

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524862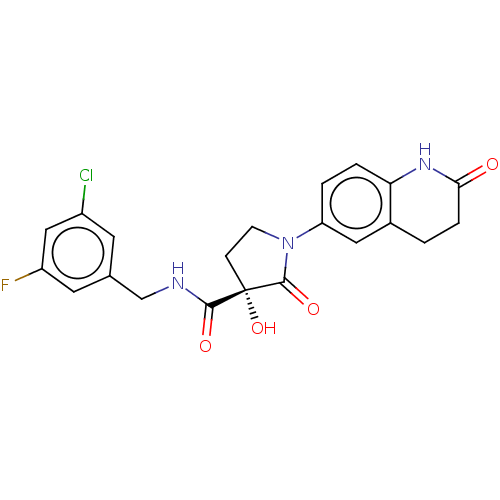 (CHEMBL4475680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524862 (CHEMBL4475680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401307 (US10005756, Compound A78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401307 (US10005756, Compound A78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531161 (CHEMBL4448724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531161 (CHEMBL4448724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531163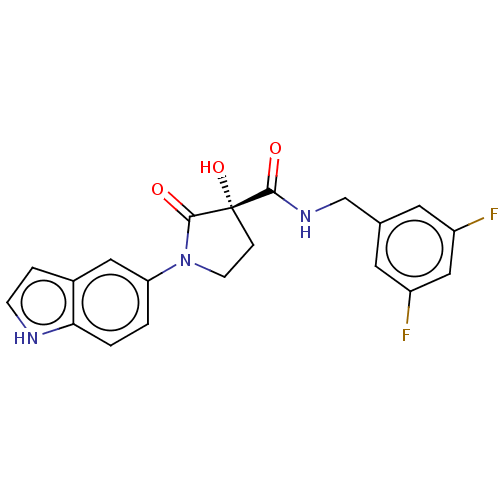 (CHEMBL4464946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531163 (CHEMBL4464946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50318884 (CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Competitive binding affinity to FAK kinase domain (410 to 689) (unknown origin) assessed as phosphorylation of p(Glu/Tyr) in presence of ATP | Bioorg Med Chem Lett 23: 5401-9 (2013) Article DOI: 10.1016/j.bmcl.2013.07.050 BindingDB Entry DOI: 10.7270/Q2891782 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531149 (CHEMBL4572028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531149 (CHEMBL4572028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531146 (CHEMBL4463138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531146 (CHEMBL4463138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401308 (US10005756, Compound A79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401308 (US10005756, Compound A79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531155 (CHEMBL4553094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531155 (CHEMBL4553094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531162 (CHEMBL4445117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531162 (CHEMBL4445117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524862 (CHEMBL4475680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524862 (CHEMBL4475680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531157 (CHEMBL4550096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531157 (CHEMBL4550096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531161 (CHEMBL4448724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531161 (CHEMBL4448724) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531163 (CHEMBL4464946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531163 (CHEMBL4464946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531150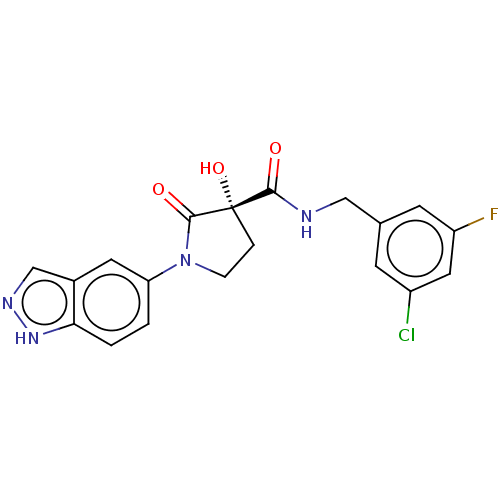 (CHEMBL4460444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531150 (CHEMBL4460444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401275 (US10005756, Compound A40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM401275 (US10005756, Compound A40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50425672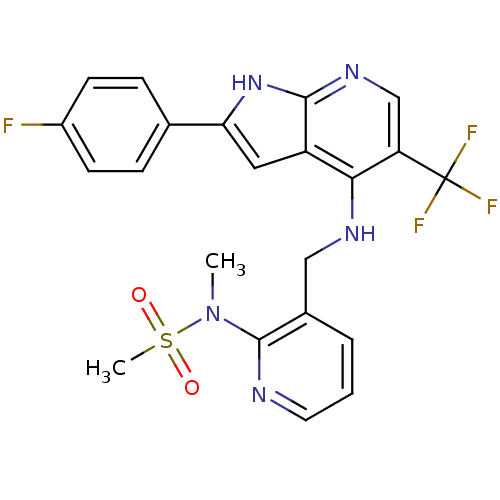 (CHEMBL2315584) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis | J Med Chem 56: 1160-70 (2013) Article DOI: 10.1021/jm3016014 BindingDB Entry DOI: 10.7270/Q2GT5PGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50425681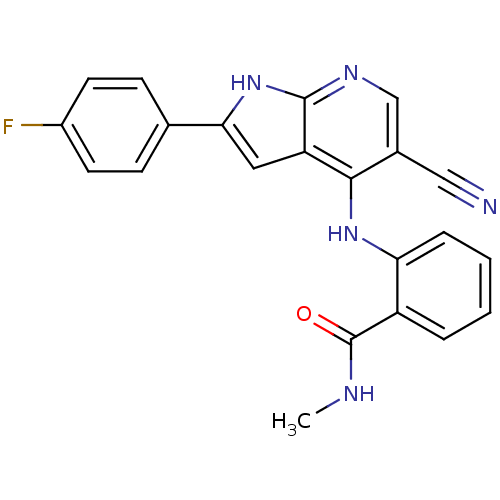 (CHEMBL2315564) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis | J Med Chem 56: 1160-70 (2013) Article DOI: 10.1021/jm3016014 BindingDB Entry DOI: 10.7270/Q2GT5PGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein 2 (Homo sapiens (Human)) | BDBM50234032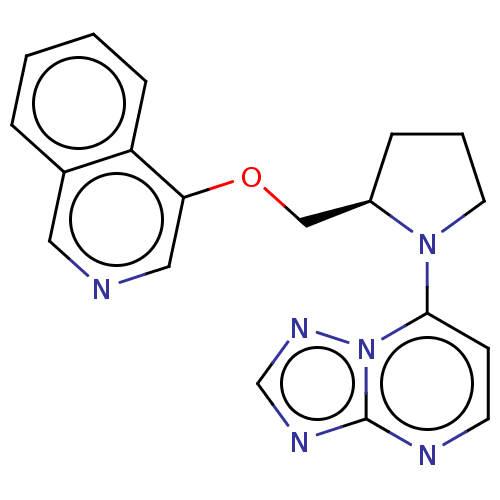 (CHEMBL4060668) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His tagged MetAP-2 using tripeptide Met-Ala-Ser as substrate preincubated for 15 mins followed by substrat... | Bioorg Med Chem Lett 27: 551-556 (2017) Article DOI: 10.1016/j.bmcl.2016.12.019 BindingDB Entry DOI: 10.7270/Q2V98BB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531162 (CHEMBL4445117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531162 (CHEMBL4445117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531148 (CHEMBL4551283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531148 (CHEMBL4551283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50425687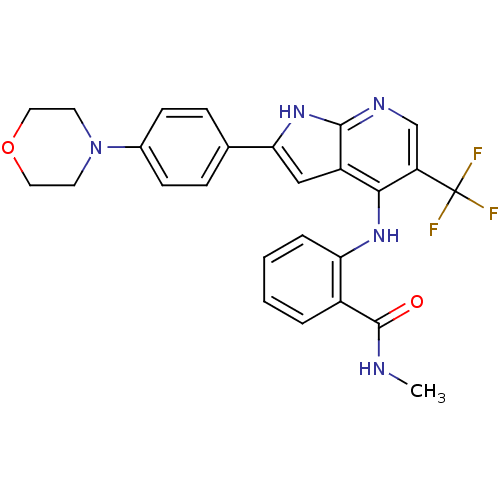 (CHEMBL2315565) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis | J Med Chem 56: 1160-70 (2013) Article DOI: 10.1021/jm3016014 BindingDB Entry DOI: 10.7270/Q2GT5PGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50425686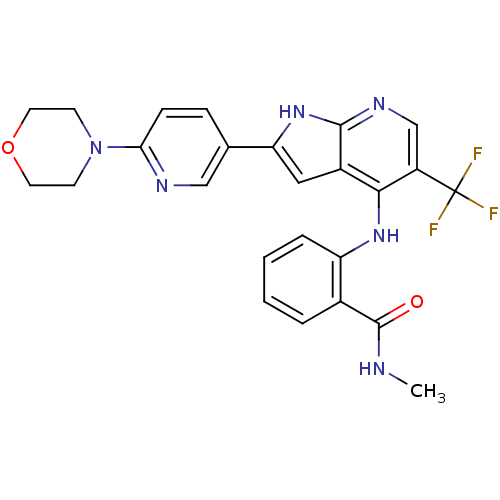 (CHEMBL2315566) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis | J Med Chem 56: 1160-70 (2013) Article DOI: 10.1021/jm3016014 BindingDB Entry DOI: 10.7270/Q2GT5PGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531154 (CHEMBL4448427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531154 (CHEMBL4448427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531148 (CHEMBL4551283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531148 (CHEMBL4551283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assay | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524871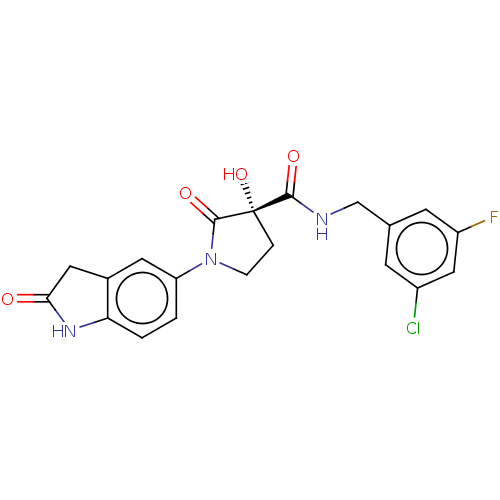 (CHEMBL4524329) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and manganese as co-facor preincubate... | J Med Chem 62: 5025-5039 (2019) Article DOI: 10.1021/acs.jmedchem.9b00041 BindingDB Entry DOI: 10.7270/Q2KP85KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50524864 (CHEMBL4566670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and manganese as co-facor preincubate... | J Med Chem 62: 5025-5039 (2019) Article DOI: 10.1021/acs.jmedchem.9b00041 BindingDB Entry DOI: 10.7270/Q2KP85KH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50425679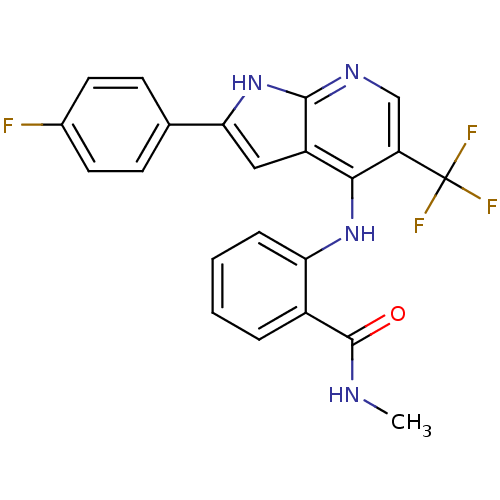 (CHEMBL2315562) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research Curated by ChEMBL | Assay Description Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis | J Med Chem 56: 1160-70 (2013) Article DOI: 10.1021/jm3016014 BindingDB Entry DOI: 10.7270/Q2GT5PGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531156 (CHEMBL4522216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531156 (CHEMBL4522216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM50531144 (CHEMBL4460950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Healthcare Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo... | J Med Chem 62: 11119-11134 (2019) Article DOI: 10.1021/acs.jmedchem.9b01070 BindingDB Entry DOI: 10.7270/Q26H4MXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 515 total ) | Next | Last >> |