

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125769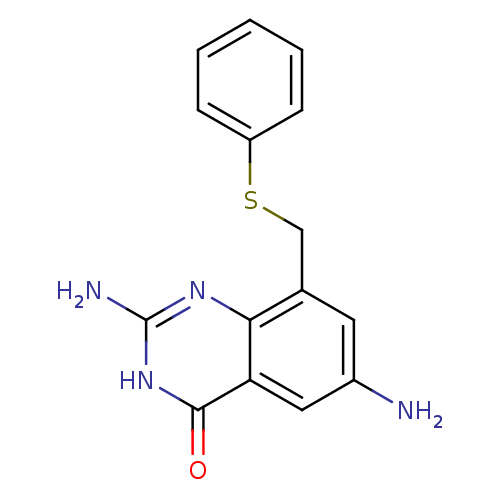 (2,6-Diamino-8-phenylsulfanylmethyl-3H-quinazolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125773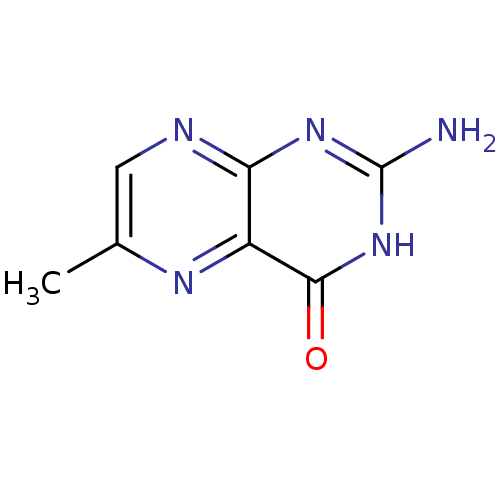 (2-Amino-6-methyl-3H-pteridin-4-one | CHEMBL14913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125771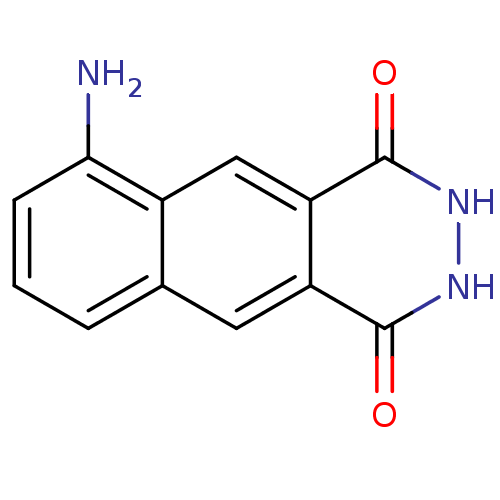 (6-Amino-2,3-dihydro-benzo[g]phthalazine-1,4-dione ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125772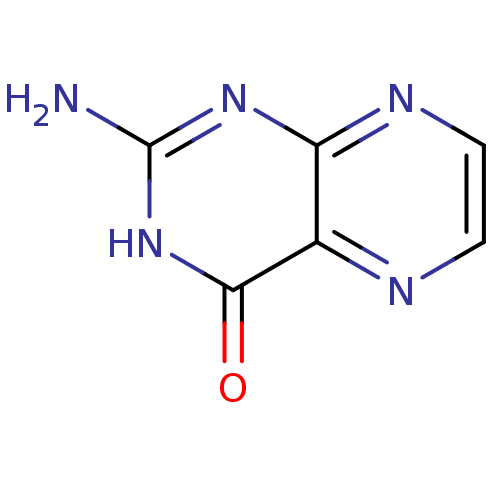 (2-amino-4-hydroxypteridine | 2-aminopteridin-4-ol ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125760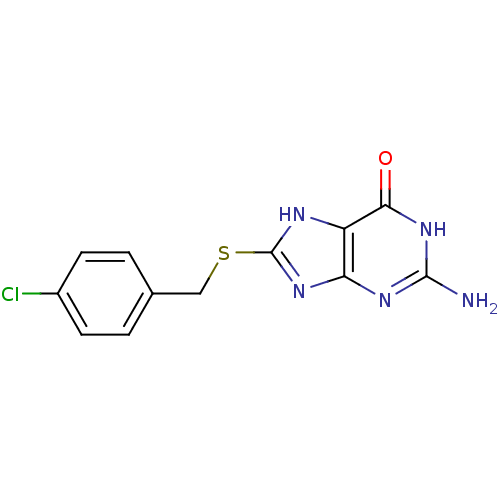 (2-Amino-8-(4-chloro-benzylsulfanyl)-1,9-dihydro-pu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125766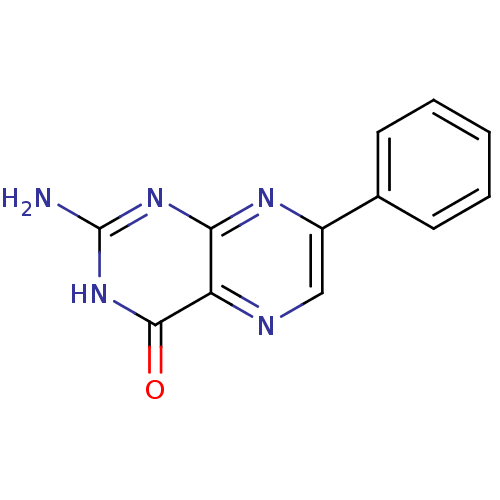 (2-Amino-7-phenyl-3H-pteridin-4-one | CHEMBL277561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125761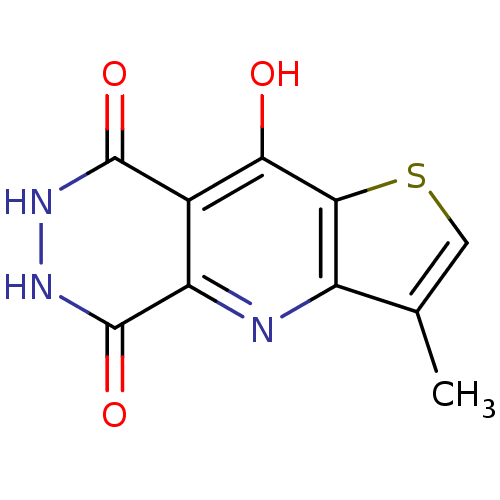 (3-Methyl-6,7-dihydro-4H-1-thia-4,6,7-triaza-cyclop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125768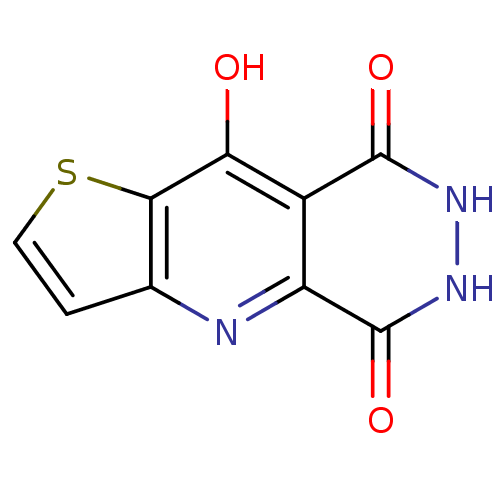 (6,7-Dihydro-4H-1-thia-4,6,7-triaza-cyclopenta[b]na...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125765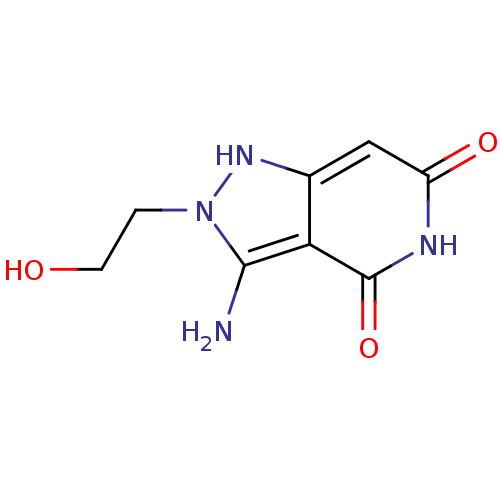 (3-Amino-6-hydroxy-2-(2-hydroxy-ethyl)-2,5-dihydro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125767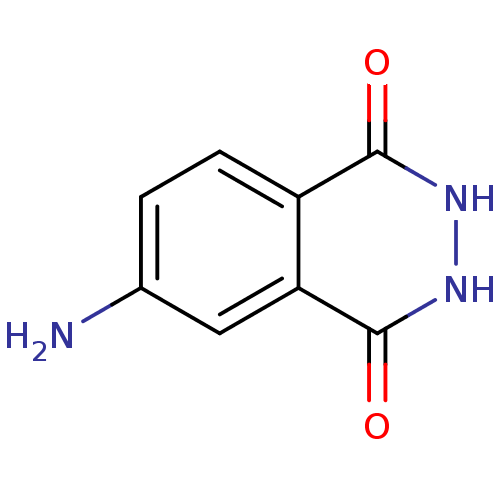 (4-AMINOPHTHALHYDRAZIDE | 6-Amino-2,3-dihydro-phtha...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125759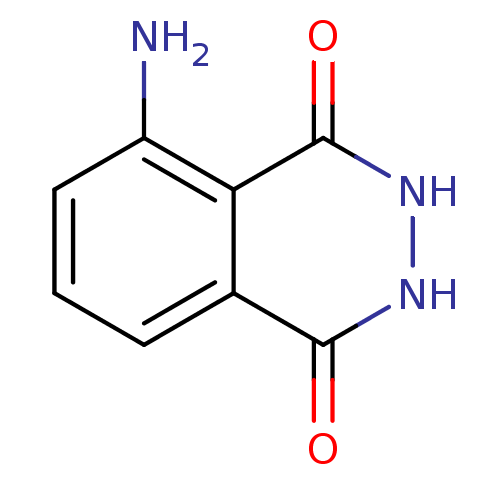 (5-Amino-2,3-dihydro-phthalazine-1,4-dione | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125775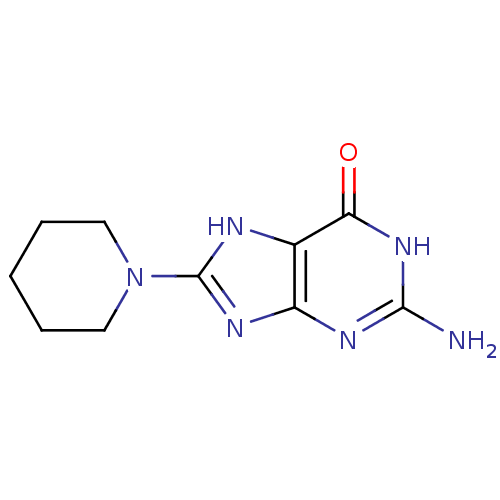 (2-Amino-8-piperidin-1-yl-1,9-dihydro-purin-6-one |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125774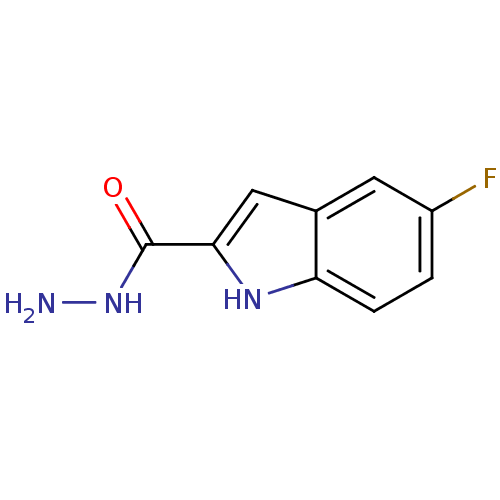 (5-Fluoro-1H-indole-2-carboxylic acid hydrazide | C...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125763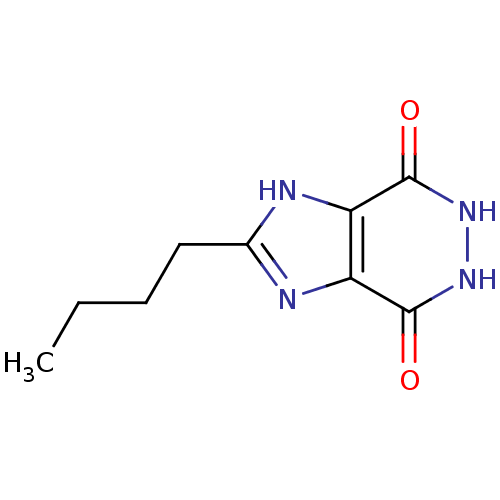 (2-BUTYL-5,6-DIHYDRO-1H-IMIDAZO[4,5-D]PYRIDAZINE-4,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125770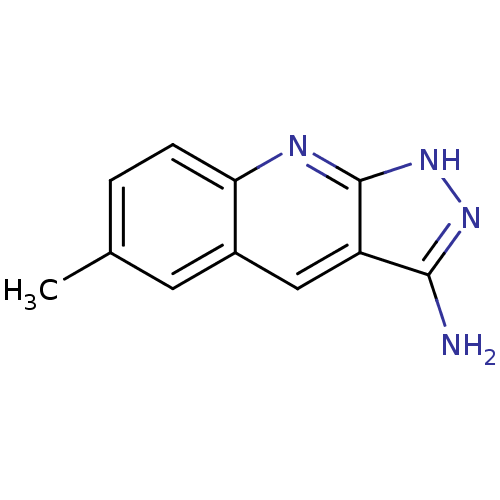 (6-Methyl-2H-pyrazolo[3,4-b]quinolin-3-ylamine | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125762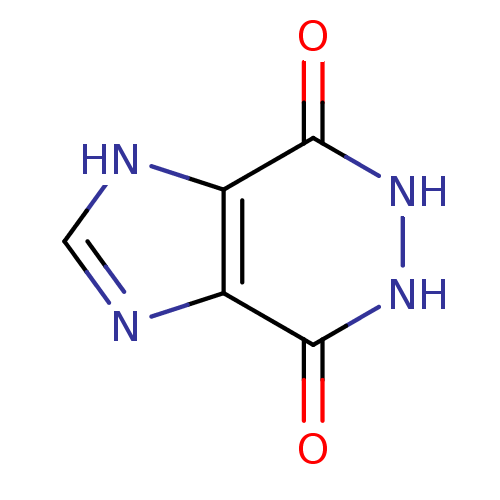 (5,6-Dihydro-1H-imidazo[4,5-d]pyridazine-4,7-dione ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Queuine tRNA-ribosyltransferase (Zymomonas mobilis) | BDBM50125764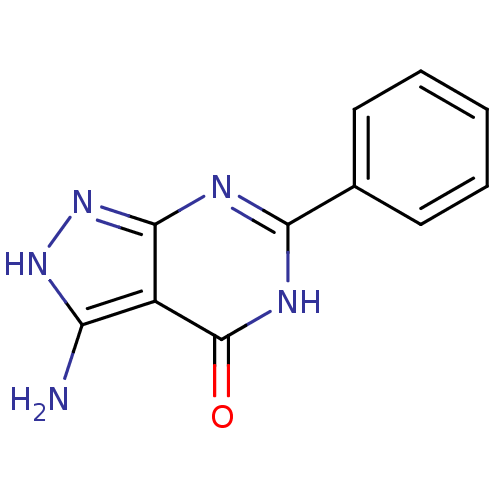 (3-Amino-6-phenyl-2,7-dihydro-pyrazolo[3,4-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg Curated by ChEMBL | Assay Description Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis | J Med Chem 46: 1133-43 (2003) Article DOI: 10.1021/jm0209937 BindingDB Entry DOI: 10.7270/Q2B27TNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355756 (CHEMBL1909658) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355755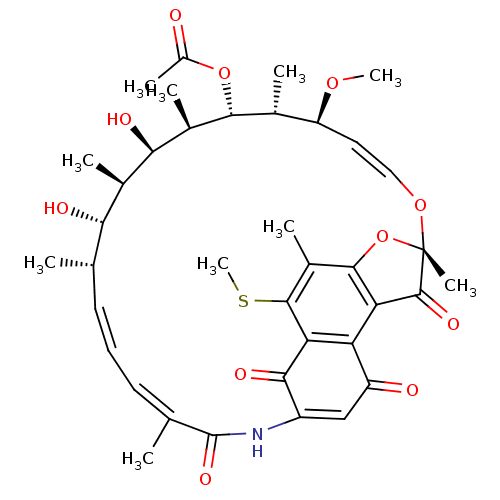 (CHEMBL1911296) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355751 (CHEMBL1911286) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355758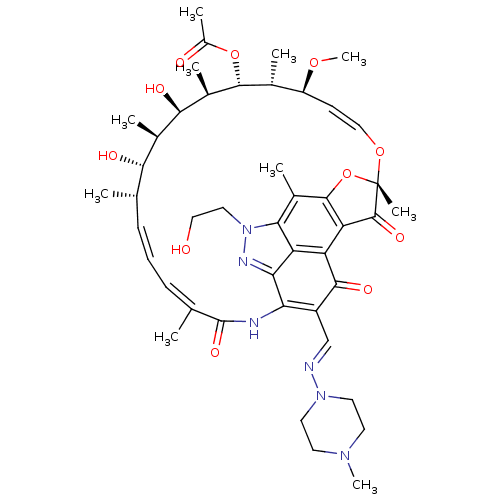 (CHEMBL1911306) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355757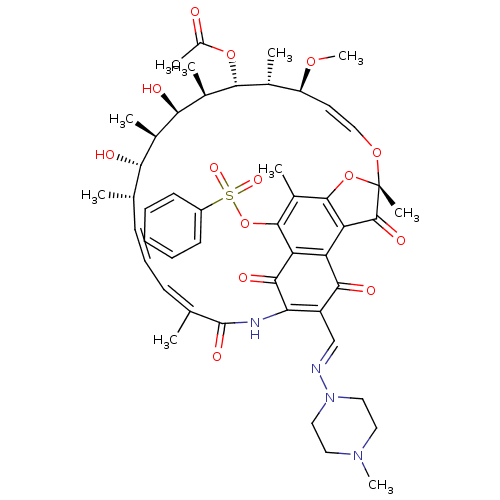 (CHEMBL1911298) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355753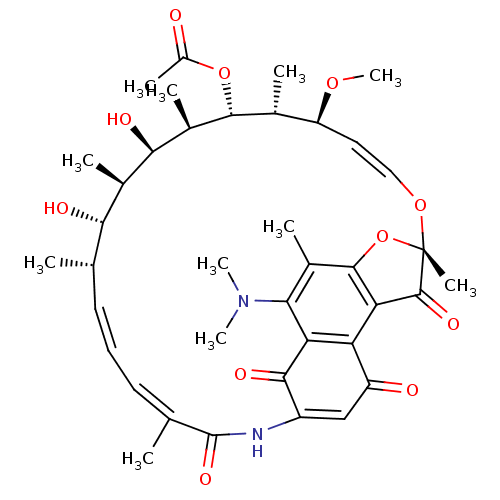 (CHEMBL1911289) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 503 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491557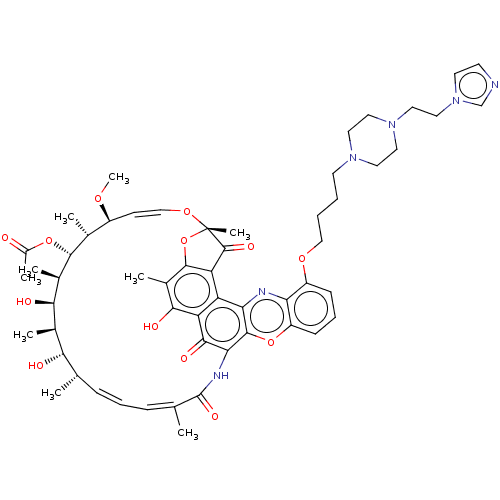 (CHEMBL2046886) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase D516V mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355752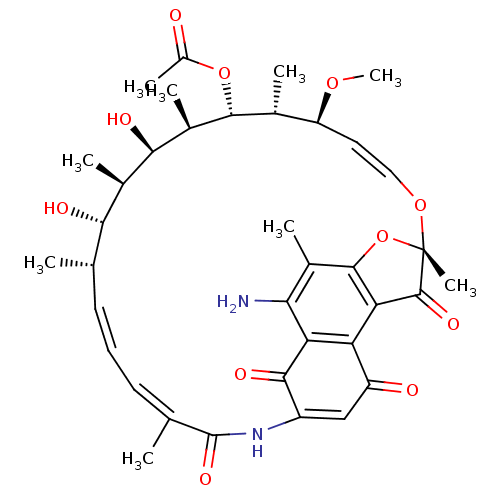 (CHEMBL1911287) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50386752 (CHEMBL2046887) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase S531L mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355754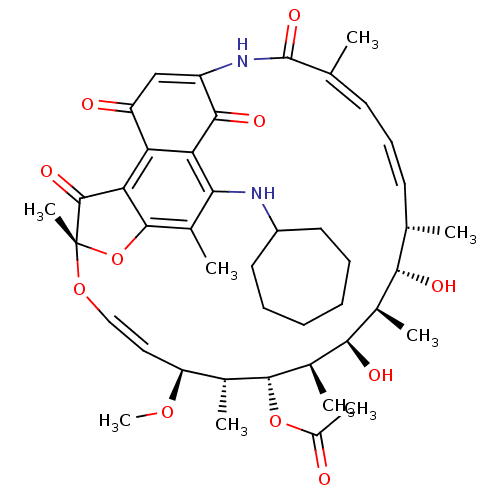 (CHEMBL1911295) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491558 (CHEMBL2046885) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase D516V mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50386752 (CHEMBL2046887) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase D516V mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491559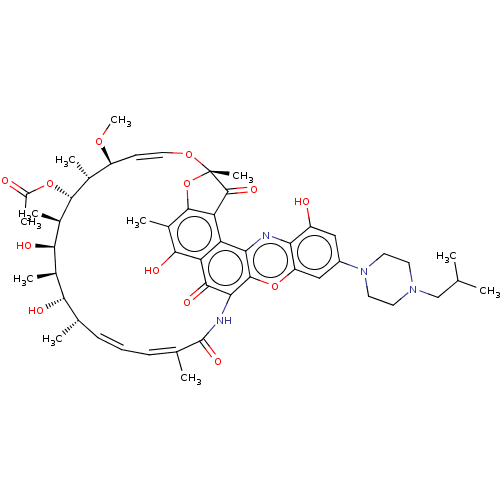 (KRM-1648 | Rifalazil) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase S531L mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491558 (CHEMBL2046885) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase S531L mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491557 (CHEMBL2046886) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 9.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase S531L mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.71E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase S531L mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491559 (KRM-1648 | Rifalazil) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase D516V mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase D516V mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491559 (KRM-1648 | Rifalazil) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase H526Y mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.13E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase H526Y mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50491558 (CHEMBL2046885) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase H526Y mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Escherichia coli) | BDBM50386752 (CHEMBL2046887) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.58E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition of rifamycin-resistant Escherichia coli RNA polymerase H526Y mutant after 10 mins by rolling circle transcription assay in presence of DNA... | J Med Chem 56: 4758-63 (2013) Article DOI: 10.1021/jm4004889 BindingDB Entry DOI: 10.7270/Q2SF303Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50355759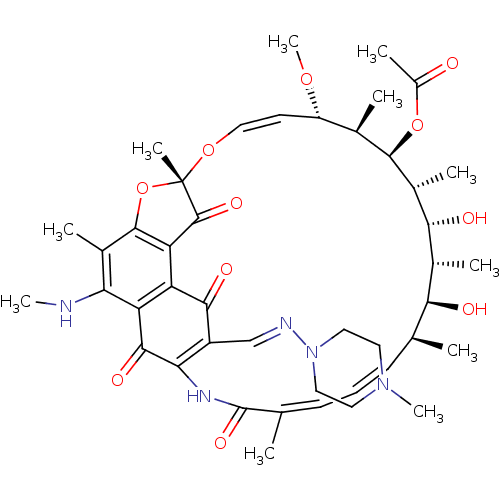 (CHEMBL1911299) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Activation of human PXR in DPX2 cells after 24 hrs by microplate reader relative to control | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Activation of human PXR in DPX2 cells after 24 hrs by microplate reader relative to control | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50386752 (CHEMBL2046887) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Activation of PXR in human DPX2 cells after 24 hrs by luciferase reporter gene assay | J Med Chem 55: 3814-26 (2012) Article DOI: 10.1021/jm201716n BindingDB Entry DOI: 10.7270/Q2GH9K0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50370232 (BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Activation of PXR in human DPX2 cells after 24 hrs by luciferase reporter gene assay | J Med Chem 55: 3814-26 (2012) Article DOI: 10.1021/jm201716n BindingDB Entry DOI: 10.7270/Q2GH9K0K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor subfamily 1 group I member 2 (Homo sapiens (Human)) | BDBM50355760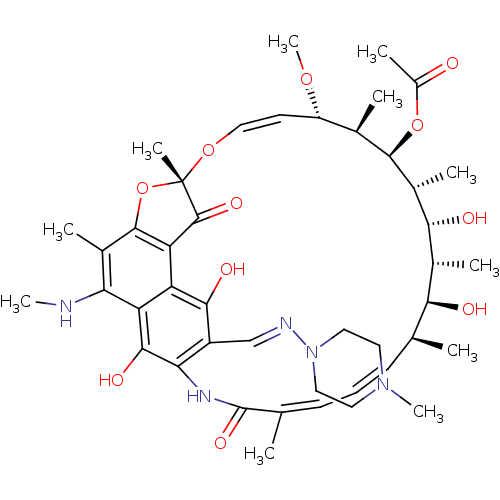 (CHEMBL1911307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Activation of human PXR in DPX2 cells after 24 hrs by microplate reader relative to control | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||