

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140783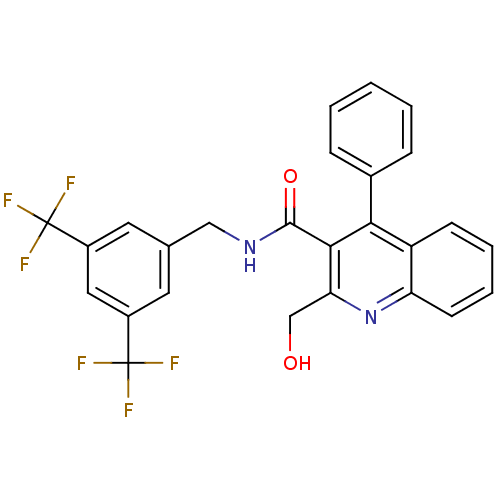 (2-Hydroxymethyl-4-phenyl-quinoline-3-carboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.000100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140770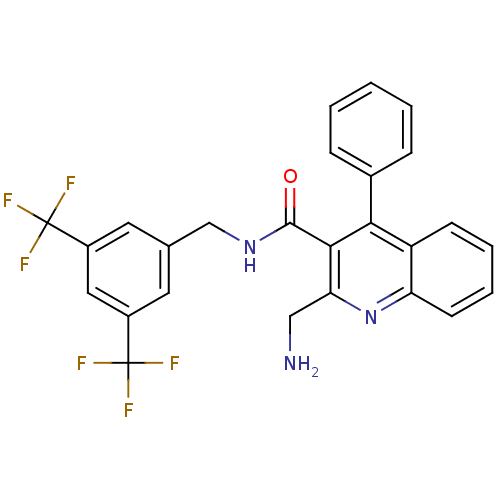 (2-Aminomethyl-4-phenyl-quinoline-3-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140773 (2-Bromomethyl-4-phenyl-quinoline-3-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140785 (2-Methylaminomethyl-4-phenyl-quinoline-3-carboxyli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140786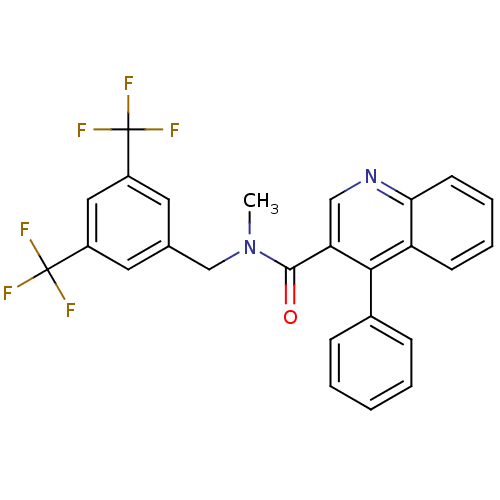 (4-Phenyl-quinoline-3-carboxylic acid (3,5-bis-trif...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140772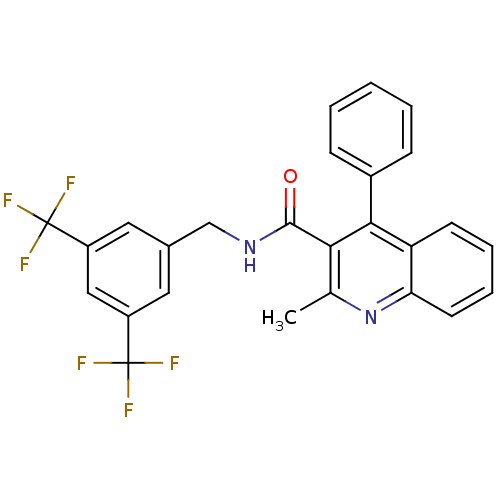 (2-Methyl-4-phenyl-quinoline-3-carboxylic acid 3,5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50418331 (SPERGUALIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to human NK1 receptor | Bioorg Med Chem 19: 2242-51 (2011) Article DOI: 10.1016/j.bmc.2011.02.031 BindingDB Entry DOI: 10.7270/Q2QJ7J8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140774 (2-Dimethylaminomethyl-4-phenyl-quinoline-3-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50009718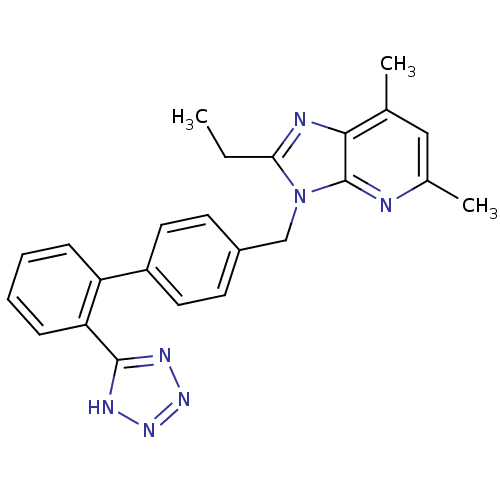 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50131724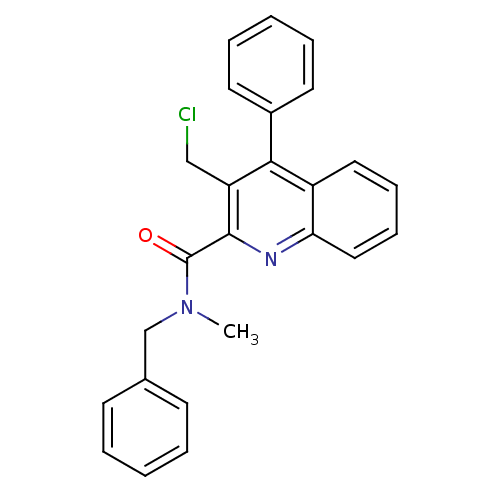 (3-Chloromethyl-4-phenyl-quinoline-2-carboxylic aci...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50009712 (5,7-Dimethyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-bip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50009719 (7-Methyl-2-propyl-3-[2'-(2H-tetrazol-5-yl)-bipheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from human recombinant NK1 receptor expressed in CHO cells after 90 mins | Bioorg Med Chem 19: 2242-51 (2011) Article DOI: 10.1016/j.bmc.2011.02.031 BindingDB Entry DOI: 10.7270/Q2QJ7J8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140771 (((2R,3R)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50049186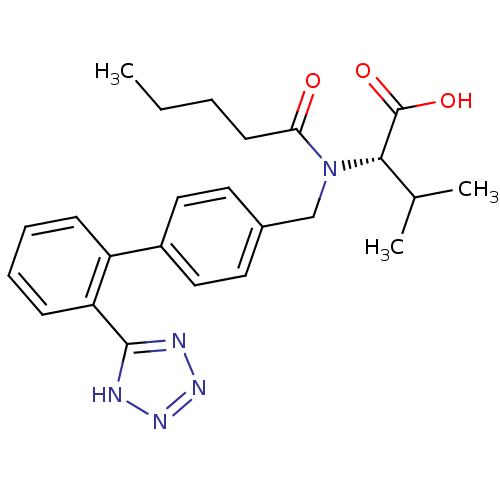 ((S)-3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50131729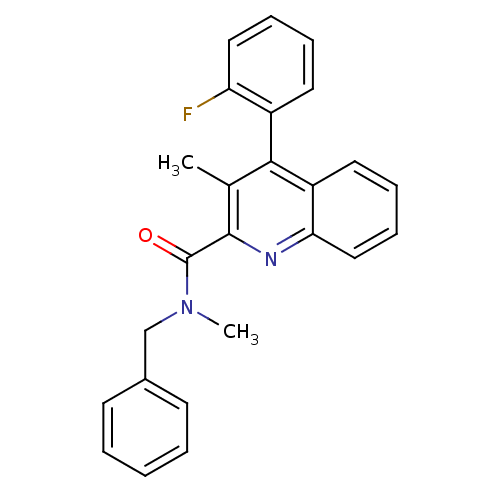 (4-(2-Fluoro-phenyl)-3-methyl-quinoline-2-carboxyli...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140776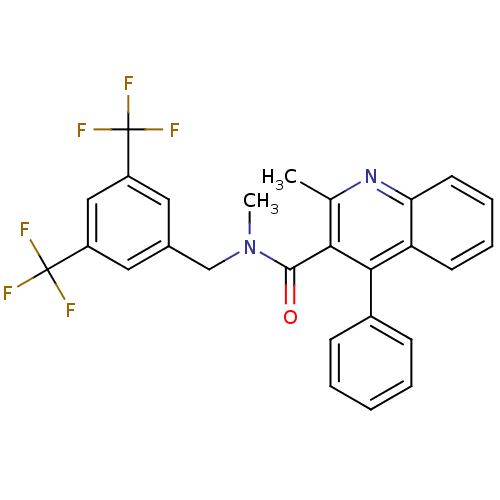 (2-Methyl-4-phenyl-quinoline-3-carboxylic acid (3,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50145972 (2-Amino-4-[4-(5,7-dimethyl-2-propyl-imidazo[4,5-b]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Antagonist activity against AT1 receptor assessed as inhibition of Ang2-induced rabit aortic strip contraction | J Med Chem 49: 6451-64 (2006) Article DOI: 10.1021/jm0603163 BindingDB Entry DOI: 10.7270/Q2WW7H9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145961 (7-Methyl-2-propyl-3-{4-[3-(2H-tetrazol-5-yl)-pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145972 (2-Amino-4-[4-(5,7-dimethyl-2-propyl-imidazo[4,5-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50059749 (3-Methyl-4-phenyl-quinoline-2-carboxylic acid benz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50197079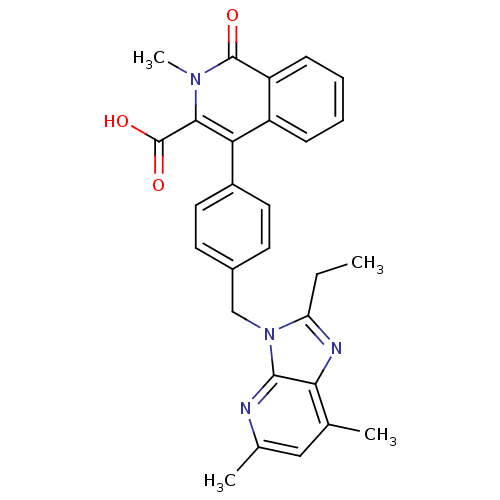 (1,2-dihydro-4-[4-[(5,7-dimethyl-2-ethyl-3H-imidazo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Antagonist activity against AT1 receptor assessed as inhibition of Ang2-induced rabit aortic strip contraction | J Med Chem 49: 6451-64 (2006) Article DOI: 10.1021/jm0603163 BindingDB Entry DOI: 10.7270/Q2WW7H9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50342013 (CHEMBL1765500 | N-[3,5-Bis(trifluoromethyl)benzyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from human recombinant NK1 receptor expressed in CHO cells after 90 mins | Bioorg Med Chem 19: 2242-51 (2011) Article DOI: 10.1016/j.bmc.2011.02.031 BindingDB Entry DOI: 10.7270/Q2QJ7J8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50046078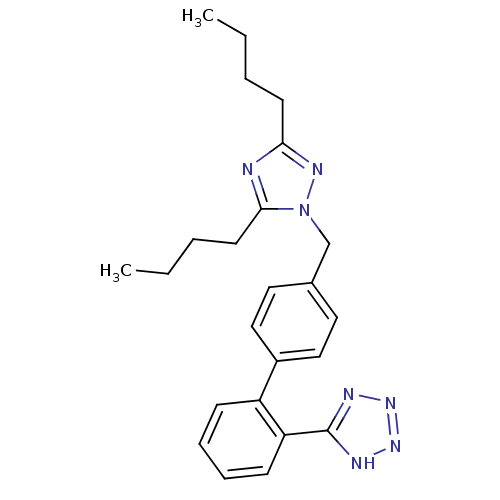 (5-[4'-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-biph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50009714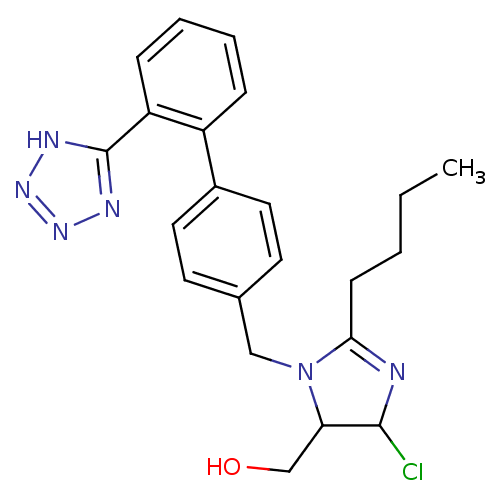 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145967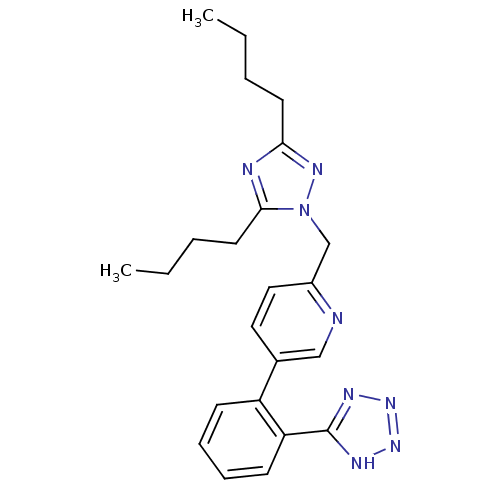 (2-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-5-[2-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50049209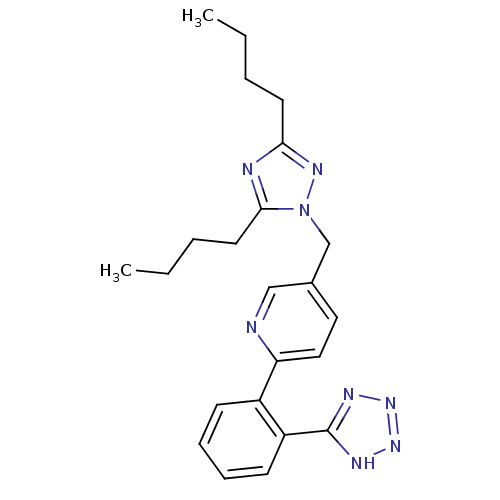 (5-(3,5-Dibutyl-[1,2,4]triazol-1-ylmethyl)-2-[2-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145979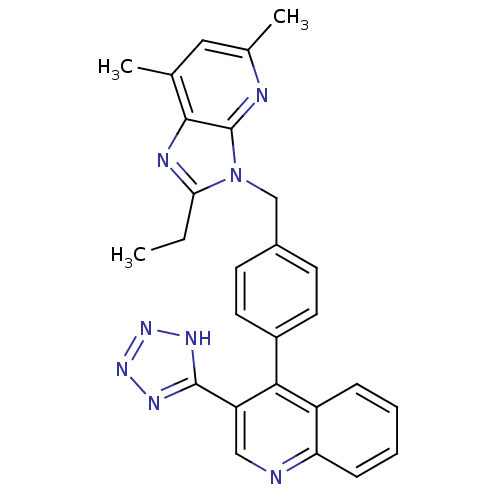 (4-(4-((2-ethyl-5,7-dimethyl-3H-imidazo[4,5-b]pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145977 (2-Chloro-4-[4-(5,7-dimethyl-2-propyl-imidazo[4,5-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032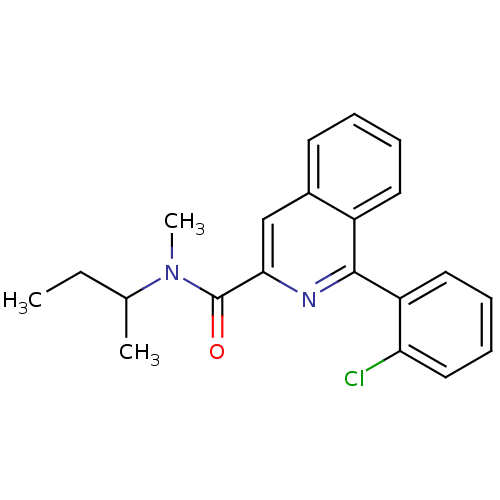 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50131721 (3-Hydroxymethyl-4-phenyl-quinoline-2-carboxylic ac...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50145979 (4-(4-((2-ethyl-5,7-dimethyl-3H-imidazo[4,5-b]pyrid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Antagonist activity against AT1 receptor assessed as inhibition of Ang2-induced rabit aortic strip contraction | J Med Chem 49: 6451-64 (2006) Article DOI: 10.1021/jm0603163 BindingDB Entry DOI: 10.7270/Q2WW7H9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140788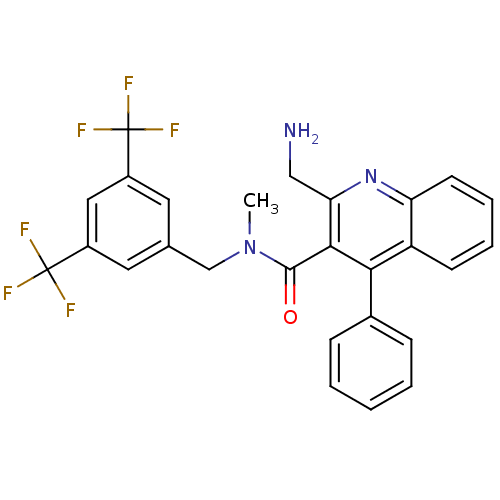 (2-Aminomethyl-4-phenyl-quinoline-3-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145981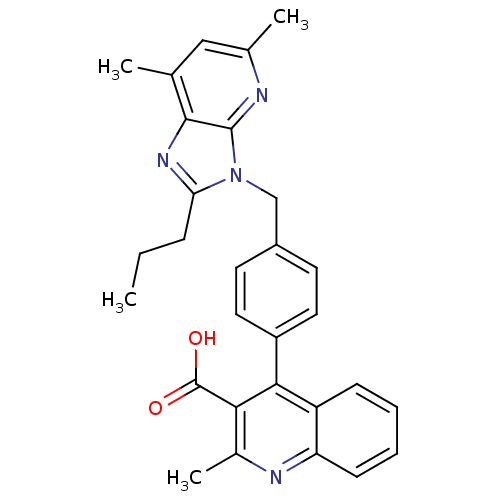 (4-(4-((5,7-dimethyl-2-propyl-3H-imidazo[4,5-b]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50131727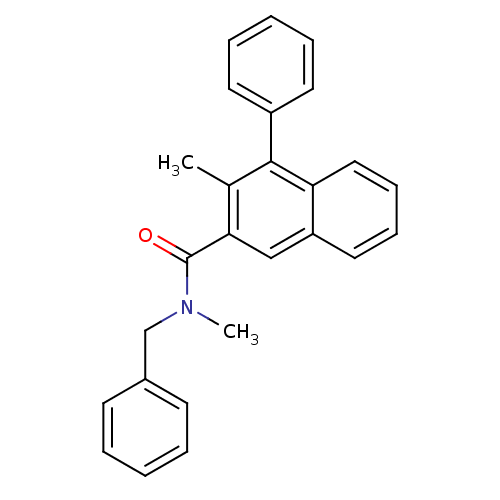 (3-Methyl-4-phenyl-naphthalene-2-carboxylic acid be...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50009714 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Antagonist activity against AT1 receptor assessed as inhibition of Ang2-induced rabit aortic strip contraction | J Med Chem 49: 6451-64 (2006) Article DOI: 10.1021/jm0603163 BindingDB Entry DOI: 10.7270/Q2WW7H9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145987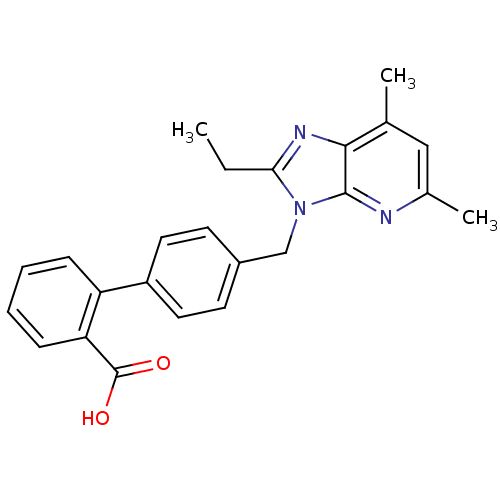 (4'-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145973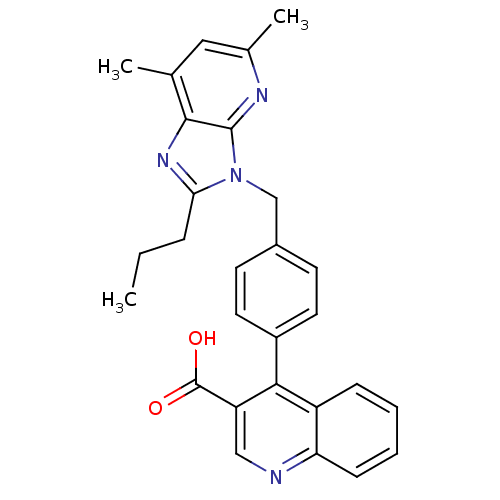 (4-[4-(5,7-Dimethyl-2-propyl-imidazo[4,5-b]pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50131730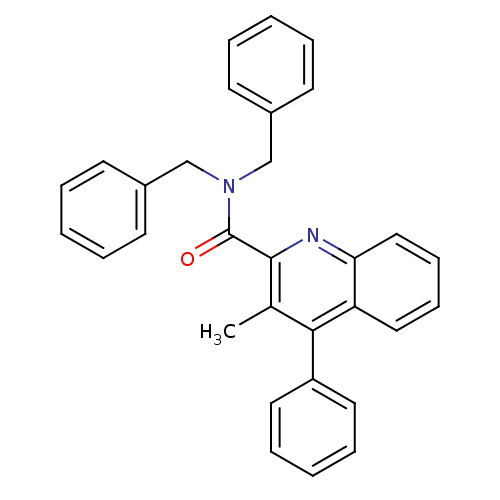 (3-Methyl-4-phenyl-quinoline-2-carboxylic acid dibe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140774 (2-Dimethylaminomethyl-4-phenyl-quinoline-3-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of labeled SP total binding against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells by the second binding component | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50140781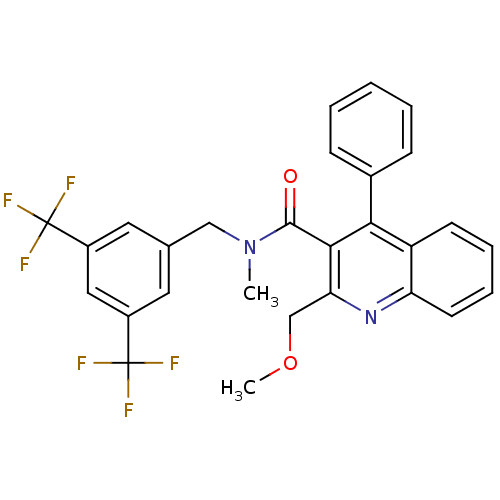 (2-Methoxymethyl-4-phenyl-quinoline-3-carboxylic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against human Tachykinin receptor 1 expressed in astrocytoma UC11MG cells using [125I]- SP radioligand | J Med Chem 47: 1315-8 (2004) Article DOI: 10.1021/jm034219a BindingDB Entry DOI: 10.7270/Q2F76C0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50131726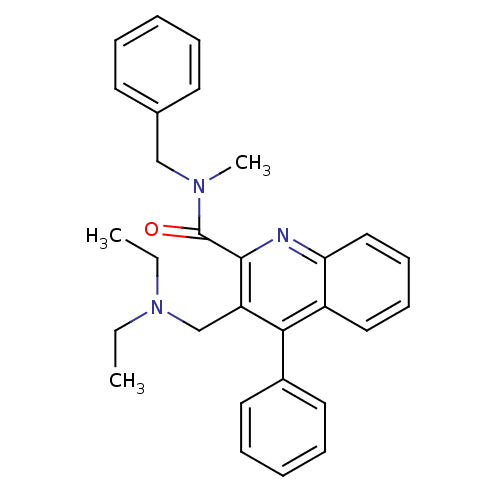 (3-Diethylaminomethyl-4-phenyl-quinoline-2-carboxyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145986 (4-[4-(5,7-Dimethyl-2-propyl-imidazo[4,5-b]pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Rattus norvegicus (rat)) | BDBM50418331 (SPERGUALIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity to gerbil NK1 receptor | Bioorg Med Chem 19: 2242-51 (2011) Article DOI: 10.1016/j.bmc.2011.02.031 BindingDB Entry DOI: 10.7270/Q2QJ7J8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50131728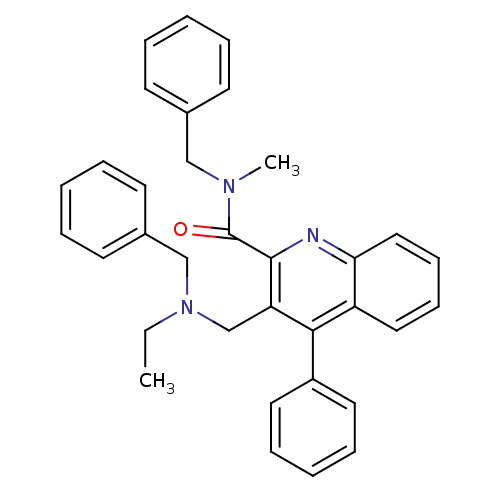 (3-[(Benzyl-ethyl-amino)-methyl]-4-phenyl-quinoline...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibitory activity against specific binding of [3H]-CB 34 to peripheral benzodiazepine receptor in rat cortical membranes was evaluated | J Med Chem 46: 3568-71 (2003) Article DOI: 10.1021/jm034068b BindingDB Entry DOI: 10.7270/Q2D79C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50145981 (4-(4-((5,7-dimethyl-2-propyl-3H-imidazo[4,5-b]pyri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Antagonist activity against AT1 receptor assessed as inhibition of Ang2-induced rabit aortic strip contraction | J Med Chem 49: 6451-64 (2006) Article DOI: 10.1021/jm0603163 BindingDB Entry DOI: 10.7270/Q2WW7H9J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145971 (2-Amino-4-[4-(2-ethyl-5,7-dimethyl-imidazo[4,5-b]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145964 (4-[4-(2-Ethyl-5,7-dimethyl-imidazo[4,5-b]pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50145976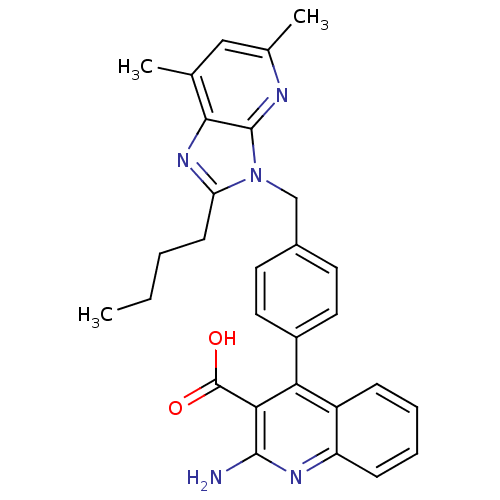 (2-Amino-4-[4-(2-butyl-5,7-dimethyl-imidazo[4,5-b]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Siena Curated by ChEMBL | Assay Description Binding affinity for rat angiotensin II receptor, type 1 | J Med Chem 47: 2574-86 (2004) Article DOI: 10.1021/jm031100t BindingDB Entry DOI: 10.7270/Q25X28DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 111 total ) | Next | Last >> |