

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049679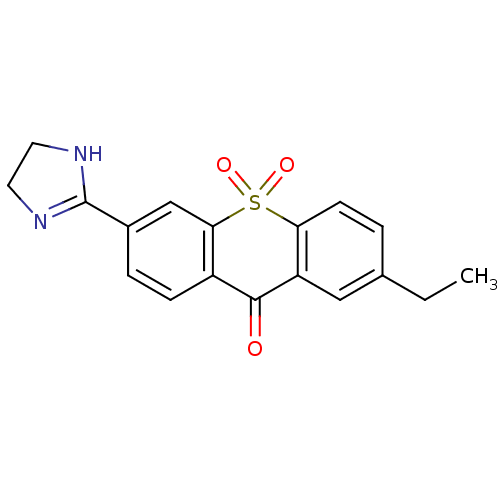 (6-(4,5-Dihydro-1H-imidazol-2-yl)-2-ethyl-10,10-dio...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Ex vivo value for inhibition of MAO A of rats sacrificed 2 hr after po administration | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049694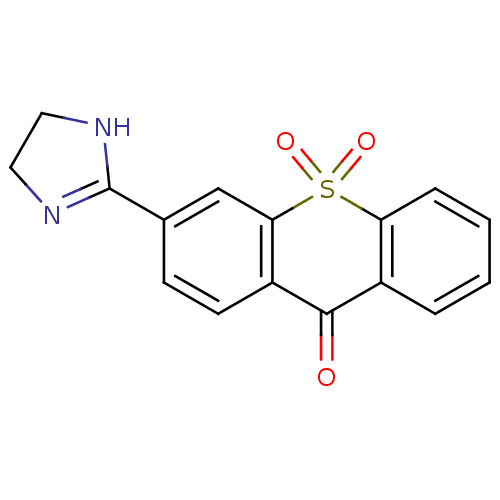 (3-(4,5-Dihydro-1H-imidazol-2-yl)-10,10-dioxo-10H-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Ex vivo value for inhibition of MAO A of rats sacrificed 2 hr after po administration | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261864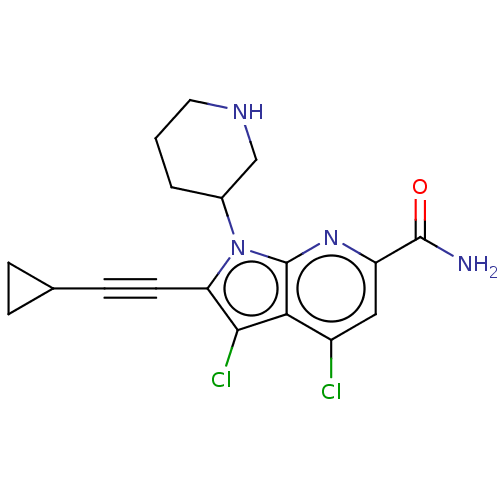 (CHEMBL4100435) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50554405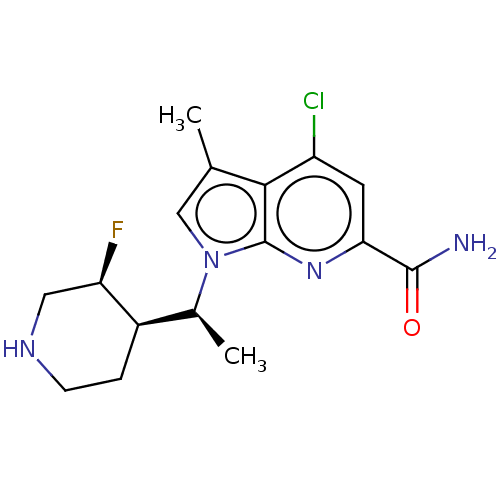 (CHEMBL4759685) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50554403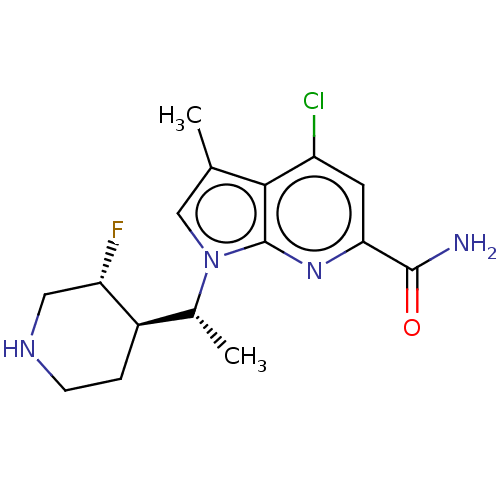 (CHEMBL4750046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50554401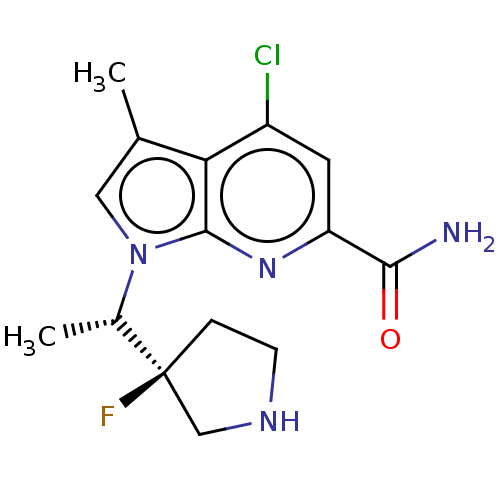 (CHEMBL4778578) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261857 (CHEMBL4082422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261826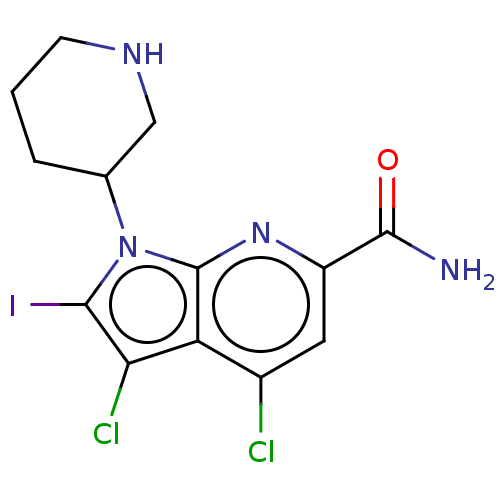 (CHEMBL4084988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261828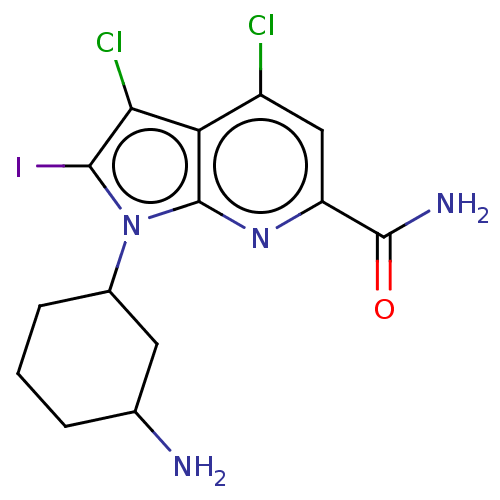 (CHEMBL4095220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261829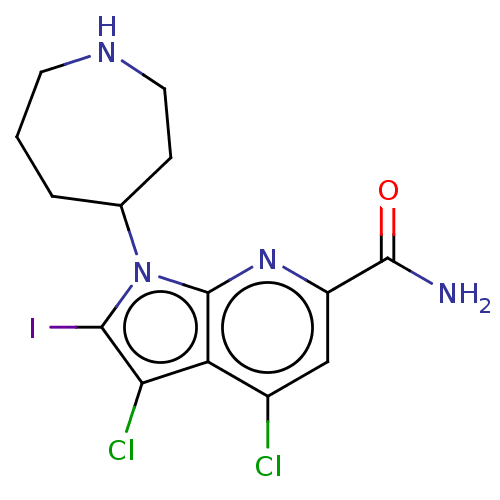 (CHEMBL4067797) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261822 (CHEMBL4093592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261822 (CHEMBL4093592) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37RV InhA | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261858 (CHEMBL4062258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261827 (CHEMBL4070441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50554404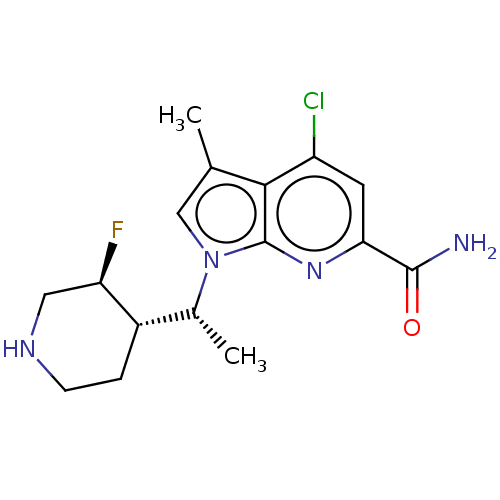 (CHEMBL4745141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049682 (2-Methoxy-6-(5-methyl-[1,3,4]oxadiazol-2-yl)-10,10...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261808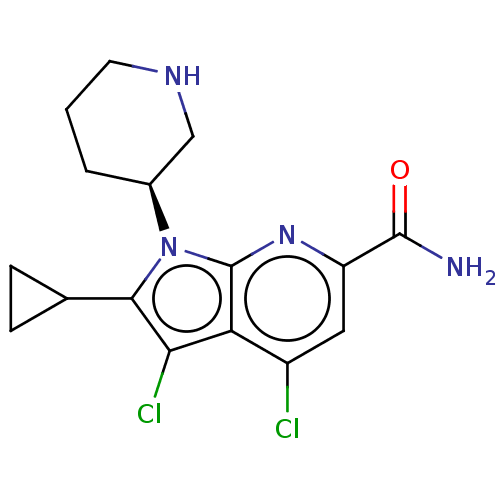 (CHEMBL4104355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50554406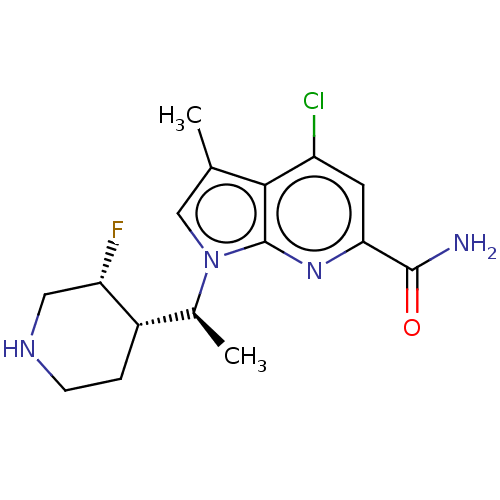 (CHEMBL4746428) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM1 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261826 (CHEMBL4084988) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261828 (CHEMBL4095220) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261825 (CHEMBL4077977) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049703 (6-(2-Methyl-2H-tetrazol-5-yl)-10,10-dioxo-2-propox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049686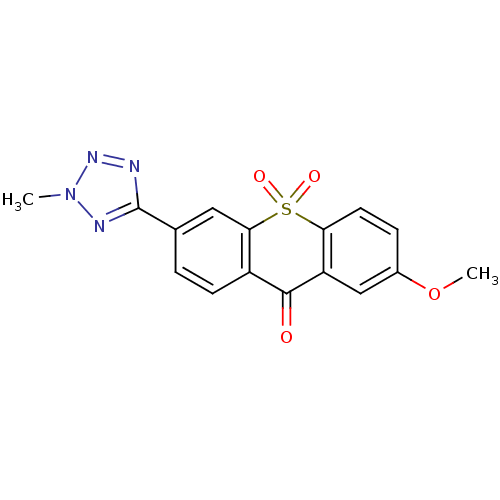 (2-Methoxy-6-(2-methyl-2H-tetrazol-5-yl)-10,10-diox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261873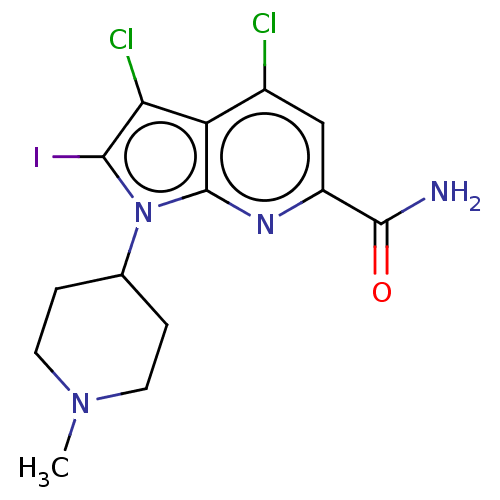 (CHEMBL4070019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049666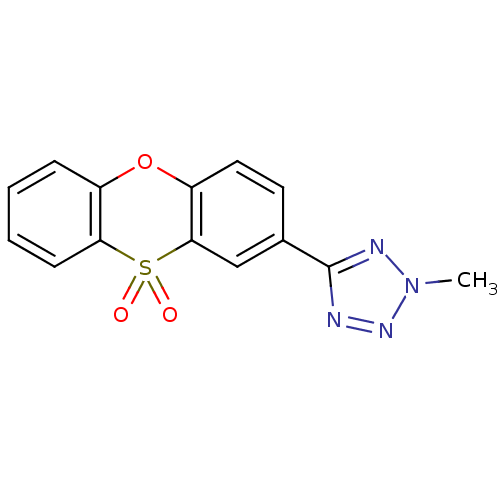 (5-(10,10-Dioxo-10H-10lambda*6*-phenoxathiin-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049671 (2-Methyl-6-(5-methyl-[1,3,4]oxadiazol-2-yl)-10,10-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50554403 (CHEMBL4750046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049701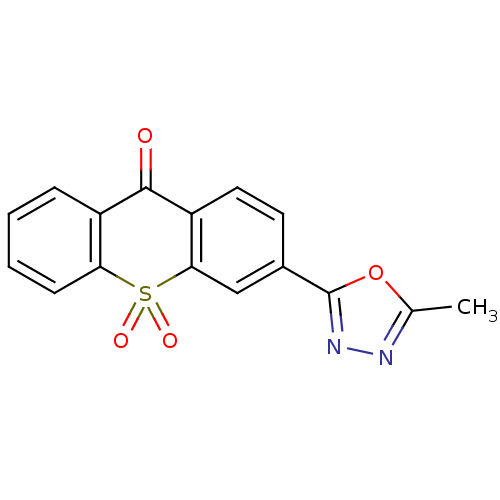 (3-(5-Methyl-[1,3,4]oxadiazol-2-yl)-10,10-dioxo-10H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049704 (2-(5-Methyl-[1,3,4]oxadiazol-2-yl)-10,11-dioxa-dib...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50554404 (CHEMBL4745141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261827 (CHEMBL4070441) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261829 (CHEMBL4067797) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261874 (CHEMBL4064302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261854 (CHEMBL4075702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261866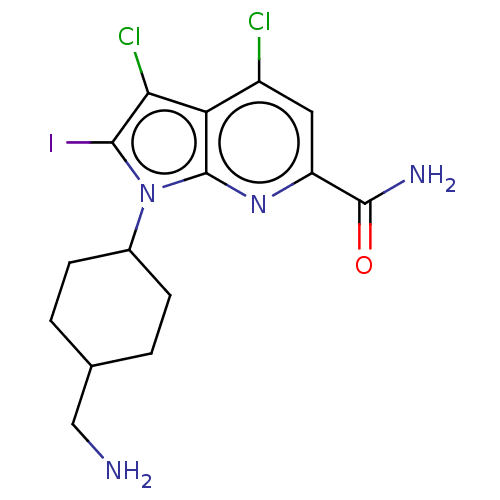 (CHEMBL4087517) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261856 (CHEMBL4071926) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37RV InhA | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261825 (CHEMBL4077977) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261823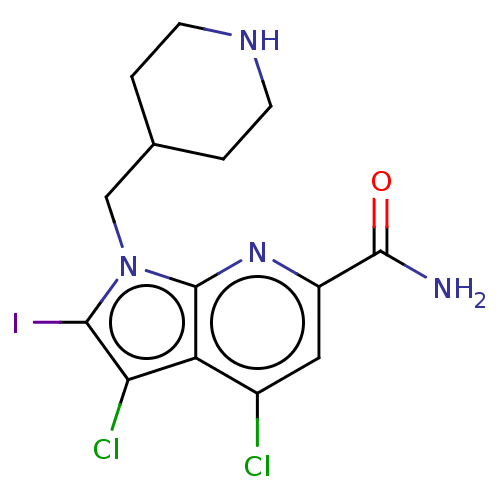 (CHEMBL4072887) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261807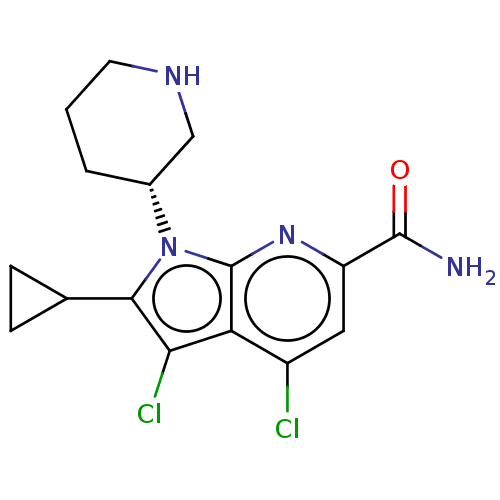 (CHEMBL4086461) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049687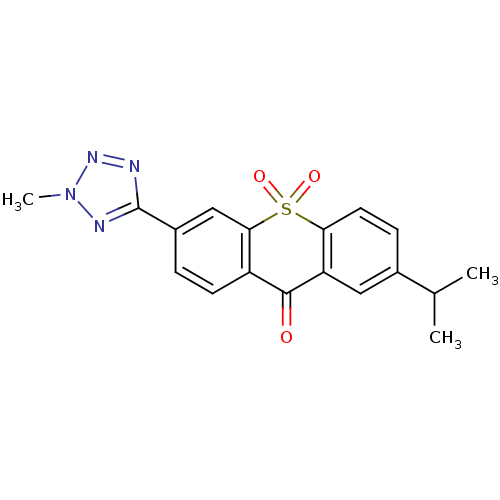 (2-Isopropyl-6-(2-methyl-2H-tetrazol-5-yl)-10,10-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM50261873 (CHEMBL4070019) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM3 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261808 (CHEMBL4104355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37RV InhA | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261830 (CHEMBL4105379) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261864 (CHEMBL4100435) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50554401 (CHEMBL4778578) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50554405 (CHEMBL4759685) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PIM2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127625 BindingDB Entry DOI: 10.7270/Q2Z3239C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261826 (CHEMBL4084988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50261827 (CHEMBL4070441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50049680 (6-(4,5-Dihydro-1H-imidazol-2-yl)-2-ethoxy-10,10-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against rat brain mitochondrial monoamine oxidase A | J Med Chem 39: 1857-63 (1996) Article DOI: 10.1021/jm950595m BindingDB Entry DOI: 10.7270/Q2TM796B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50261832 (CHEMBL4086532) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IDD Medicinal Chemistry, Sanofi Genzyme, 153 Second Avenue, Waltham, MA 02451, USA. Electronic address: claude.barberis2@sanofi.com. Curated by ChEMBL | Assay Description Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues by TR-FRET assay | Bioorg Med Chem Lett 27: 4735-4740 (2017) Article DOI: 10.1016/j.bmcl.2017.08.068 BindingDB Entry DOI: 10.7270/Q2NG4T34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |