 Found 248 hits with Last Name = 'pepe' and Initial = 'g'
Found 248 hits with Last Name = 'pepe' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221
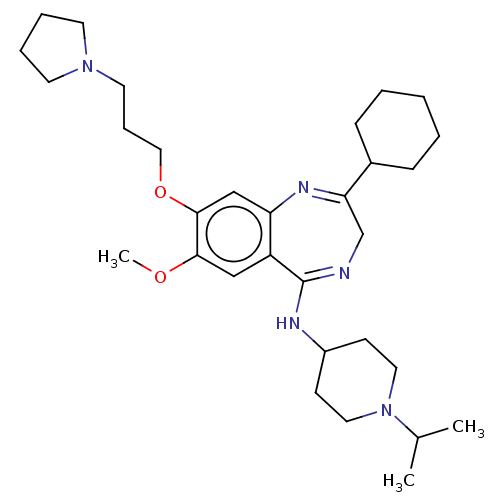
(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) as substrate after 30 mins in presence of va... |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221

(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) as substrate at varying concentration af... |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50465610

(CHEMBL4295040)Show SMILES COC(=O)[C@H](Cc1c[nH]c2ccccc12)N(Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C26H26N2O2/c1-30-26(29)25(16-22-17-27-24-15-9-8-14-23(22)24)28(18-20-10-4-2-5-11-20)19-21-12-6-3-7-13-21/h2-15,17,25,27H,16,18-19H2,1H3/t25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as reduction in menthol-induced channel currents by whole cell patch clamp method |
J Med Chem 61: 6140-6152 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00545
BindingDB Entry DOI: 10.7270/Q2FN18V3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50465611

(CHEMBL4284808)Show InChI InChI=1S/C24H30N2/c1-3-9-20(10-4-1)18-26(19-21-11-5-2-6-12-21)16-15-22-17-25-24-14-8-7-13-23(22)24/h1,3-4,7-10,13-14,17,21,25H,2,5-6,11-12,15-16,18-19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as reduction in menthol-induced channel currents by whole cell patch clamp method |
J Med Chem 61: 6140-6152 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00545
BindingDB Entry DOI: 10.7270/Q2FN18V3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544523
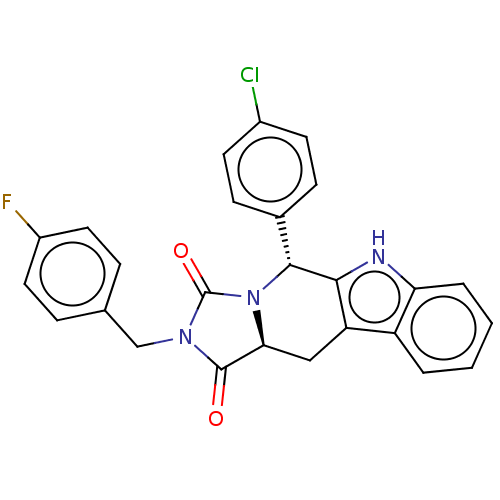
(CHEMBL4647674)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)N(Cc1ccc(F)cc1)C2=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H19ClFN3O2/c27-17-9-7-16(8-10-17)24-23-20(19-3-1-2-4-21(19)29-23)13-22-25(32)30(26(33)31(22)24)14-15-5-11-18(28)12-6-15/h1-12,22,24,29H,13-14H2/t22-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544513
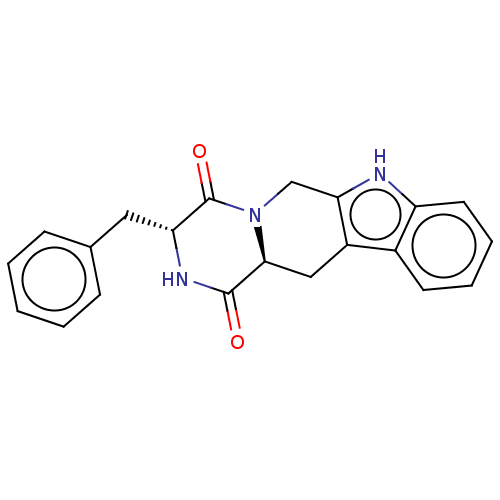
(CHEMBL4649088)Show SMILES [H][C@@]12Cc3c(CN1C(=O)[C@@H](Cc1ccccc1)NC2=O)[nH]c1ccccc31 |r| Show InChI InChI=1S/C21H19N3O2/c25-20-19-11-15-14-8-4-5-9-16(14)22-18(15)12-24(19)21(26)17(23-20)10-13-6-2-1-3-7-13/h1-9,17,19,22H,10-12H2,(H,23,25)/t17-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544531
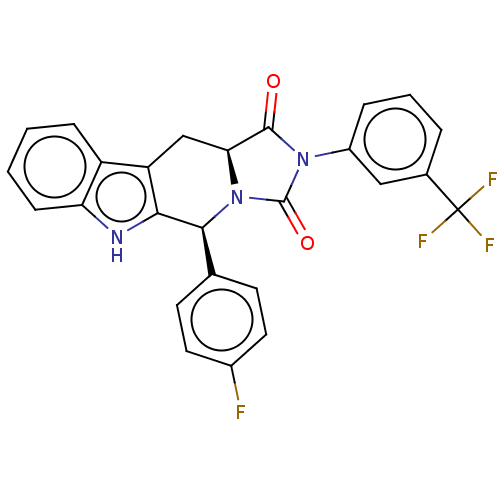
(CHEMBL4648953)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@@H](N1C(=O)N(C2=O)c1cccc(c1)C(F)(F)F)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H17F4N3O2/c27-16-10-8-14(9-11-16)23-22-19(18-6-1-2-7-20(18)31-22)13-21-24(34)32(25(35)33(21)23)17-5-3-4-15(12-17)26(28,29)30/h1-12,21,23,31H,13H2/t21-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544501

(CHEMBL4647444)Show SMILES COC(=O)[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H24N2O2/c1-30-26(29)23-16-21-20-14-8-9-15-22(20)27-24(21)25(19-12-6-3-7-13-19)28(23)17-18-10-4-2-5-11-18/h2-15,23,25,27H,16-17H2,1H3/t23-,25+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544506

(CHEMBL4647464)Show SMILES COC(=O)CC[C@H]1N[C@@H](Cc2c1[nH]c1ccccc21)C(=O)NCc1ccc(F)cc1 |r| Show InChI InChI=1S/C23H24FN3O3/c1-30-21(28)11-10-19-22-17(16-4-2-3-5-18(16)27-22)12-20(26-19)23(29)25-13-14-6-8-15(24)9-7-14/h2-9,19-20,26-27H,10-13H2,1H3,(H,25,29)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition G9a (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of GLP (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50526221

(CHEMBL4454542)Show SMILES COc1cc2c(cc1OCCCN1CCCC1)N=C(CN=C2NC1CCN(CC1)C(C)C)C1CCCCC1 |c:19,22| Show InChI InChI=1S/C31H49N5O2/c1-23(2)36-17-12-25(13-18-36)33-31-26-20-29(37-3)30(38-19-9-16-35-14-7-8-15-35)21-27(26)34-28(22-32-31)24-10-5-4-6-11-24/h20-21,23-25H,4-19,22H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) after 30 mins in presence of SAM by AlphaLISA assay |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50353128

(CHEMBL1231795)Show SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1 Show InChI InChI=1S/C30H47N5O2/c1-22(2)35-17-12-24(13-18-35)31-30-25-20-27(36-3)28(37-19-9-16-34-14-7-8-15-34)21-26(25)32-29(33-30)23-10-5-4-6-11-23/h20-24H,4-19H2,1-3H3,(H,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human G9a using biotinylated-Histone H3 peptide (1 to 21 residues) after 30 mins in presence of SAM by AlphaLISA assay |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50544503
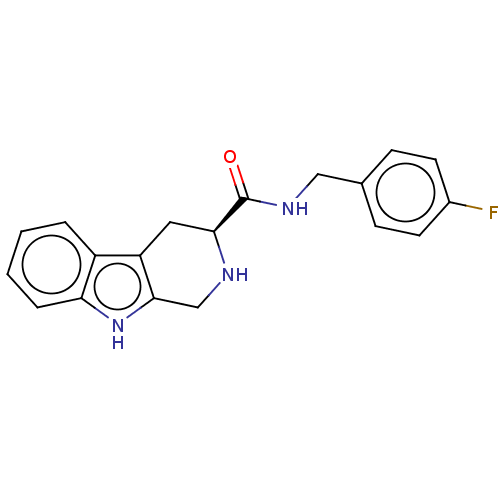
(CHEMBL4644158)Show SMILES Fc1ccc(CNC(=O)[C@@H]2Cc3c(CN2)[nH]c2ccccc32)cc1 |r| Show InChI InChI=1S/C19H18FN3O/c20-13-7-5-12(6-8-13)10-22-19(24)17-9-15-14-3-1-2-4-16(14)23-18(15)11-21-17/h1-8,17,21,23H,9-11H2,(H,22,24)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPM8 expressed in HEK-293/TRPM8 exon1 K3 cells assessed as inhibition of menthol-induced current response by whole-cell... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50465610

(CHEMBL4295040)Show SMILES COC(=O)[C@H](Cc1c[nH]c2ccccc12)N(Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C26H26N2O2/c1-30-26(29)25(16-22-17-27-24-15-9-8-14-23(22)24)28(18-20-10-4-2-5-11-20)19-21-12-6-3-7-13-21/h2-15,17,25,27H,16,18-19H2,1H3/t25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as reduction in menthol-induced calcium flux incubated for 60 mins by Fluo-4 NW-d... |
J Med Chem 61: 6140-6152 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00545
BindingDB Entry DOI: 10.7270/Q2FN18V3 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591532

(CHEMBL4590950) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544500
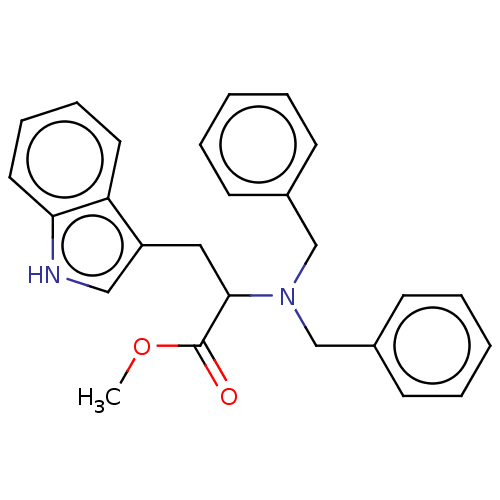
(CHEMBL4649195)Show SMILES COC(=O)C(Cc1c[nH]c2ccccc12)N(Cc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C26H26N2O2/c1-30-26(29)25(16-22-17-27-24-15-9-8-14-23(22)24)28(18-20-10-4-2-5-11-20)19-21-12-6-3-7-13-21/h2-15,17,25,27H,16,18-19H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50396024
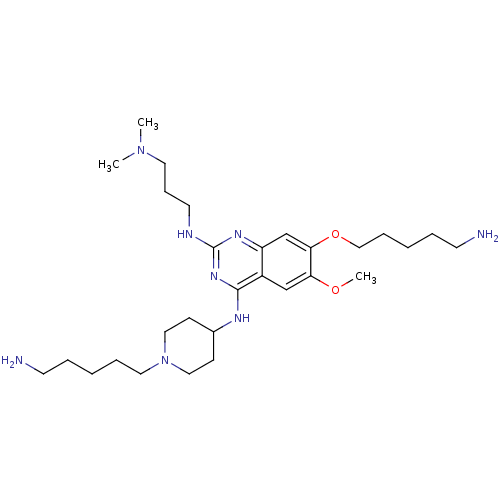
(CHEMBL1232453)Show SMILES COc1cc2c(NC3CCN(CCCCCN)CC3)nc(NCCCN(C)C)nc2cc1OCCCCCN Show InChI InChI=1S/C29H52N8O2/c1-36(2)16-10-15-32-29-34-25-22-27(39-20-9-5-7-14-31)26(38-3)21-24(25)28(35-29)33-23-11-18-37(19-12-23)17-8-4-6-13-30/h21-23H,4-20,30-31H2,1-3H3,(H2,32,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of GLP (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591533
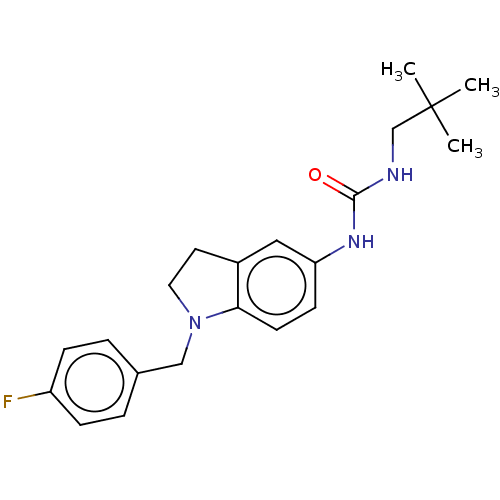
(CHEMBL4449485)Show SMILES CC(C)(C)CNC(=O)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591535
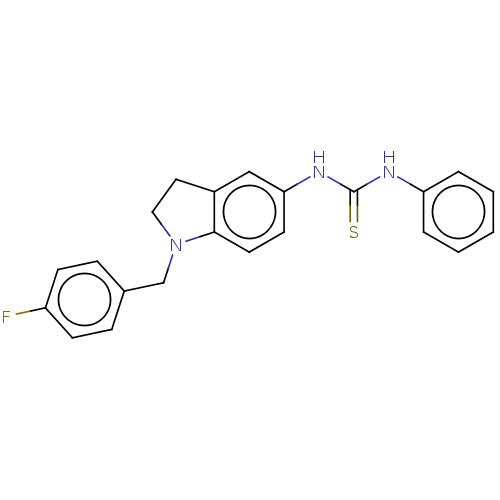
(CHEMBL5185907) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50396024

(CHEMBL1232453)Show SMILES COc1cc2c(NC3CCN(CCCCCN)CC3)nc(NCCCN(C)C)nc2cc1OCCCCCN Show InChI InChI=1S/C29H52N8O2/c1-36(2)16-10-15-32-29-34-25-22-27(39-20-9-5-7-14-31)26(38-3)21-24(25)28(35-29)33-23-11-18-37(19-12-23)17-8-4-6-13-30/h21-23H,4-20,30-31H2,1-3H3,(H2,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition G9a (unknown origin) |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591533

(CHEMBL4449485)Show SMILES CC(C)(C)CNC(=O)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544531

(CHEMBL4648953)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@@H](N1C(=O)N(C2=O)c1cccc(c1)C(F)(F)F)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H17F4N3O2/c27-16-10-8-14(9-11-16)23-22-19(18-6-1-2-7-20(18)31-22)13-21-24(34)32(25(35)33(21)23)17-5-3-4-15(12-17)26(28,29)30/h1-12,21,23,31H,13H2/t21-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591532

(CHEMBL4590950) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591524
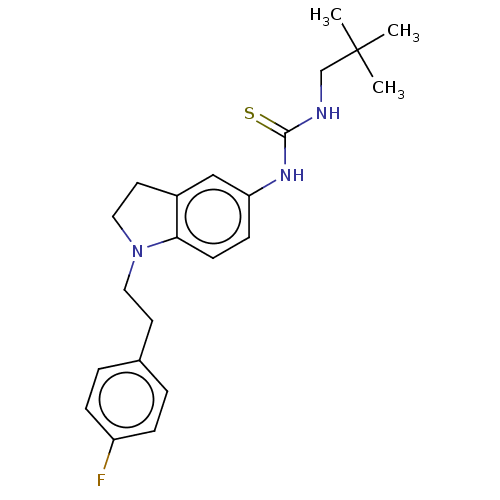
(CHEMBL5202375)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(CCc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544513

(CHEMBL4649088)Show SMILES [H][C@@]12Cc3c(CN1C(=O)[C@@H](Cc1ccccc1)NC2=O)[nH]c1ccccc31 |r| Show InChI InChI=1S/C21H19N3O2/c25-20-19-11-15-14-8-4-5-9-16(14)22-18(15)12-24(19)21(26)17(23-20)10-13-6-2-1-3-7-13/h1-9,17,19,22H,10-12H2,(H,23,25)/t17-,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50529951
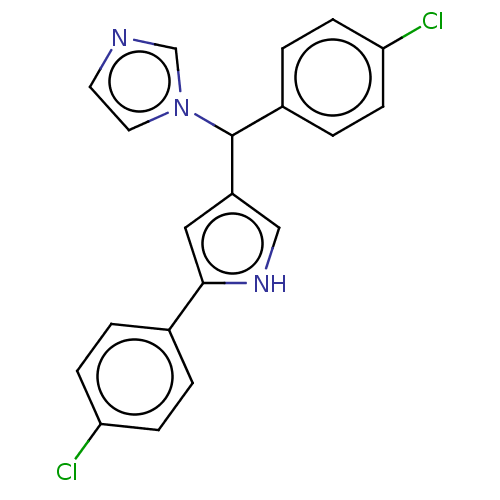
(CHEMBL4468270)Show SMILES Clc1ccc(cc1)C(c1c[nH]c(c1)-c1ccc(Cl)cc1)n1ccnc1 Show InChI InChI=1S/C20H15Cl2N3/c21-17-5-1-14(2-6-17)19-11-16(12-24-19)20(25-10-9-23-13-25)15-3-7-18(22)8-4-15/h1-13,20,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50529951

(CHEMBL4468270)Show SMILES Clc1ccc(cc1)C(c1c[nH]c(c1)-c1ccc(Cl)cc1)n1ccnc1 Show InChI InChI=1S/C20H15Cl2N3/c21-17-5-1-14(2-6-17)19-11-16(12-24-19)20(25-10-9-23-13-25)15-3-7-18(22)8-4-15/h1-13,20,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591539
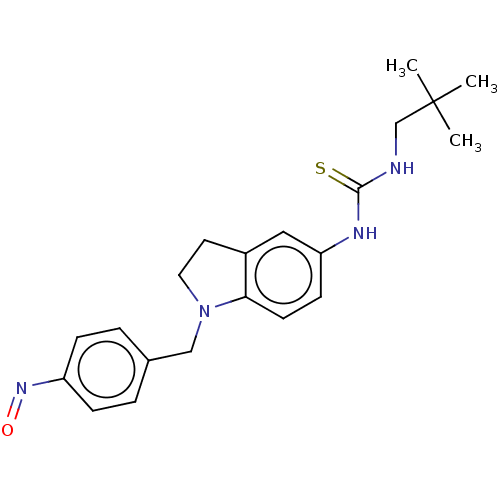
(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591539

(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591522
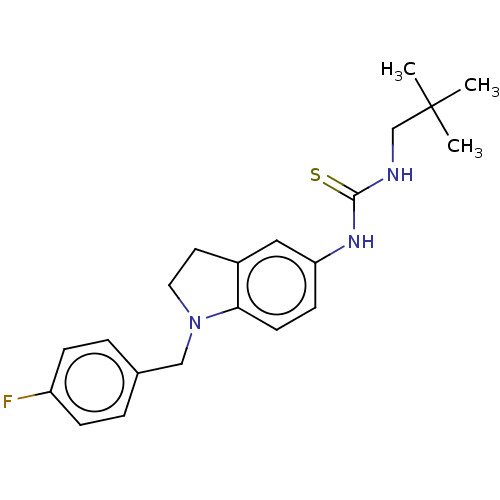
(CHEMBL4473010)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(F)cc3)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Homo sapiens (Human)) | BDBM50133817
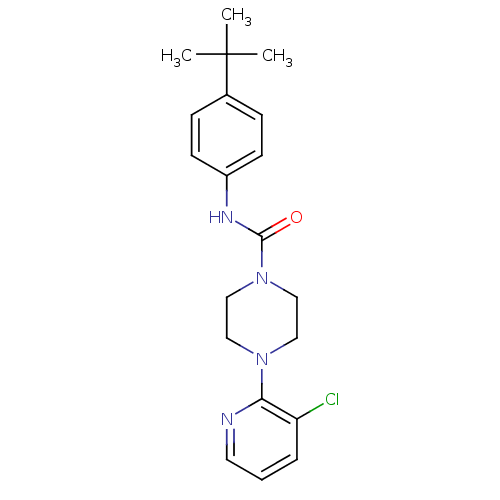
(4-(3-Chloro-pyridin-2-yl)-piperazine-1-carboxylic ...)Show SMILES CC(C)(C)c1ccc(NC(=O)N2CCN(CC2)c2ncccc2Cl)cc1 Show InChI InChI=1S/C20H25ClN4O/c1-20(2,3)15-6-8-16(9-7-15)23-19(26)25-13-11-24(12-14-25)18-17(21)5-4-10-22-18/h4-10H,11-14H2,1-3H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 475 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at TRPM8 (unknown origin) assessed as reduction in menthol-induced calcium influx |
J Med Chem 61: 6140-6152 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00545
BindingDB Entry DOI: 10.7270/Q2FN18V3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544523

(CHEMBL4647674)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)N(Cc1ccc(F)cc1)C2=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C26H19ClFN3O2/c27-17-9-7-16(8-10-17)24-23-20(19-3-1-2-4-21(19)29-23)13-22-25(32)30(26(33)31(22)24)14-15-5-11-18(28)12-6-15/h1-12,22,24,29H,13-14H2/t22-,24+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591534
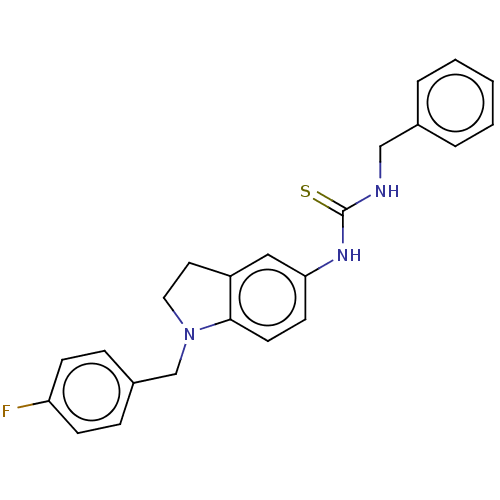
(CHEMBL5199450)Show SMILES Fc1ccc(CN2CCc3cc(NC(=S)NCc4ccccc4)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50591539

(CHEMBL5170803)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(cc3)N=O)CCc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50465611

(CHEMBL4284808)Show InChI InChI=1S/C24H30N2/c1-3-9-20(10-4-1)18-26(19-21-11-5-2-6-12-21)16-15-22-17-25-24-14-8-7-13-23(22)24/h1,3-4,7-10,13-14,17,21,25H,2,5-6,11-12,15-16,18-19H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as reduction in menthol-induced calcium flux incubated for 60 mins by Fluo-4 NW-d... |
J Med Chem 61: 6140-6152 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00545
BindingDB Entry DOI: 10.7270/Q2FN18V3 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT1
(Homo sapiens (Human)) | BDBM50300028
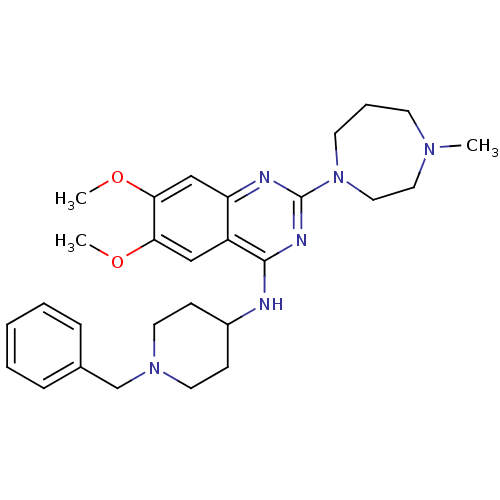
(CHEMBL569864 | N-(1-benzylpiperidin-4-yl)-6,7-dime...)Show SMILES COc1cc2nc(nc(NC3CCN(Cc4ccccc4)CC3)c2cc1OC)N1CCCN(C)CC1 Show InChI InChI=1S/C28H38N6O2/c1-32-12-7-13-34(17-16-32)28-30-24-19-26(36-3)25(35-2)18-23(24)27(31-28)29-22-10-14-33(15-11-22)20-21-8-5-4-6-9-21/h4-6,8-9,18-19,22H,7,10-17,20H2,1-3H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas M.D. Anderson Cancer Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GLP (610 to 917 residues) using H3 peptide (1 to 20 residues) by mass spectrometry |
J Med Chem 62: 2666-2689 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02008
BindingDB Entry DOI: 10.7270/Q2280C10 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50591531
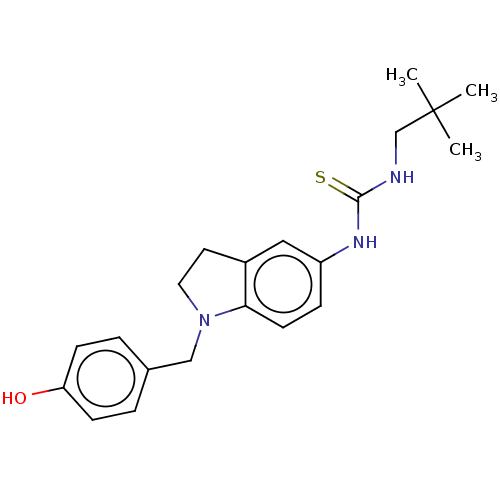
(CHEMBL5193145)Show SMILES CC(C)(C)CNC(=S)Nc1ccc2N(Cc3ccc(O)cc3)CCc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00817
BindingDB Entry DOI: 10.7270/Q2GT5S5W |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544521
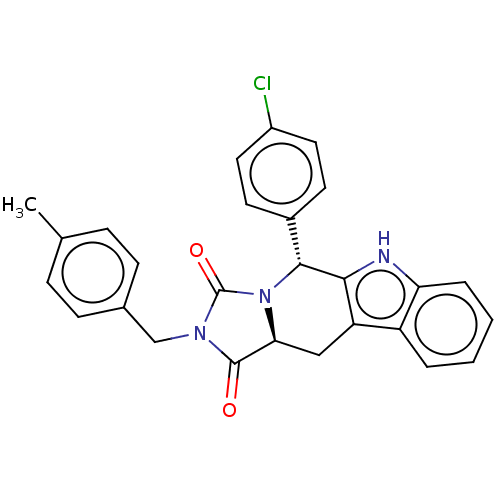
(CHEMBL4634053)Show SMILES [H][C@@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)N(Cc1ccc(C)cc1)C2=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H22ClN3O2/c1-16-6-8-17(9-7-16)15-30-26(32)23-14-21-20-4-2-3-5-22(20)29-24(21)25(31(23)27(30)33)18-10-12-19(28)13-11-18/h2-13,23,25,29H,14-15H2,1H3/t23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50529952

(CHEMBL315874)Show SMILES Clc1ccc(cc1)-c1c[nH]cc1C(c1ccc(Cl)cc1Cl)n1ccnc1 Show InChI InChI=1S/C20H14Cl3N3/c21-14-3-1-13(2-4-14)17-10-25-11-18(17)20(26-8-7-24-12-26)16-6-5-15(22)9-19(16)23/h1-12,20,25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50529952

(CHEMBL315874)Show SMILES Clc1ccc(cc1)-c1c[nH]cc1C(c1ccc(Cl)cc1Cl)n1ccnc1 Show InChI InChI=1S/C20H14Cl3N3/c21-14-3-1-13(2-4-14)17-10-25-11-18(17)20(26-8-7-24-12-26)16-6-5-15(22)9-19(16)23/h1-12,20,25H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50465615
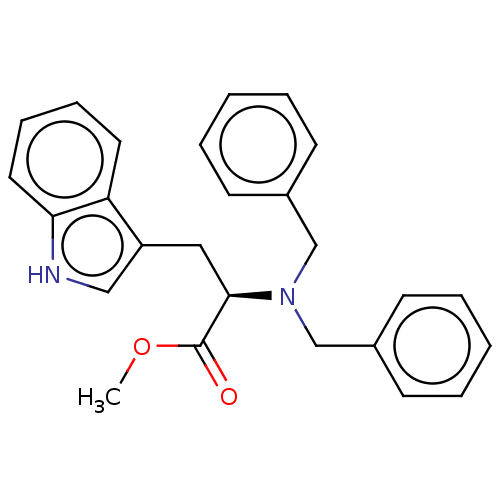
(CHEMBL4282067)Show SMILES COC(=O)[C@@H](Cc1c[nH]c2ccccc12)N(Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C26H26N2O2/c1-30-26(29)25(16-22-17-27-24-15-9-8-14-23(22)24)28(18-20-10-4-2-5-11-20)19-21-12-6-3-7-13-21/h2-15,17,25,27H,16,18-19H2,1H3/t25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 865 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as reduction in menthol-induced channel currents by whole cell patch clamp method |
J Med Chem 61: 6140-6152 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00545
BindingDB Entry DOI: 10.7270/Q2FN18V3 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 8
(Rattus norvegicus (Rat)) | BDBM50544501

(CHEMBL4647444)Show SMILES COC(=O)[C@@H]1Cc2c([nH]c3ccccc23)[C@H](N1Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H24N2O2/c1-30-26(29)23-16-21-20-14-8-9-15-22(20)27-24(21)25(19-12-6-3-7-13-19)28(23)17-18-10-4-2-5-11-18/h2-15,23,25,27H,16-17H2,1H3/t23-,25+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Salerno
Curated by ChEMBL
| Assay Description
Antagonist activity at rat TRPM8 expressed in HEK293 cells assessed as inhibition of menthol-induced calcium flux preincubated for 60 mins followed b... |
J Med Chem 63: 9672-9694 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00816
BindingDB Entry DOI: 10.7270/Q2GF0Z2X |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50529950
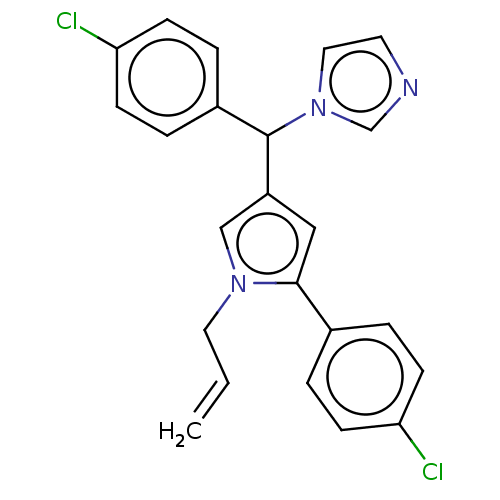
(CHEMBL4551139)Show SMILES Clc1ccc(cc1)C(c1cc(-c2ccc(Cl)cc2)n(CC=C)c1)n1ccnc1 Show InChI InChI=1S/C23H19Cl2N3/c1-2-12-27-15-19(14-22(27)17-3-7-20(24)8-4-17)23(28-13-11-26-16-28)18-5-9-21(25)10-6-18/h2-11,13-16,23H,1,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50529949
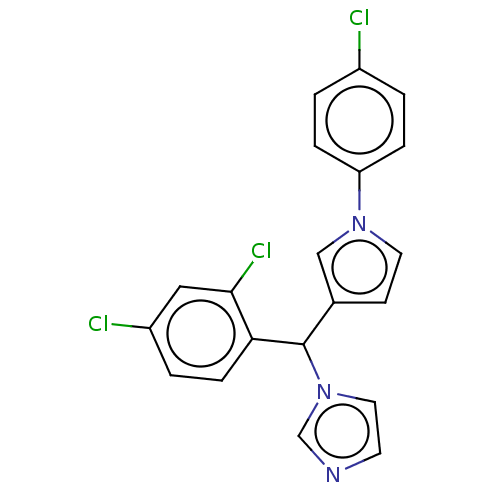
(CHEMBL4438726)Show SMILES Clc1ccc(cc1)-n1ccc(c1)C(c1ccc(Cl)cc1Cl)n1ccnc1 Show InChI InChI=1S/C20H14Cl3N3/c21-15-1-4-17(5-2-15)25-9-7-14(12-25)20(26-10-8-24-13-26)18-6-3-16(22)11-19(18)23/h1-13,20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50529949

(CHEMBL4438726)Show SMILES Clc1ccc(cc1)-n1ccc(c1)C(c1ccc(Cl)cc1Cl)n1ccnc1 Show InChI InChI=1S/C20H14Cl3N3/c21-15-1-4-17(5-2-15)25-9-7-14(12-25)20(26-10-8-24-13-26)18-6-3-16(22)11-19(18)23/h1-13,20H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 in presence of NADPH by luciferase reporter gene assay |
J Med Chem 62: 1330-1347 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01464
BindingDB Entry DOI: 10.7270/Q2CF9TKG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data