15 mins by fluorescence assay |
Bioorg Med Chem 18: 3252-60 (2010)
Article DOI: 10.1016/j.bmc.2010.03.022
BindingDB Entry DOI: 10.7270/Q2BC3ZP6 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591533

(CHEMBL4449485)Show SMILES CC(C)(C)CNC(=O)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464480
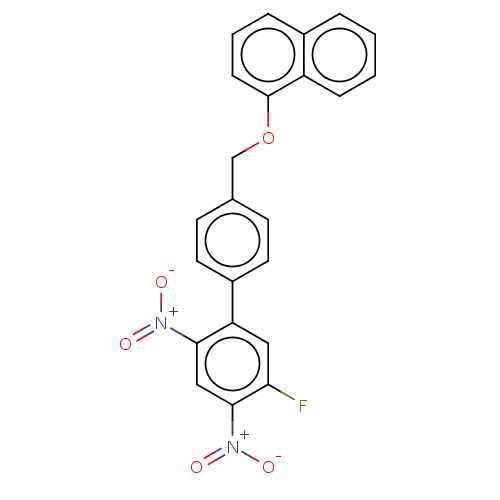
(CHEMBL4282628)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccc(COc2cccc3ccccc23)cc1)[N+]([O-])=O Show InChI InChI=1S/C23H15FN2O5/c24-20-12-19(21(25(27)28)13-22(20)26(29)30)17-10-8-15(9-11-17)14-31-23-7-3-5-16-4-1-2-6-18(16)23/h1-13H,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544531

(CHEMBL4648953)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@@H](N1C(=O)N(C2=O)c1cccc(c1)C(F)(F)F)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H17F4N3O2/c27-16-10-8-14(9-11-16)23-22-19(18-6-1-2-7-20(18)31-22)13-21-24(34)32(25(35)33(21)23)17-5-3-4-15(12-17)26(28,29)30/h1-12,21,23,31H,13H2/t21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50405760

(CHEMBL5284133)Show SMILES COc1ccccc1CNCCCCCCNCCCCCCCCCCCNCCCCCCNCc1ccccc1OC Show InChI InChI=1S/C39H68N4O2/c1-44-38-26-16-14-24-36(38)34-42-32-22-12-10-20-30-40-28-18-8-6-4-3-5-7-9-19-29-41-31-21-11-13-23-33-43-35-37-25-15-17-27-39(37)45-2/h14-17,24-27,40-43H,3-13,18-23,28-35H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist potency against muscarinic receptors was assed by antagonism of carbachol induced inhibition of electrically stimulated guinea pig atria |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592165

(CHEMBL5198707)Show SMILES COC(=O)[C@H](Cc1ccc(N)cc1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50140172

(CHEBI:3962 | CHEMBL140 | Curcumin | US9409845, Tab...)Show SMILES COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)c(OC)c2)ccc1O Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Mashhad University of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 preincubated for 15 mins by... |
J Nat Prod 78: 2867-79 (2015)
Article DOI: 10.1021/acs.jnatprod.5b00700
BindingDB Entry DOI: 10.7270/Q2PG1VR7 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Bos taurus (bovine)) | BDBM50365328
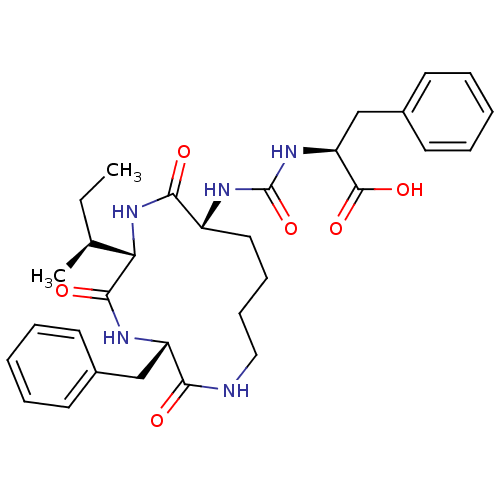
(CHEMBL1955927)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CCCCNC(=O)[C@H](Cc2ccccc2)NC1=O)NC(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H41N5O6/c1-3-20(2)26-29(39)33-24(18-21-12-6-4-7-13-21)27(37)32-17-11-10-16-23(28(38)36-26)34-31(42)35-25(30(40)41)19-22-14-8-5-9-15-22/h4-9,12-15,20,23-26H,3,10-11,16-19H2,1-2H3,(H,32,37)(H,33,39)(H,36,38)(H,40,41)(H2,34,35,42)/t20-,23+,24-,25-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreas carboxypeptidase A using N-(4-methoxyphenylazoformyl)-phenylalanine as substrate after 5 mins by colorimetry |
J Med Chem 55: 735-42 (2012)
Article DOI: 10.1021/jm201238p
BindingDB Entry DOI: 10.7270/Q24F1R7Z |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592163
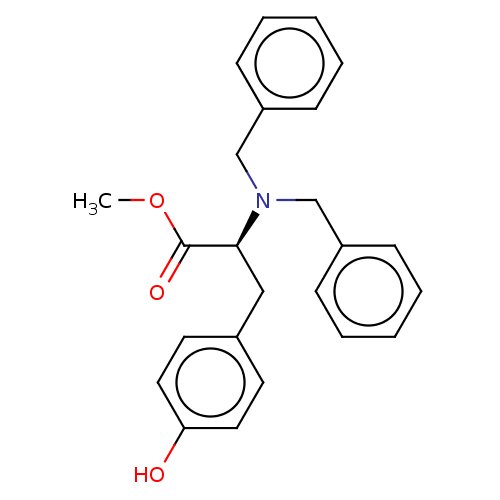
(CHEMBL5169807)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464484
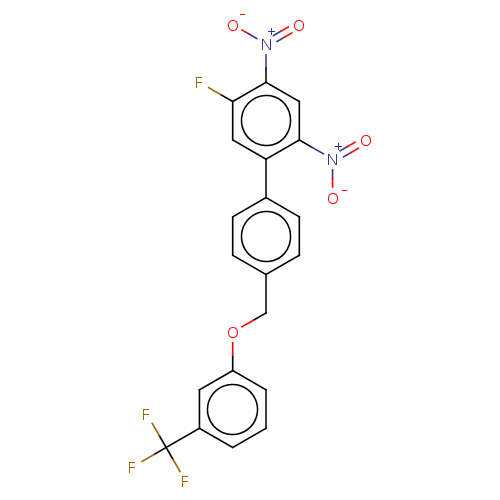
(CHEMBL4290910)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccc(COc2cccc(c2)C(F)(F)F)cc1)[N+]([O-])=O Show InChI InChI=1S/C20H12F4N2O5/c21-17-9-16(18(25(27)28)10-19(17)26(29)30)13-6-4-12(5-7-13)11-31-15-3-1-2-14(8-15)20(22,23)24/h1-10H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592159

(CHEMBL5193429)Show SMILES COC(=O)[C@H](Cc1ccc(Cl)cc1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591532

(CHEMBL4590950) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592160
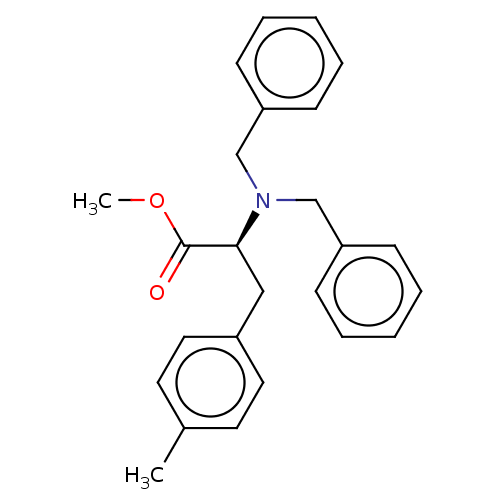
(CHEMBL5182352)Show SMILES COC(=O)[C@H](Cc1ccc(C)cc1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592162
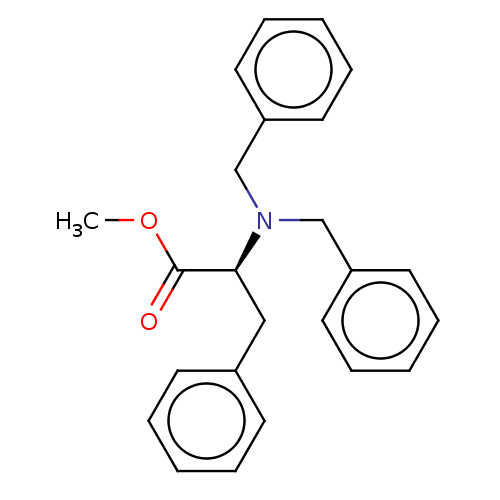
(CHEMBL5205458)Show SMILES COC(=O)[C@H](Cc1ccccc1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021461

(CHEMBL3289927)Show SMILES CCOC(OCC)c1ccc(\C=C2/CN(C)C\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C30H39NO5/c1-6-33-29(34-7-2)24-14-10-22(11-15-24)18-26-20-31(5)21-27(28(26)32)19-23-12-16-25(17-13-23)30(35-8-3)36-9-4/h10-19,29-30H,6-9,20-21H2,1-5H3/b26-18+,27-19+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591524
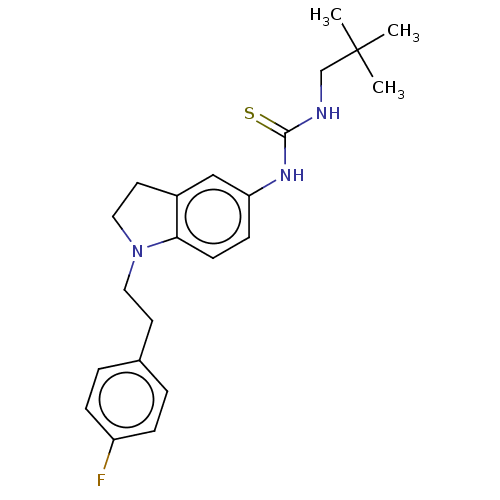
(CHEMBL5202375)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(CCc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50339570
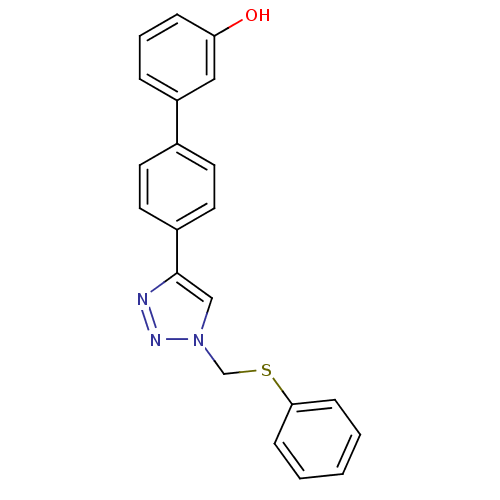
(4'-(1-Phenylsulfanylmethyl-1H-[1,2,3]triazol-4-yl)...)Show InChI InChI=1S/C21H17N3OS/c25-19-6-4-5-18(13-19)16-9-11-17(12-10-16)21-14-24(23-22-21)15-26-20-7-2-1-3-8-20/h1-14,25H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human neutrophils |
J Med Chem 54: 1565-75 (2011)
Article DOI: 10.1021/jm101238d
BindingDB Entry DOI: 10.7270/Q2SN098M |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544513

(CHEMBL4649088)Show SMILES [H][C@@]12Cc3c(CN1C(=O)[C@@H](Cc1ccccc1)NC2=O)[nH]c1ccccc31 |r| Show InChI InChI=1S/C21H19N3O2/c25-20-19-11-15-14-8-4-5-9-16(14)22-18(15)12-24(19)21(26)17(23-20)10-13-6-2-1-3-7-13/h1-9,17,19,22H,10-12H2,(H,23,25)/t17-,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50061980

(CHEMBL3394323)Show SMILES CC1=C(C(NC(=O)N1CCC(O)=O)c1ccc(o1)-c1cccc(c1)C(F)(F)F)C(=O)OCc1ccccc1 |t:1| Show InChI InChI=1S/C27H23F3N2O6/c1-16-23(25(35)37-15-17-6-3-2-4-7-17)24(31-26(36)32(16)13-12-22(33)34)21-11-10-20(38-21)18-8-5-9-19(14-18)27(28,29)30/h2-11,14,24H,12-13,15H2,1H3,(H,31,36)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in interleukin-1beta-stimulated human A549 cells microsomal membranes assessed as reduction in PGE2 formation incubated for 15 ... |
ACS Med Chem Lett 6: 187-91 (2015)
Article DOI: 10.1021/ml500433j
BindingDB Entry DOI: 10.7270/Q2474CJH |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591539
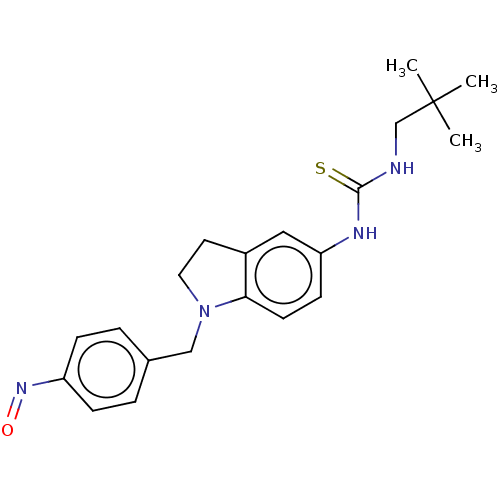
(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021468

(CHEMBL3289934)Show SMILES CN(C)c1ccc(\C=C2\CCC\C(=C/c3ccc(cc3[N+]([O-])=O)N(C)C)C2=O)c(c1)[N+]([O-])=O Show InChI InChI=1S/C24H26N4O5/c1-25(2)20-10-8-16(22(14-20)27(30)31)12-18-6-5-7-19(24(18)29)13-17-9-11-21(26(3)4)15-23(17)28(32)33/h8-15H,5-7H2,1-4H3/b18-12-,19-13+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591539

(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591522
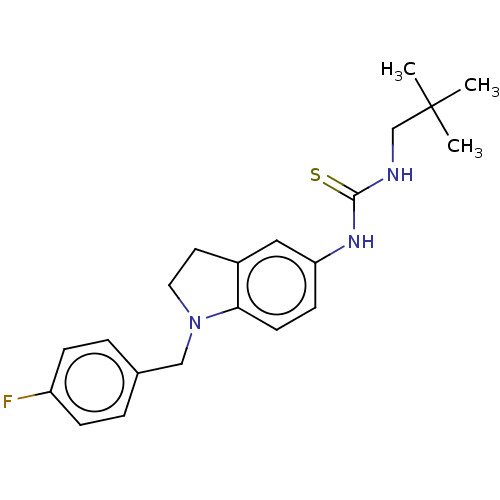
(CHEMBL4473010)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50021447
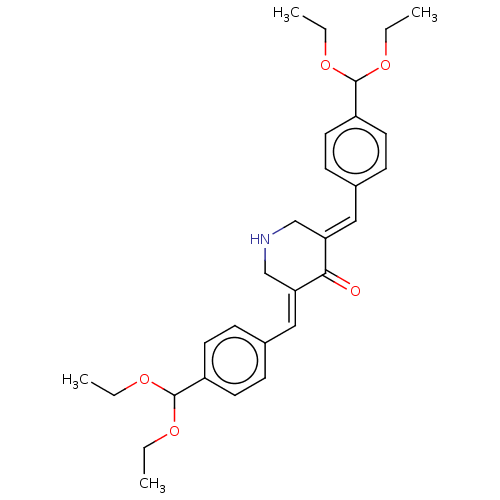
(CHEMBL3289926)Show SMILES CCOC(OCC)c1ccc(\C=C2/CNC\C(=C/c3ccc(cc3)C(OCC)OCC)C2=O)cc1 Show InChI InChI=1S/C29H37NO5/c1-5-32-28(33-6-2)23-13-9-21(10-14-23)17-25-19-30-20-26(27(25)31)18-22-11-15-24(16-12-22)29(34-7-3)35-8-4/h9-18,28-30H,5-8,19-20H2,1-4H3/b25-17+,26-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 using arachidonic acid substrate assessed as PGE2 production ELISA method after 2 mins |
Bioorg Med Chem 22: 4151-61 (2014)
Article DOI: 10.1016/j.bmc.2014.05.052
BindingDB Entry DOI: 10.7270/Q2GX4D6F |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50592164

(CHEMBL5208212)Show SMILES COC(=O)[C@H](Cc1ccc(O)c(O)c1)N(Cc1ccccc1)Cc1ccccc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114435
BindingDB Entry DOI: 10.7270/Q2ZP4B36 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi del Piemonte Orientale A. Avogadro
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human SHSY5Y cells by fluorimetric cellular activity assay |
J Med Chem 52: 2776-85 (2009)
Article DOI: 10.1021/jm801529c
BindingDB Entry DOI: 10.7270/Q2D221F1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544523

(CHEMBL4647674)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)N(Cc1ccc(F)cc1)C2=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H19ClFN3O2/c27-17-9-7-16(8-10-17)24-23-20(19-3-1-2-4-21(19)29-23)13-22-25(32)30(26(33)31(22)24)14-15-5-11-18(28)12-6-15/h1-12,22,24,29H,13-14H2/t22-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50464486
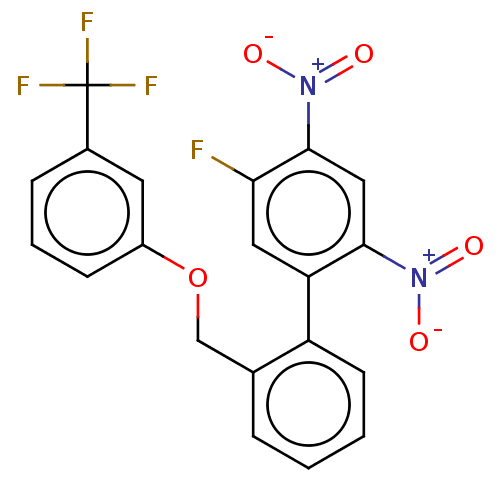
(CHEMBL4293214)Show SMILES [O-][N+](=O)c1cc(c(cc1F)-c1ccccc1COc1cccc(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C20H12F4N2O5/c21-17-9-16(18(25(27)28)10-19(17)26(29)30)15-7-2-1-4-12(15)11-31-14-6-3-5-13(8-14)20(22,23)24/h1-10H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL-1beta-stimulated human A549 cell microsomes using PGH2 as substrate assessed as reduction in PGE2 production preincubated ... |
Eur J Med Chem 143: 1419-1427 (2018)
Article DOI: 10.1016/j.ejmech.2017.10.039
BindingDB Entry DOI: 10.7270/Q2G44SXG |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591534
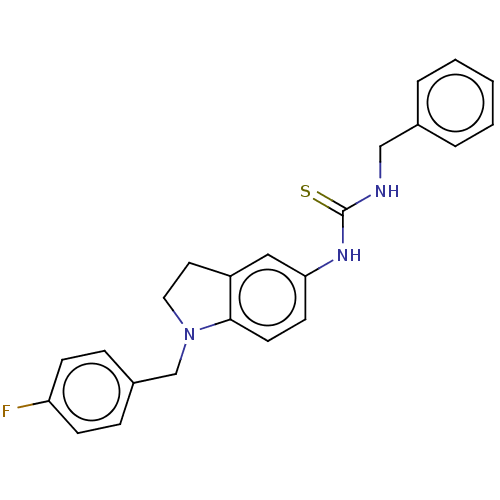
(CHEMBL5199450)Show SMILES Fc1ccc(CN2CCc3cc(NC(=S)NCc4ccccc4)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591539

(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50339571
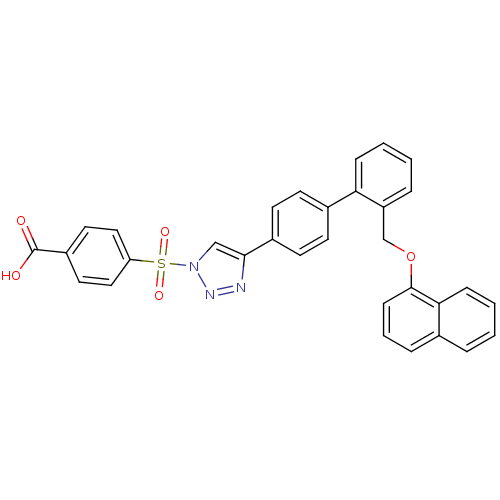
(4-{4-[2'-(Naphthalen-1-yloxymethyl)-biphenyl-4-yl]...)Show SMILES OC(=O)c1ccc(cc1)S(=O)(=O)n1cc(nn1)-c1ccc(cc1)-c1ccccc1COc1cccc2ccccc12 Show InChI InChI=1S/C32H23N3O5S/c36-32(37)25-16-18-27(19-17-25)41(38,39)35-20-30(33-34-35)24-14-12-23(13-15-24)28-9-3-2-7-26(28)21-40-31-11-5-8-22-6-1-4-10-29(22)31/h1-20H,21H2,(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in human neutrophils |
J Med Chem 54: 1565-75 (2011)
Article DOI: 10.1021/jm101238d
BindingDB Entry DOI: 10.7270/Q2SN098M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data