 Found 23 hits with Last Name = 'liparulo' and Initial = 'i'
Found 23 hits with Last Name = 'liparulo' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515598

(CHEMBL4526049)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(Cc2ccc(O)c(O)c2)CC1 Show InChI InChI=1S/C22H27ClN2O4/c1-29-22-12-18(24)17(23)11-16(22)19(26)4-2-14-6-8-25(9-7-14)13-15-3-5-20(27)21(28)10-15/h3,5,10-12,14,27-28H,2,4,6-9,13,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515600
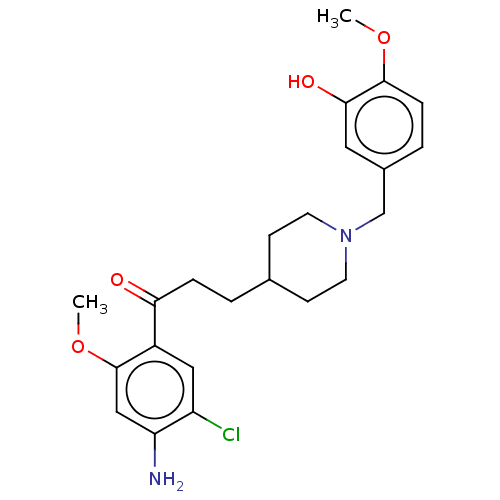
(CHEMBL4580044)Show SMILES COc1ccc(CN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)cc1O Show InChI InChI=1S/C23H29ClN2O4/c1-29-22-6-4-16(11-21(22)28)14-26-9-7-15(8-10-26)3-5-20(27)17-12-18(24)19(25)13-23(17)30-2/h4,6,11-13,15,28H,3,5,7-10,14,25H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515599

(CHEMBL4457426)Show SMILES COc1cc(\C=C\C(=O)NCCN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)ccc1O Show InChI InChI=1S/C27H34ClN3O5/c1-35-25-17-22(29)21(28)16-20(25)23(32)6-3-18-9-12-31(13-10-18)14-11-30-27(34)8-5-19-4-7-24(33)26(15-19)36-2/h4-5,7-8,15-18,33H,3,6,9-14,29H2,1-2H3,(H,30,34)/b8-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515598

(CHEMBL4526049)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(Cc2ccc(O)c(O)c2)CC1 Show InChI InChI=1S/C22H27ClN2O4/c1-29-22-12-18(24)17(23)11-16(22)19(26)4-2-14-6-8-25(9-7-14)13-15-3-5-20(27)21(28)10-15/h3,5,10-12,14,27-28H,2,4,6-9,13,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Displacement of [3H]-GR 113808 from recombinant human brain 5HT4 receptor measured after 60 mins by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE assessed as reduction in 5-thio-2-nitrobenzoate anion formation using acetylthiocholine iodide as substrate pre... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515600

(CHEMBL4580044)Show SMILES COc1ccc(CN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)cc1O Show InChI InChI=1S/C23H29ClN2O4/c1-29-22-6-4-16(11-21(22)28)14-26-9-7-15(8-10-26)3-5-20(27)17-12-18(24)19(25)13-23(17)30-2/h4,6,11-13,15,28H,3,5,7-10,14,25H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Displacement of [3H]-GR 113808 from recombinant human brain 5HT4 receptor measured after 60 mins by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515599

(CHEMBL4457426)Show SMILES COc1cc(\C=C\C(=O)NCCN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)ccc1O Show InChI InChI=1S/C27H34ClN3O5/c1-35-25-17-22(29)21(28)16-20(25)23(32)6-3-18-9-12-31(13-10-18)14-11-30-27(34)8-5-19-4-7-24(33)26(15-19)36-2/h4-5,7-8,15-18,33H,3,6,9-14,29H2,1-2H3,(H,30,34)/b8-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Displacement of [3H]-GR 113808 from recombinant human brain 5HT4 receptor measured after 60 mins by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50515598

(CHEMBL4526049)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(Cc2ccc(O)c(O)c2)CC1 Show InChI InChI=1S/C22H27ClN2O4/c1-29-22-12-18(24)17(23)11-16(22)19(26)4-2-14-6-8-25(9-7-14)13-15-3-5-20(27)21(28)10-15/h3,5,10-12,14,27-28H,2,4,6-9,13,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE assessed as reduction in 5-thio-2-nitrobenzoate anion formation using acetylthiocholine iodide as substrate pre... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Displacement of [3H]-GR 113808 from recombinant human brain 5HT4 receptor measured after 60 mins by radio ligand binding assay |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50515601

(CHEMBL4527729)Show SMILES COc1cc(CN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)ccc1O Show InChI InChI=1S/C23H29ClN2O4/c1-29-22-13-19(25)18(24)12-17(22)20(27)5-3-15-7-9-26(10-8-15)14-16-4-6-21(28)23(11-16)30-2/h4,6,11-13,15,28H,3,5,7-10,14,25H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 535 | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE assessed as reduction in 5-thio-2-nitrobenzoate anion formation using acetylthiocholine iodide as substrate pre... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50597595
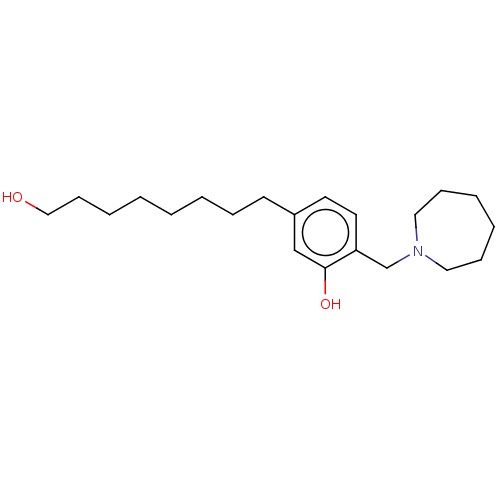
(CHEMBL5190179) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50139053
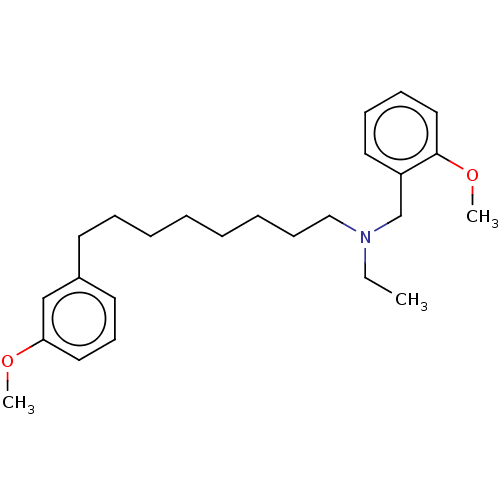
(CHEMBL3752227)Show InChI InChI=1S/C25H37NO2/c1-4-26(21-23-16-10-11-18-25(23)28-3)19-12-8-6-5-7-9-14-22-15-13-17-24(20-22)27-2/h10-11,13,15-18,20H,4-9,12,14,19,21H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50597594
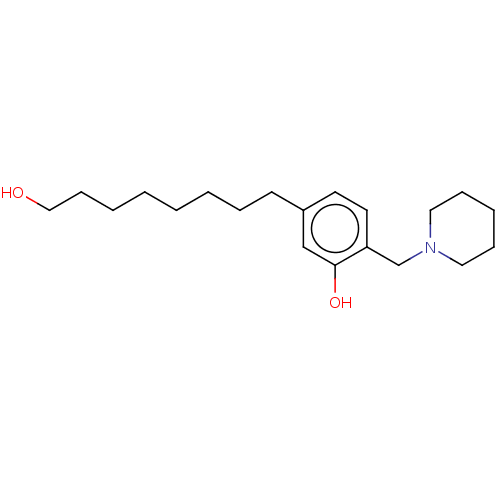
(CHEMBL5183866) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50597596
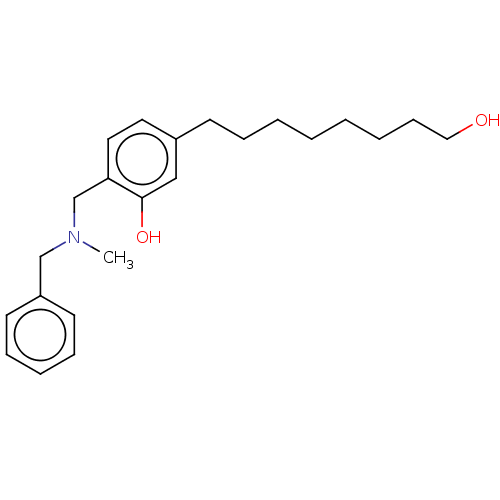
(CHEMBL5195029) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50597593
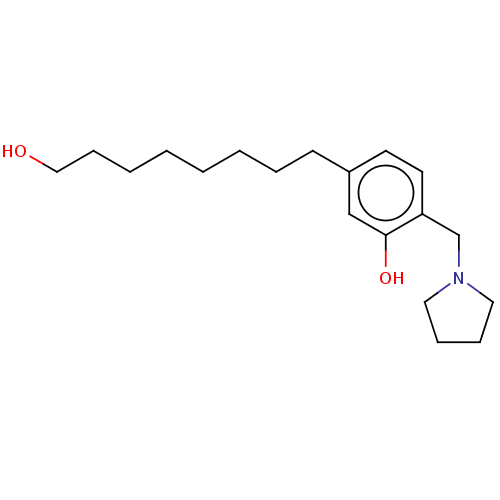
(CHEMBL5192958) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50597597

(CHEMBL5176423) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50597594

(CHEMBL5183866) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50597593

(CHEMBL5192958) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50597595

(CHEMBL5190179) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00046b
BindingDB Entry DOI: 10.7270/Q2WM1JG5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515600

(CHEMBL4580044)Show SMILES COc1ccc(CN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)cc1O Show InChI InChI=1S/C23H29ClN2O4/c1-29-22-6-4-16(11-21(22)28)14-26-9-7-15(8-10-26)3-5-20(27)17-12-18(24)19(25)13-23(17)30-2/h4,6,11-13,15,28H,3,5,7-10,14,25H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Partial agonist activity at human HA-tagged 5HT4 receptor expressed in COS7 cells assessed as induction of cAMP production measured after 10 mins by ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515598

(CHEMBL4526049)Show SMILES COc1cc(N)c(Cl)cc1C(=O)CCC1CCN(Cc2ccc(O)c(O)c2)CC1 Show InChI InChI=1S/C22H27ClN2O4/c1-29-22-12-18(24)17(23)11-16(22)19(26)4-2-14-6-8-25(9-7-14)13-15-3-5-20(27)21(28)10-15/h3,5,10-12,14,27-28H,2,4,6-9,13,24H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Partial agonist activity at human HA-tagged 5HT4 receptor expressed in COS7 cells assessed as induction of cAMP production measured after 10 mins by ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(Homo sapiens (Human)) | BDBM50515599

(CHEMBL4457426)Show SMILES COc1cc(\C=C\C(=O)NCCN2CCC(CCC(=O)c3cc(Cl)c(N)cc3OC)CC2)ccc1O Show InChI InChI=1S/C27H34ClN3O5/c1-35-25-17-22(29)21(28)16-20(25)23(32)6-3-18-9-12-31(13-10-18)14-11-30-27(34)8-5-19-4-7-24(33)26(15-19)36-2/h4-5,7-8,15-18,33H,3,6,9-14,29H2,1-2H3,(H,30,34)/b8-5+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Normandie Univ
Curated by ChEMBL
| Assay Description
Partial agonist activity at human HA-tagged 5HT4 receptor expressed in COS7 cells assessed as induction of cAMP production measured after 10 mins by ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111596
BindingDB Entry DOI: 10.7270/Q2988BC8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data