 Found 558 hits with Last Name = 'shannon' and Initial = 'he'
Found 558 hits with Last Name = 'shannon' and Initial = 'he' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471498
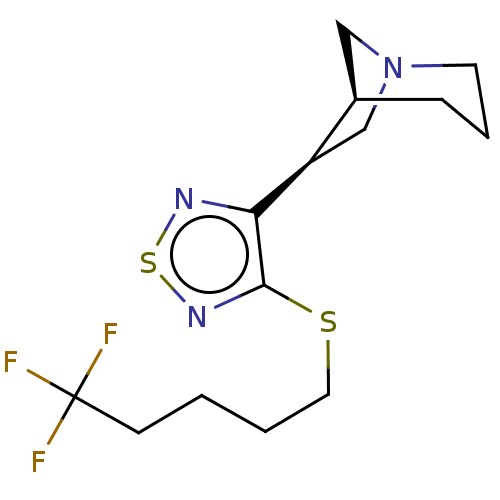
(CHEMBL150450)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C14H20F3N3S2/c15-14(16,17)5-1-2-7-21-13-12(18-22-19-13)11-9-20-6-3-4-10(11)8-20/h10-11H,1-9H2/t10-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471487
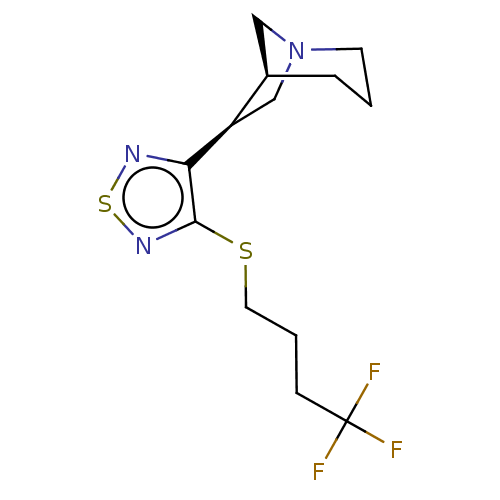
(CHEMBL436075)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471494
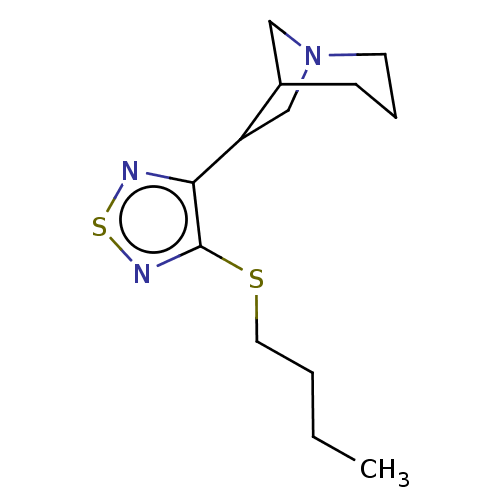
(CHEMBL153723)Show InChI InChI=1S/C13H21N3S2/c1-2-3-7-17-13-12(14-18-15-13)11-9-16-6-4-5-10(11)8-16/h10-11H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471485
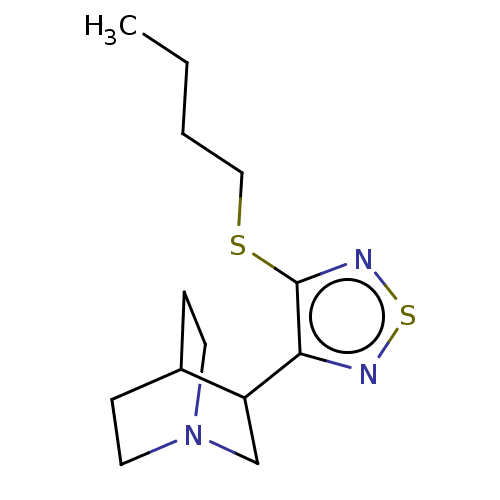
(Vedaclidine)Show SMILES CCCCSc1nsnc1C1CN2CCC1CC2 |(16.46,-2.74,;15.65,-4.05,;14.12,-3.99,;13.3,-5.3,;11.76,-5.24,;10.94,-6.55,;11.52,-7.98,;10.35,-8.98,;9.04,-8.15,;9.4,-6.67,;8.41,-5.49,;6.9,-5.77,;5.93,-4.58,;6.45,-3.12,;7.96,-2.86,;8.95,-4.05,;7.8,-3.47,;7.42,-4.98,)| Show InChI InChI=1S/C13H21N3S2/c1-2-3-8-17-13-12(14-18-15-13)11-9-16-6-4-10(11)5-7-16/h10-11H,2-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471499
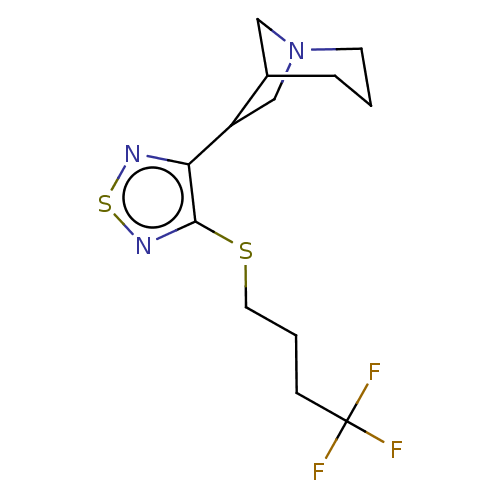
(CHEMBL345774)Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471492
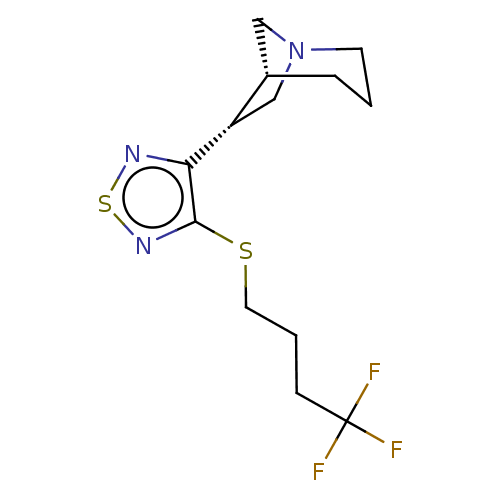
(CHEMBL150293)Show SMILES [H][C@]12CN(C[C@@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471495
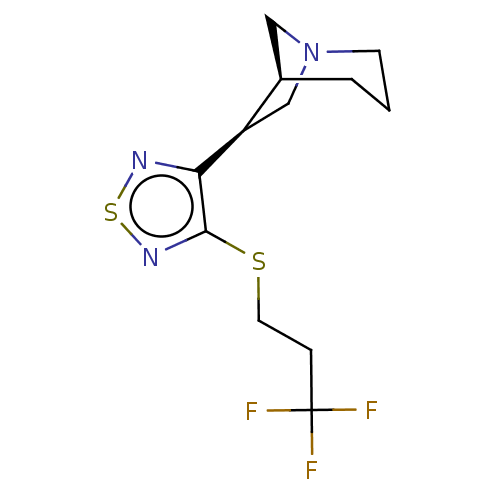
(CHEMBL153478)Show SMILES [H][C@@]12CN(C[C@H]1c1nsnc1SCCC(F)(F)F)CCC2 Show InChI InChI=1S/C12H16F3N3S2/c13-12(14,15)3-5-19-11-10(16-20-17-11)9-7-18-4-1-2-8(9)6-18/h8-9H,1-7H2/t8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471486
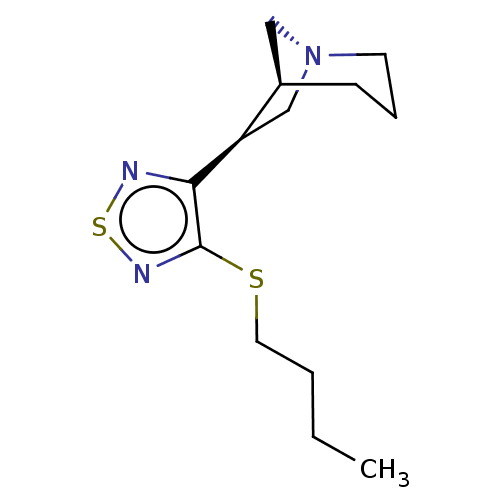
(CHEMBL343731)Show SMILES [H][C@@]12C[N@@](C[C@@]1([H])c1nsnc1SCCCC)CCC2 Show InChI InChI=1S/C13H21N3S2/c1-2-3-7-17-13-12(14-18-15-13)11-9-16-6-4-5-10(11)8-16/h10-11H,2-9H2,1H3/t10-,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50033155
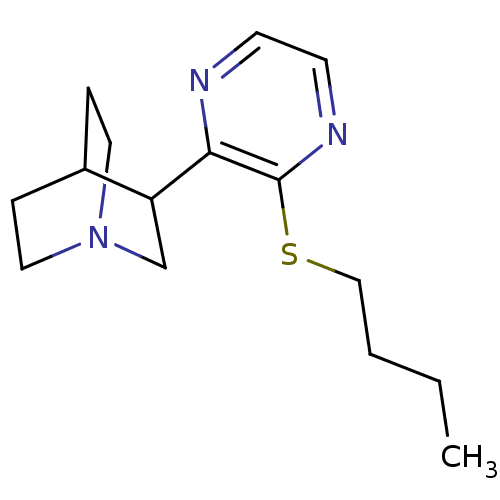
(3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...)Show SMILES CCCCSc1nccnc1C1CN2CCC1CC2 |(4.41,-8.01,;5.75,-8.78,;5.74,-10.32,;7.07,-11.09,;7.07,-12.63,;8.4,-13.4,;9.74,-12.65,;11.07,-13.42,;11.07,-14.96,;9.73,-15.71,;8.41,-14.94,;7.07,-15.71,;7.07,-17.25,;5.74,-18.01,;6.28,-16.47,;5,-16.36,;5.74,-14.93,;4.42,-15.71,;4.42,-17.25,)| Show InChI InChI=1S/C15H23N3S/c1-2-3-10-19-15-14(16-6-7-17-15)13-11-18-8-4-12(13)5-9-18/h6-7,12-13H,2-5,8-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50403547

(ATROPEN | ATROPINE)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)C(CO)c1ccccc1 |r,THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3/t13-,14+,15+,16? | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471491
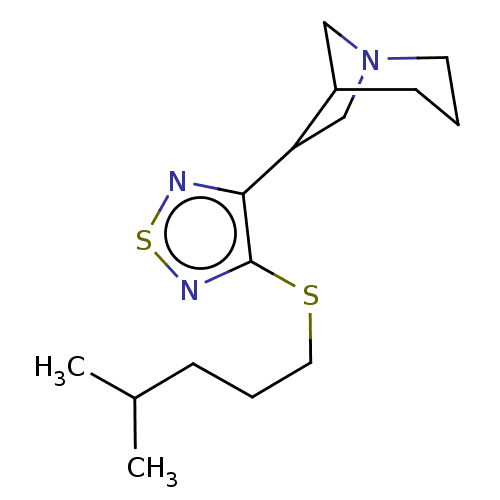
(CHEMBL357279)Show InChI InChI=1S/C15H25N3S2/c1-11(2)5-4-8-19-15-14(16-20-17-15)13-10-18-7-3-6-12(13)9-18/h11-13H,3-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070725
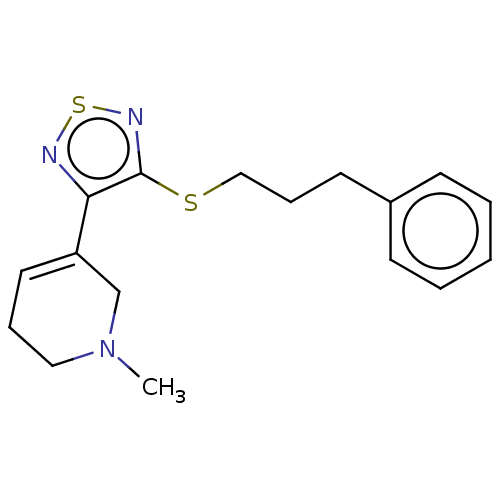
(CHEMBL318403)Show InChI InChI=1S/C28H43N5O8/c1-4-16(3)23(26(38)30-19(5-2)28(40)41)32-25(37)21-10-8-14-33(21)27(39)20(9-6-7-13-29)31-24(36)18-15-17(34)11-12-22(18)35/h11-12,15-16,19-21,23,34-35H,4-10,13-14,29H2,1-3H3,(H,30,38)(H,31,36)(H,32,37)(H,40,41)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003351
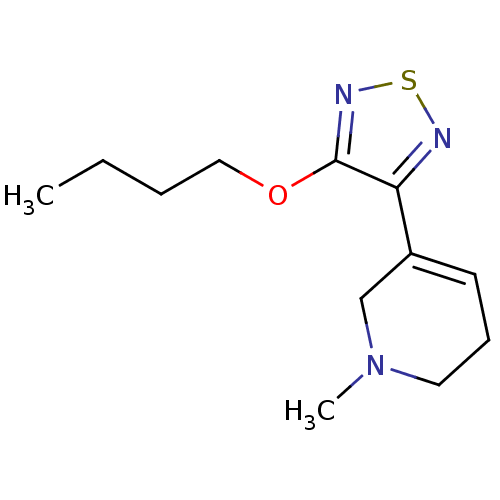
(3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...)Show InChI InChI=1S/C12H19N3OS/c1-3-4-8-16-12-11(13-17-14-12)10-6-5-7-15(2)9-10/h6H,3-5,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50494356
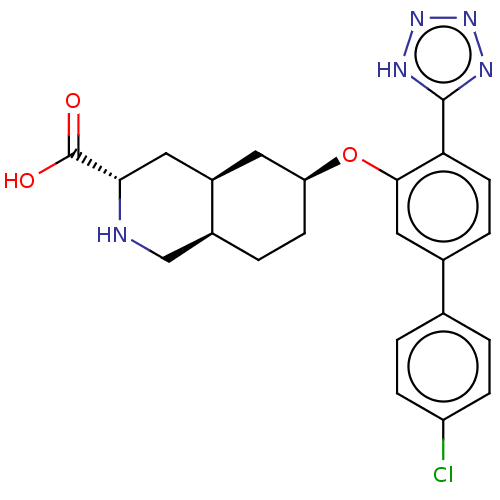
(CHEMBL3088070)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H24ClN5O3.ClH/c24-17-5-1-13(2-6-17)14-4-8-19(22-26-28-29-27-22)21(11-14)32-18-7-3-15-12-25-20(23(30)31)10-16(15)9-18;/h1-2,4-6,8,11,15-16,18,20,25H,3,7,9-10,12H2,(H,30,31)(H,26,27,28,29);1H/t15-,16+,18-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from homomeric recombinant GluA2 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471489
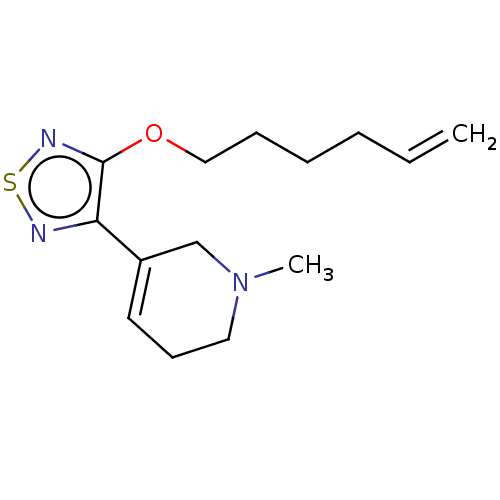
(CHEMBL153150)Show InChI InChI=1S/C14H21N3OS/c1-3-4-5-6-10-18-14-13(15-19-16-14)12-8-7-9-17(2)11-12/h3,8H,1,4-7,9-11H2,2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471496
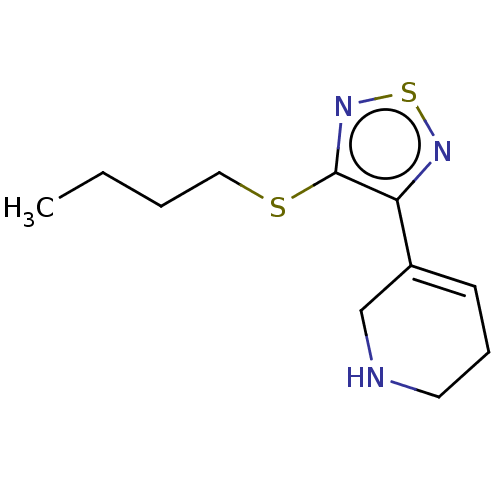
(CHEMBL153031)Show InChI InChI=1S/C11H17N3S2/c1-2-3-7-15-11-10(13-16-14-11)9-5-4-6-12-8-9/h5,12H,2-4,6-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50033151
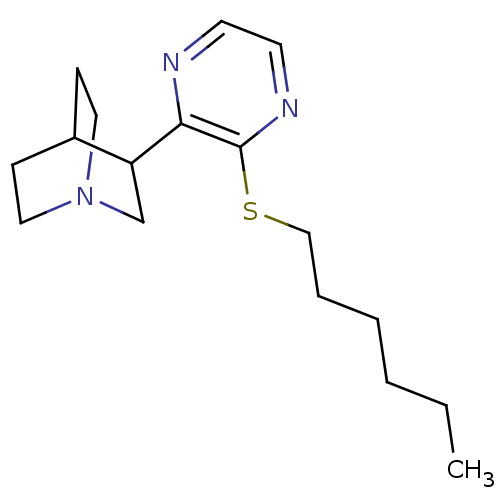
(3-(3-Hexylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...)Show SMILES CCCCCCSc1nccnc1C1CN2CCC1CC2 |(4.09,3.27,;5.44,2.49,;5.44,.95,;6.77,.18,;6.77,-1.36,;8.1,-2.13,;8.1,-3.67,;9.43,-4.44,;10.76,-3.69,;12.09,-4.46,;12.09,-6,;10.76,-6.75,;9.43,-5.98,;8.1,-6.75,;8.1,-8.29,;6.77,-9.05,;7.31,-7.51,;6.03,-7.4,;6.77,-5.97,;5.44,-6.75,;5.44,-8.29,)| Show InChI InChI=1S/C17H27N3S/c1-2-3-4-5-12-21-17-16(18-8-9-19-17)15-13-20-10-6-14(15)7-11-20/h8-9,14-15H,2-7,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471497
(CHEMBL150501)Show SMILES [H][C@@]12CN(C[C@@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471488
(CHEMBL152892)Show InChI InChI=1S/C15H19N3S3/c1-18-8-2-5-12(11-18)14-15(17-21-16-14)20-10-4-7-13-6-3-9-19-13/h3,5-6,9H,2,4,7-8,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471493
(CHEMBL152919)Show InChI InChI=1S/C15H25N3S2/c1-3-5-6-7-11-19-15-14(16-20-17-15)13-9-8-10-18(4-2)12-13/h9H,3-8,10-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494356

(CHEMBL3088070)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H24ClN5O3.ClH/c24-17-5-1-13(2-6-17)14-4-8-19(22-26-28-29-27-22)21(11-14)32-18-7-3-15-12-25-20(23(30)31)10-16(15)9-18;/h1-2,4-6,8,11,15-16,18,20,25H,3,7,9-10,12H2,(H,30,31)(H,26,27,28,29);1H/t15-,16+,18-,20-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471490
(CHEMBL154923)Show SMILES [H][C@]12CN(C[C@H]1c1nsnc1SCCCC(F)(F)F)CCC2 Show InChI InChI=1S/C13H18F3N3S2/c14-13(15,16)4-2-6-20-12-11(17-21-18-12)10-8-19-5-1-3-9(10)7-19/h9-10H,1-8H2/t9-,10+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex |
J Med Chem 40: 538-46 (1997)
Article DOI: 10.1021/jm9602470
BindingDB Entry DOI: 10.7270/Q2SQ934F |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494342

(CHEMBL3088072)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1cccs1 |r| Show InChI InChI=1S/C21H23N5O3S.ClH/c27-21(28)17-9-14-8-15(5-3-13(14)11-22-17)29-18-10-12(19-2-1-7-30-19)4-6-16(18)20-23-25-26-24-20;/h1-2,4,6-7,10,13-15,17,22H,3,5,8-9,11H2,(H,27,28)(H,23,24,25,26);1H/t13-,14+,15-,17-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494350
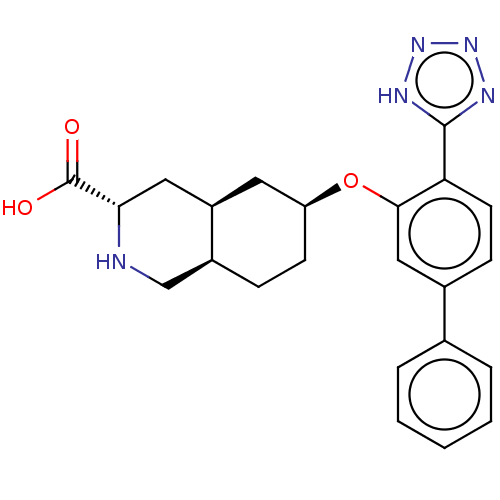
(CHEMBL3088064)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C23H25N5O3.ClH/c29-23(30)20-11-17-10-18(8-6-16(17)13-24-20)31-21-12-15(14-4-2-1-3-5-14)7-9-19(21)22-25-27-28-26-22;/h1-5,7,9,12,16-18,20,24H,6,8,10-11,13H2,(H,29,30)(H,25,26,27,28);1H/t16-,17+,18-,20-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494365

(CHEMBL3085837)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(Cc2ccccc2)ccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C24H27N5O3.ClH/c30-24(31)21-13-18-12-19(8-7-17(18)14-25-21)32-22-11-16(10-15-4-2-1-3-5-15)6-9-20(22)23-26-28-29-27-23;/h1-6,9,11,17-19,21,25H,7-8,10,12-14H2,(H,30,31)(H,26,27,28,29);1H/t17-,18+,19-,21-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494355
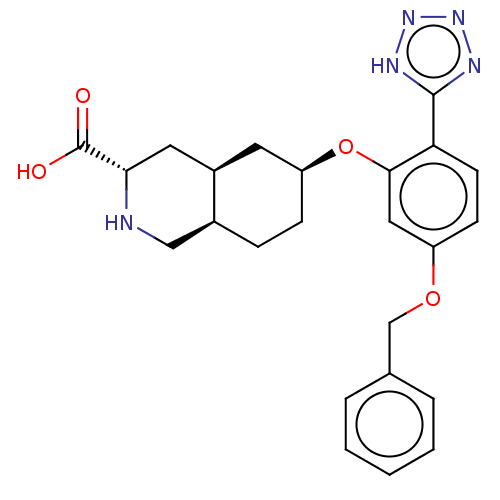
(CHEMBL3085836)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(OCc2ccccc2)ccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C24H27N5O4.ClH/c30-24(31)21-11-17-10-19(7-6-16(17)13-25-21)33-22-12-18(32-14-15-4-2-1-3-5-15)8-9-20(22)23-26-28-29-27-23;/h1-5,8-9,12,16-17,19,21,25H,6-7,10-11,13-14H2,(H,30,31)(H,26,27,28,29);1H/t16-,17+,19-,21-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494358

(CHEMBL3088058)Show SMILES [H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Nc1cccc(Cl)c1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H21ClN6O2/c18-12-2-1-3-13(15(12)16-21-23-24-22-16)20-11-5-4-9-8-19-14(17(25)26)7-10(9)6-11/h1-3,9-11,14,19-20H,4-8H2,(H,25,26)(H,21,22,23,24)/t9-,10+,11-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494364

(CHEMBL3088054)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cccc(Cl)c1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H20ClN5O3.ClH/c18-12-2-1-3-14(15(12)16-20-22-23-21-16)26-11-5-4-9-8-19-13(17(24)25)7-10(9)6-11;/h1-3,9-11,13,19H,4-8H2,(H,24,25)(H,20,21,22,23);1H/t9-,10+,11-,13-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Lilly
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-AMPA binding to human GluR2 receptors expressed in HEK 293 cells |
J Med Chem 48: 4200-3 (2005)
Article DOI: 10.1021/jm0491952
BindingDB Entry DOI: 10.7270/Q2HQ3ZDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50494351
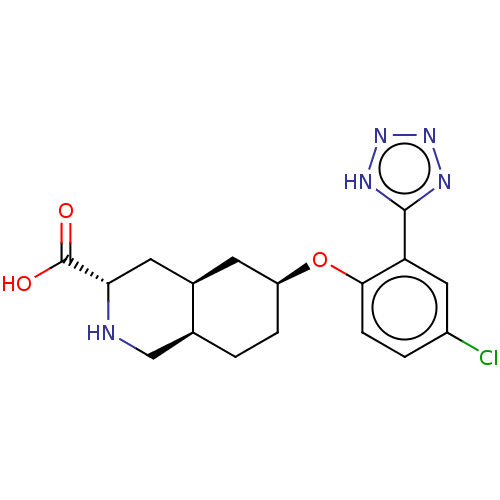
(CHEMBL3088060)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1ccc(Cl)cc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H20ClN5O3.ClH/c18-11-2-4-15(13(7-11)16-20-22-23-21-16)26-12-3-1-9-8-19-14(17(24)25)6-10(9)5-12;/h2,4,7,9-10,12,14,19H,1,3,5-6,8H2,(H,24,25)(H,20,21,22,23);1H/t9-,10+,12-,14-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]AMPA from homomeric recombinant GluA2 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494351

(CHEMBL3088060)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1ccc(Cl)cc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H20ClN5O3.ClH/c18-11-2-4-15(13(7-11)16-20-22-23-21-16)26-12-3-1-9-8-19-14(17(24)25)6-10(9)5-12;/h2,4,7,9-10,12,14,19H,1,3,5-6,8H2,(H,24,25)(H,20,21,22,23);1H/t9-,10+,12-,14-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494055

(CHEMBL2440699)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1C(O)=O)-c1ccccc1 |r| Show InChI InChI=1S/C23H25NO5.ClH/c25-22(26)19-9-7-15(14-4-2-1-3-5-14)12-21(19)29-18-8-6-16-13-24-20(23(27)28)11-17(16)10-18;/h1-5,7,9,12,16-18,20,24H,6,8,10-11,13H2,(H,25,26)(H,27,28);1H/t16-,17+,18-,20-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Binding affinity to GluK1 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6459-62 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.046
BindingDB Entry DOI: 10.7270/Q2862KDB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494367

(CHEMBL3087685)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Sc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H21N5O2S.ClH/c23-17(24)14-8-11-7-12(6-5-10(11)9-18-14)25-15-4-2-1-3-13(15)16-19-21-22-20-16;/h1-4,10-12,14,18H,5-9H2,(H,23,24)(H,19,20,21,22);1H/t10-,11+,12-,14-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494348
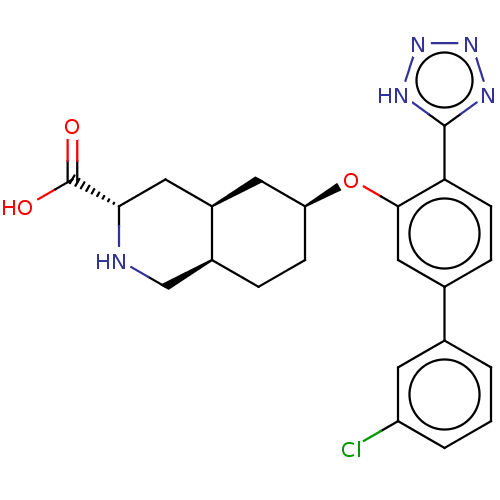
(CHEMBL3088069)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C23H24ClN5O3.ClH/c24-17-3-1-2-13(8-17)14-5-7-19(22-26-28-29-27-22)21(11-14)32-18-6-4-15-12-25-20(23(30)31)10-16(15)9-18;/h1-3,5,7-8,11,15-16,18,20,25H,4,6,9-10,12H2,(H,30,31)(H,26,27,28,29);1H/t15-,16+,18-,20-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50168964

((3S,4aR,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenylamin...)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Nc1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C17H22N6O2/c24-17(25)15-8-11-7-12(6-5-10(11)9-18-15)19-14-4-2-1-3-13(14)16-20-22-23-21-16/h1-4,10-12,15,18-19H,5-9H2,(H,24,25)(H,20,21,22,23)/t10-,11+,12-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494068
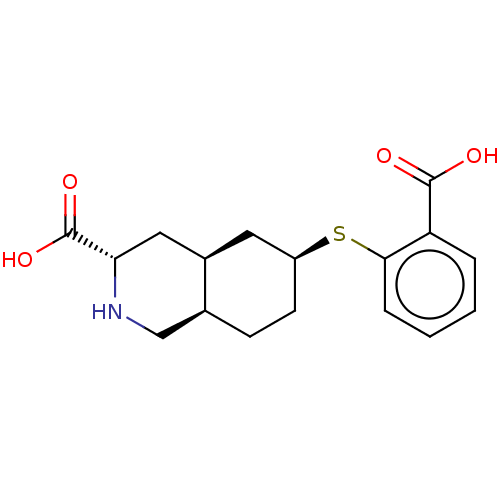
(CHEMBL2440688)Show SMILES [H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Sc1ccccc1C(O)=O |r| Show InChI InChI=1S/C17H21NO4S/c19-16(20)13-3-1-2-4-15(13)23-12-6-5-10-9-18-14(17(21)22)8-11(10)7-12/h1-4,10-12,14,18H,5-9H2,(H,19,20)(H,21,22)/t10-,11+,12-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Binding affinity to GluK1 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6459-62 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.046
BindingDB Entry DOI: 10.7270/Q2862KDB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494346
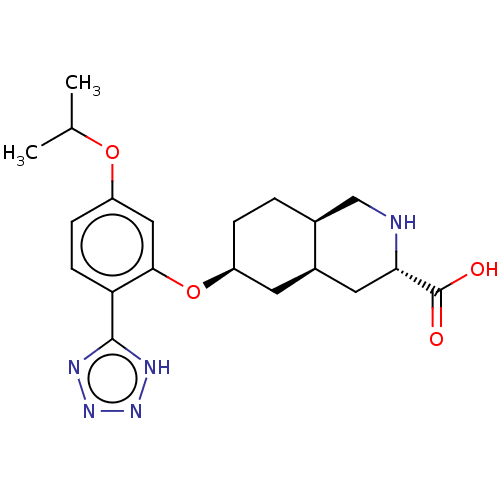
(CHEMBL3088074)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(OC(C)C)ccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C20H27N5O4.ClH/c1-11(2)28-15-5-6-16(19-22-24-25-23-19)18(9-15)29-14-4-3-12-10-21-17(20(26)27)8-13(12)7-14;/h5-6,9,11-14,17,21H,3-4,7-8,10H2,1-2H3,(H,26,27)(H,22,23,24,25);1H/t12-,13+,14-,17-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494347
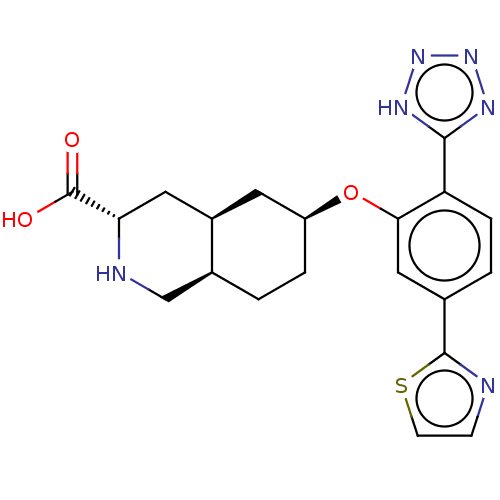
(CHEMBL3088071)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1nccs1 |r| Show InChI InChI=1S/C20H22N6O3S.ClH/c27-20(28)16-8-13-7-14(3-1-12(13)10-22-16)29-17-9-11(19-21-5-6-30-19)2-4-15(17)18-23-25-26-24-18;/h2,4-6,9,12-14,16,22H,1,3,7-8,10H2,(H,27,28)(H,23,24,25,26);1H/t12-,13+,14-,16-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494061
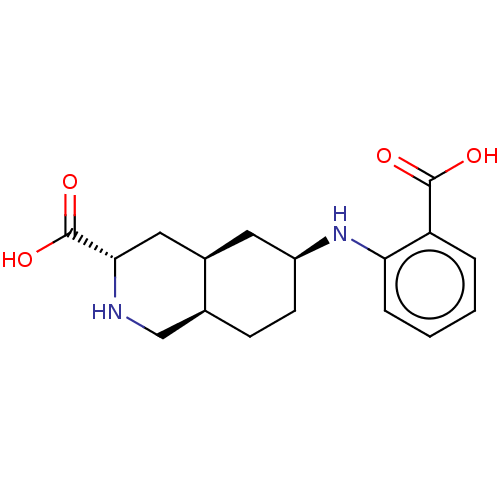
(CHEMBL2440689)Show SMILES [H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Nc1ccccc1C(O)=O |r| Show InChI InChI=1S/C17H22N2O4/c20-16(21)13-3-1-2-4-14(13)19-12-6-5-10-9-18-15(17(22)23)8-11(10)7-12/h1-4,10-12,15,18-19H,5-9H2,(H,20,21)(H,22,23)/t10-,11+,12-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Binding affinity to GluK1 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6459-62 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.046
BindingDB Entry DOI: 10.7270/Q2862KDB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494352
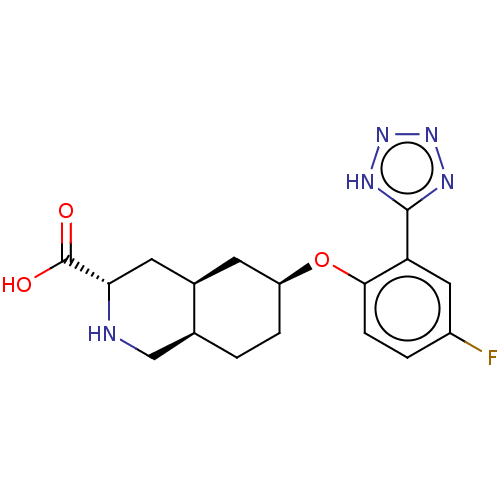
(CHEMBL3088059)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1ccc(F)cc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H20FN5O3.ClH/c18-11-2-4-15(13(7-11)16-20-22-23-21-16)26-12-3-1-9-8-19-14(17(24)25)6-10(9)5-12;/h2,4,7,9-10,12,14,19H,1,3,5-6,8H2,(H,24,25)(H,20,21,22,23);1H/t9-,10+,12-,14-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494366

(CHEMBL3088068)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(ccc1-c1nnn[nH]1)-c1ccccc1Cl |r| Show InChI InChI=1S/C23H24ClN5O3.ClH/c24-19-4-2-1-3-17(19)13-6-8-18(22-26-28-29-27-22)21(11-13)32-16-7-5-14-12-25-20(23(30)31)10-15(14)9-16;/h1-4,6,8,11,14-16,20,25H,5,7,9-10,12H2,(H,30,31)(H,26,27,28,29);1H/t14-,15+,16-,20-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494361
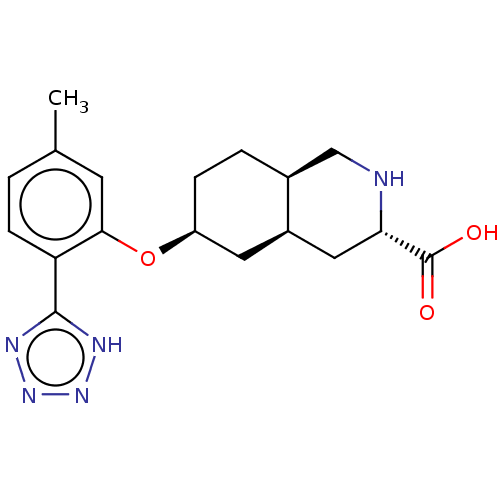
(CHEMBL3088062)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(C)ccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C18H23N5O3.ClH/c1-10-2-5-14(17-20-22-23-21-17)16(6-10)26-13-4-3-11-9-19-15(18(24)25)8-12(11)7-13;/h2,5-6,11-13,15,19H,3-4,7-9H2,1H3,(H,24,25)(H,20,21,22,23);1H/t11-,12+,13-,15-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494349
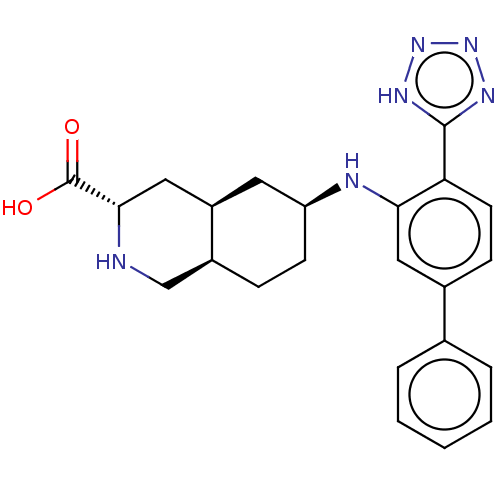
(CHEMBL3088066)Show SMILES [H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Nc1cc(ccc1-c1nnn[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C23H26N6O2/c30-23(31)21-12-17-10-18(8-6-16(17)13-24-21)25-20-11-15(14-4-2-1-3-5-14)7-9-19(20)22-26-28-29-27-22/h1-5,7,9,11,16-18,21,24-25H,6,8,10,12-13H2,(H,30,31)(H,26,27,28,29)/t16-,17+,18-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494359

(CHEMBL3088055)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cccc(OC)c1-c1nnn[nH]1 |r| Show InChI InChI=1S/C18H23N5O4.ClH/c1-26-14-3-2-4-15(16(14)17-20-22-23-21-17)27-12-6-5-10-9-19-13(18(24)25)8-11(10)7-12;/h2-4,10-13,19H,5-9H2,1H3,(H,24,25)(H,20,21,22,23);1H/t10-,11+,12-,13-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50168965
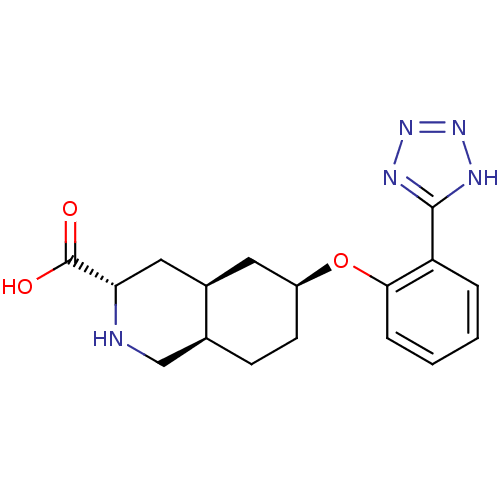
((3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-d...)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C17H21N5O3/c23-17(24)14-8-11-7-12(6-5-10(11)9-18-14)25-15-4-2-1-3-13(15)16-19-21-22-20-16/h1-4,10-12,14,18H,5-9H2,(H,23,24)(H,19,20,21,22)/t10-,11+,12-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494051

(CHEMBL2440702)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Sc1cc(Cl)ccc1C(O)=O |r| Show InChI InChI=1S/C17H20ClNO4S.ClH/c18-11-2-4-13(16(20)21)15(7-11)24-12-3-1-9-8-19-14(17(22)23)6-10(9)5-12;/h2,4,7,9-10,12,14,19H,1,3,5-6,8H2,(H,20,21)(H,22,23);1H/t9-,10+,12-,14-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Binding affinity to GluK1 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6459-62 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.046
BindingDB Entry DOI: 10.7270/Q2862KDB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494362

(CHEMBL3088063)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1cc(OC)ccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C18H23N5O4.ClH/c1-26-12-4-5-14(17-20-22-23-21-17)16(8-12)27-13-3-2-10-9-19-15(18(24)25)7-11(10)6-13;/h4-5,8,10-11,13,15,19H,2-3,6-7,9H2,1H3,(H,24,25)(H,20,21,22,23);1H/t10-,11+,13-,15-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]ATPA from human Gluk1 receptor |
Bioorg Med Chem Lett 23: 6463-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.045
BindingDB Entry DOI: 10.7270/Q2XS5ZBW |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50494059
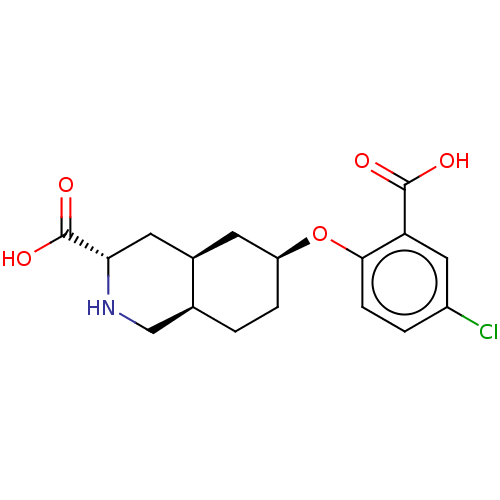
(CHEMBL2440685)Show SMILES Cl.[H][C@@]12CC[C@@H](C[C@]1([H])C[C@H](NC2)C(O)=O)Oc1ccc(Cl)cc1C(O)=O |r| Show InChI InChI=1S/C17H20ClNO5.ClH/c18-11-2-4-15(13(7-11)16(20)21)24-12-3-1-9-8-19-14(17(22)23)6-10(9)5-12;/h2,4,7,9-10,12,14,19H,1,3,5-6,8H2,(H,20,21)(H,22,23);1H/t9-,10+,12-,14-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Binding affinity to GluK1 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6459-62 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.046
BindingDB Entry DOI: 10.7270/Q2862KDB |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50168964

((3S,4aR,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenylamin...)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Nc1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C17H22N6O2/c24-17(25)15-8-11-7-12(6-5-10(11)9-18-15)19-14-4-2-1-3-13(14)16-20-22-23-21-16/h1-4,10-12,15,18-19H,5-9H2,(H,24,25)(H,20,21,22,23)/t10-,11+,12-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Lilly
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-KA binding to iontropic glutamate receptor 5 |
J Med Chem 48: 4200-3 (2005)
Article DOI: 10.1021/jm0491952
BindingDB Entry DOI: 10.7270/Q2HQ3ZDM |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 1
(Homo sapiens (Human)) | BDBM50168965

((3S,4aS,6S,8aR)-6-[2-(1H-Tetrazol-5-yl)-phenoxy]-d...)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C17H21N5O3/c23-17(24)14-8-11-7-12(6-5-10(11)9-18-14)25-15-4-2-1-3-13(15)16-19-21-22-20-16/h1-4,10-12,14,18H,5-9H2,(H,23,24)(H,19,20,21,22)/t10-,11+,12-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigación Lilly
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-KA binding to iontropic glutamate receptor 5 |
J Med Chem 48: 4200-3 (2005)
Article DOI: 10.1021/jm0491952
BindingDB Entry DOI: 10.7270/Q2HQ3ZDM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data