

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391716 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 1 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50539955 (CHEMBL4638938) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391726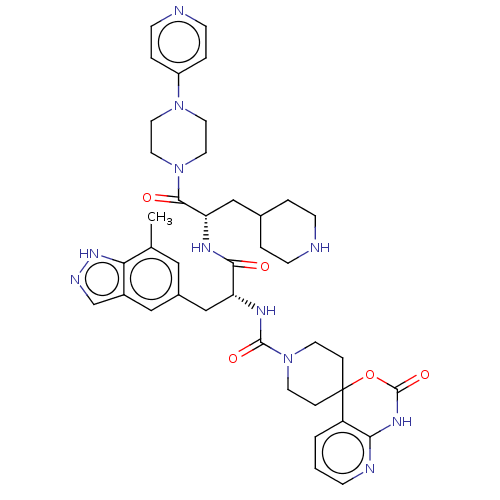 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 11 |...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50184069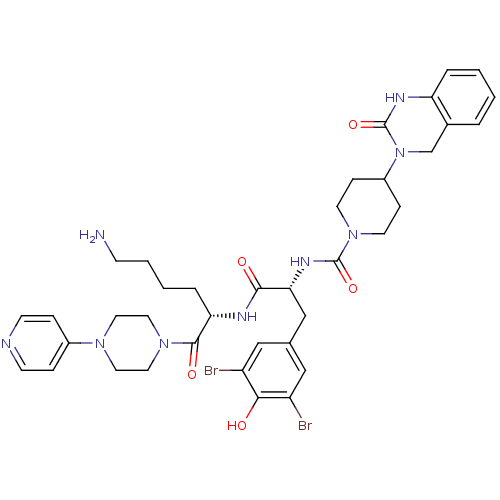 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391723 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 8...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391725 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 10 |...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391718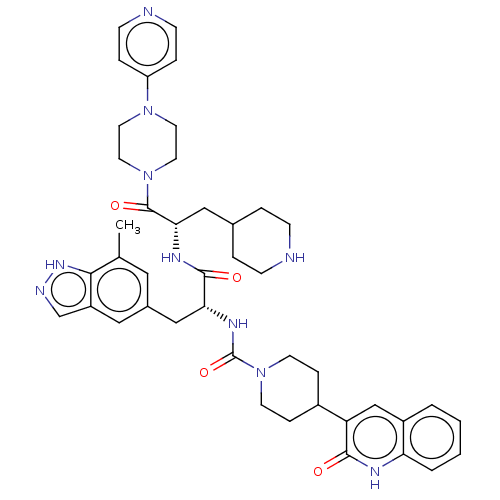 (N-[(2R)-3-(7-methyl-1H- | US10300056, Example 3 | ...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM391724 (3,5-dibromo-Nalpha-{[4-(2- | US10300056, Example 9...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1 (Homo sapiens (Human)) | BDBM50539954 (CHEMBL4636143) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sosei Heptares Curated by ChEMBL | Assay Description Displacement of [3H]telcagepant from recombinant human CLR/RAMP1 expressed in Sf21 insect cell membranes measured after 60 mins by microbeta scintill... | J Med Chem 63: 7906-7920 (2020) Article DOI: 10.1021/acs.jmedchem.0c01003 BindingDB Entry DOI: 10.7270/Q2NK3JKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50229322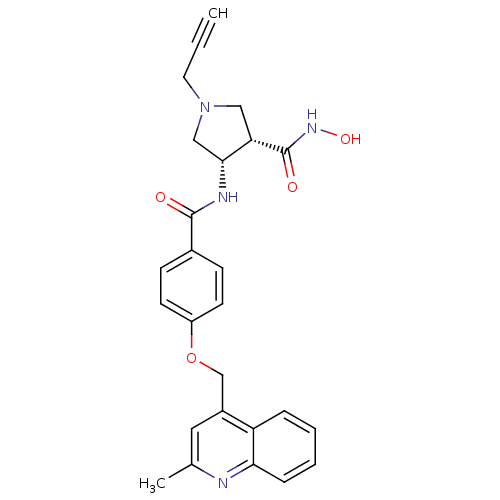 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP7 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26567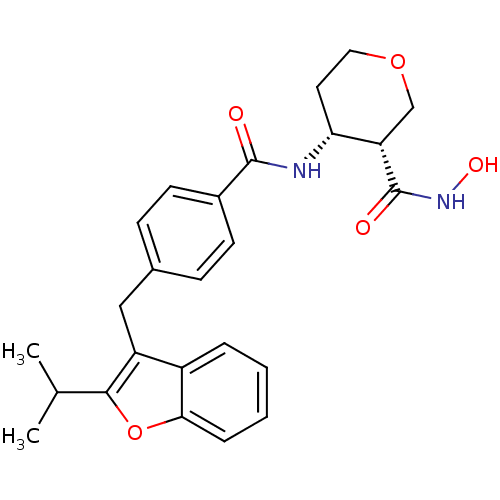 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)-1-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP12 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26565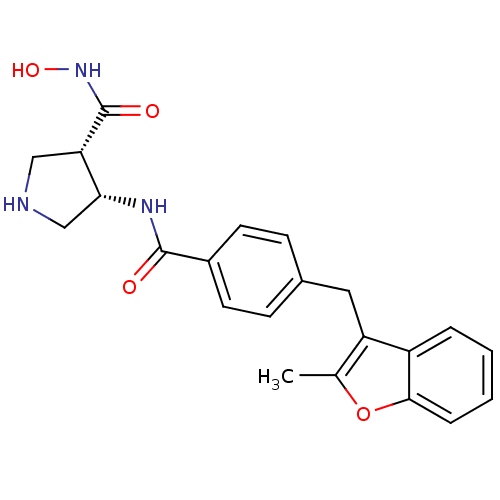 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26564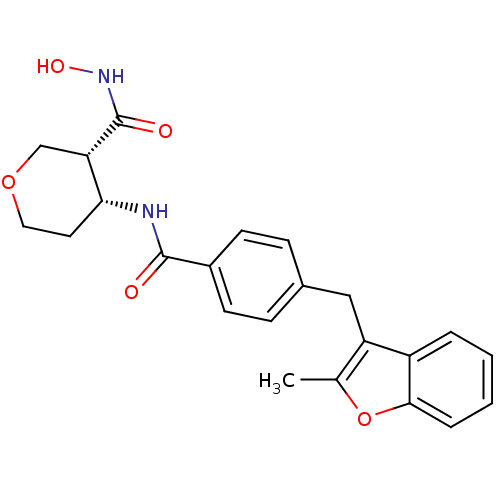 ((3R,4R)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP3 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26562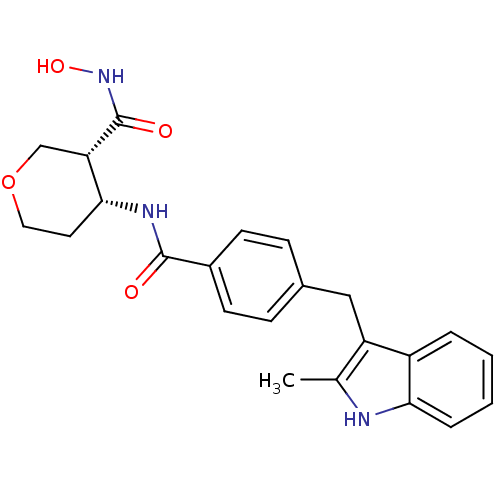 ((3R,4R)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26561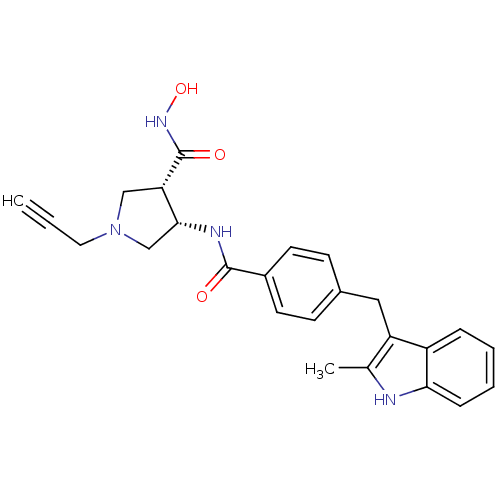 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26566 ((3S,4S)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)-1-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26563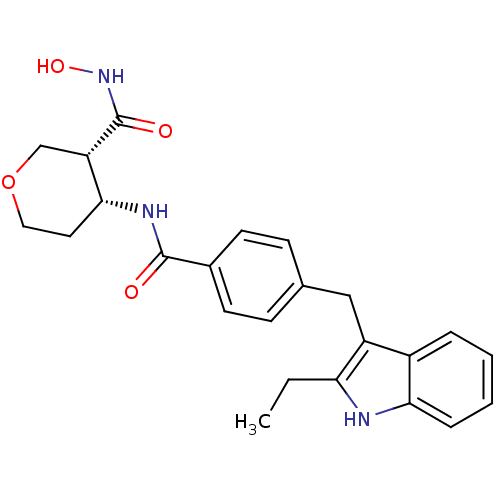 ((3R,4R)-4-N-{4-[(2-ethyl-1H-indol-3-yl)methyl]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26573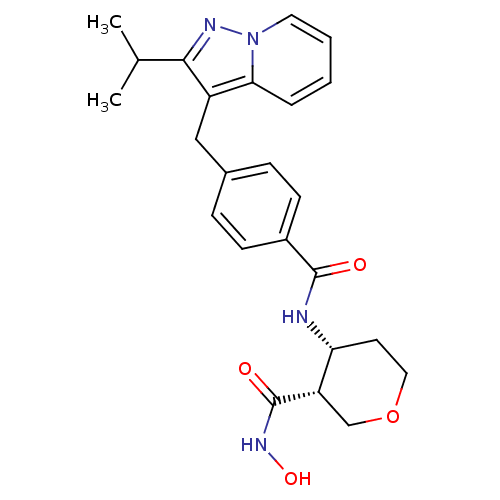 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26560 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP10 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM26561 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26572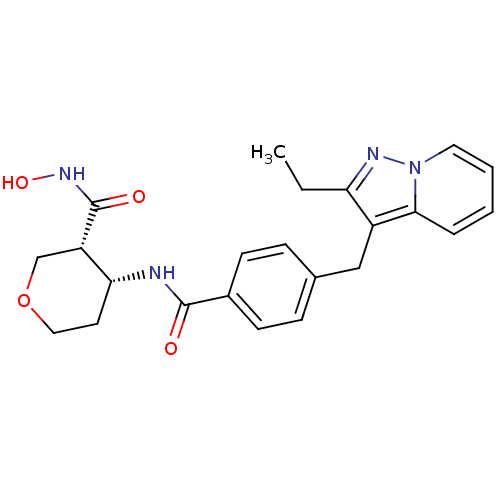 ((3R,4R)-4-N-[4-({2-ethylpyrazolo[1,5-a]pyridin-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 327 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP13 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26570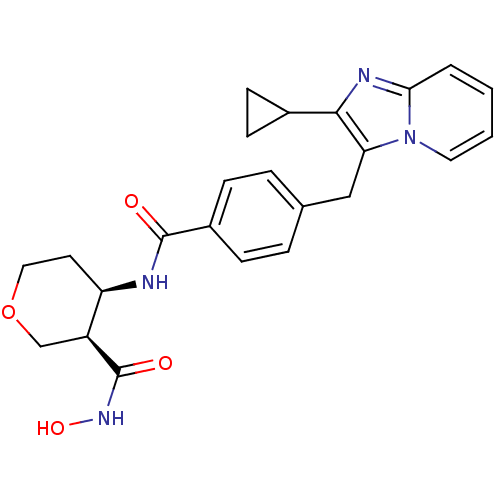 ((3R,4R)-4-N-[4-({2-cyclopropylimidazo[1,2-a]pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26569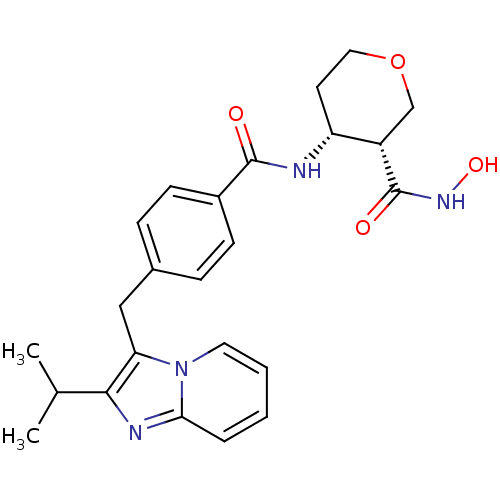 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)imidaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM26565 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 627 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26574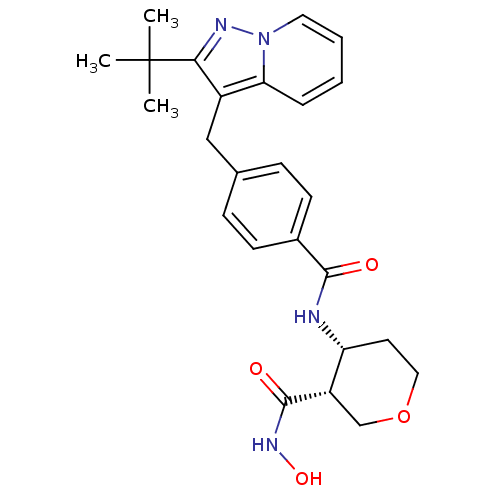 ((3R,4R)-4-N-[4-({2-tert-butylpyrazolo[1,5-a]pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 726 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM26567 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)-1-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 766 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM26567 ((3R,4R)-3-N-hydroxy-4-N-(4-{[2-(propan-2-yl)-1-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 811 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM26561 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 852 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM26568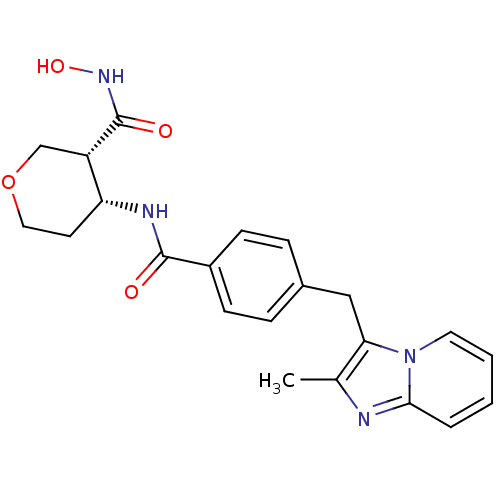 ((3R,4R)-3-N-hydroxy-4-N-[4-({2-methylimidazo[1,2-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP2 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM26564 ((3R,4R)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM26565 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM26810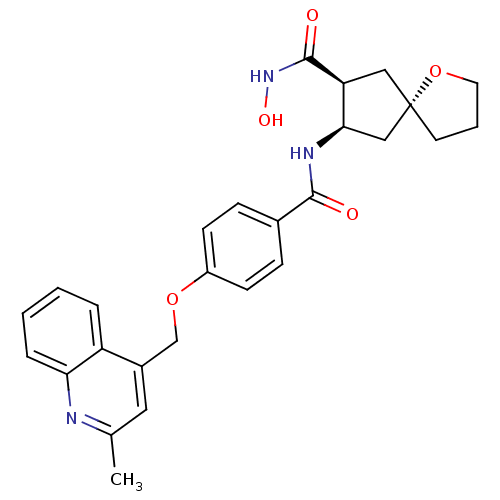 ((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.25E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM26560 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26809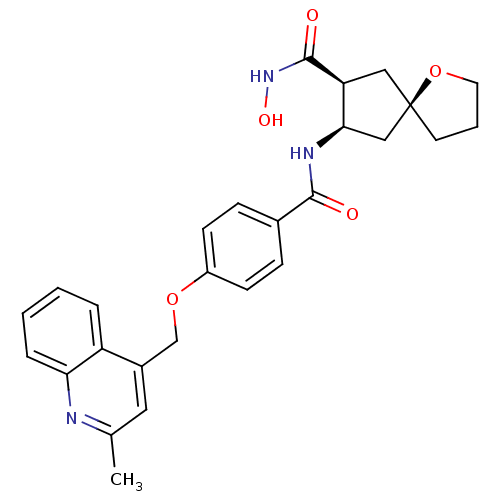 ((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.36E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26565 ((3S,4S)-3-N-hydroxy-4-N-{4-[(2-methyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.37E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM26809 ((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.45E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50371346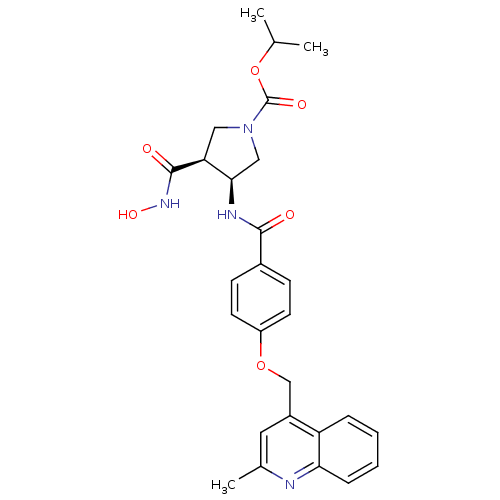 (CHEMBL256598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP2 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM26562 ((3R,4R)-3-N-hydroxy-4-N-{4-[(2-methyl-1H-indol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1958-62 (2008) Article DOI: 10.1016/j.bmcl.2008.01.120 BindingDB Entry DOI: 10.7270/Q25B00SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50371349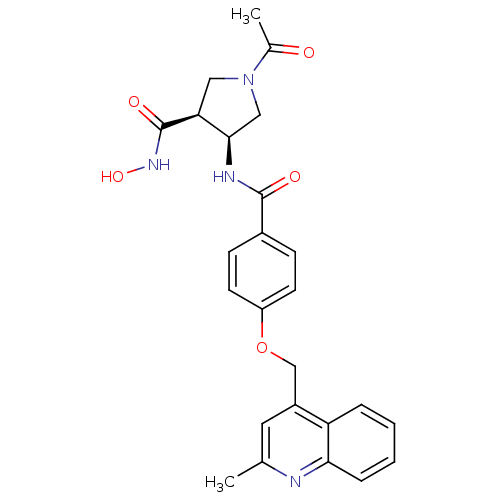 (CHEMBL401979) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP2 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP9 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50371346 (CHEMBL256598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP9 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM26791 (benzimidazole analog., 17 | tert-butyl (3S,4S)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.87E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1577-82 (2008) Article DOI: 10.1016/j.bmcl.2008.01.075 BindingDB Entry DOI: 10.7270/Q2GM85NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM26810 ((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1288-92 (2008) Article DOI: 10.1016/j.bmcl.2008.01.030 BindingDB Entry DOI: 10.7270/Q2BV7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM26789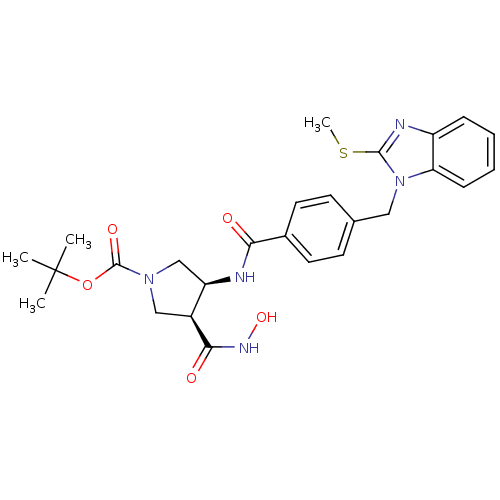 (benzimidazole analog., 15 | tert-butyl (3S,4S)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.96E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company | Assay Description The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... | Bioorg Med Chem Lett 18: 1577-82 (2008) Article DOI: 10.1016/j.bmcl.2008.01.075 BindingDB Entry DOI: 10.7270/Q2GM85NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50229322 ((3S,4S)-N-hydroxy-4-(4-((2-methylquinolin-4-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to MMP8 | Bioorg Med Chem Lett 18: 694-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.059 BindingDB Entry DOI: 10.7270/Q2BV7HGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 827 total ) | Next | Last >> |