

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541911 (CHEMBL4639983) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541907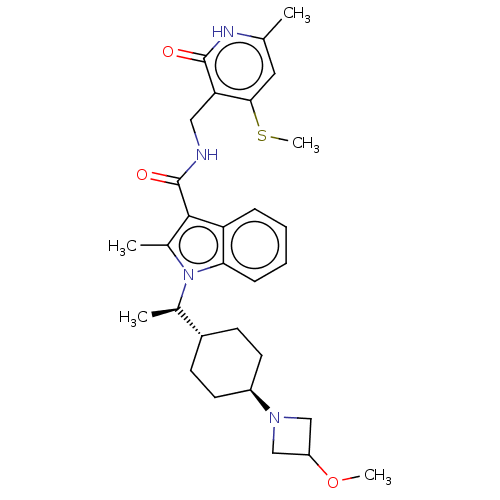 (CHEMBL4640994) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541915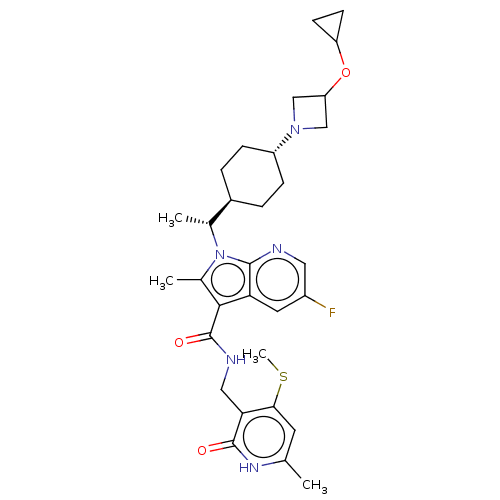 (CHEMBL4638754) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541912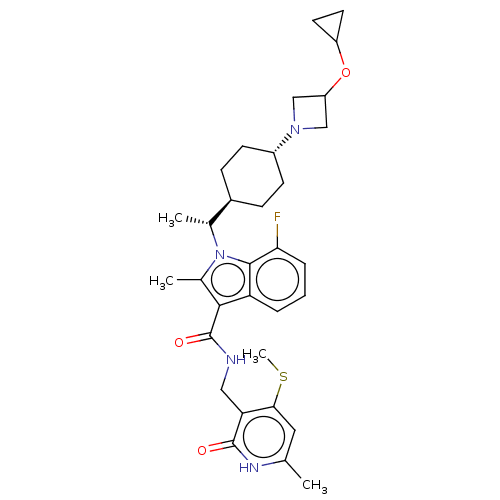 (CHEMBL4637572) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541902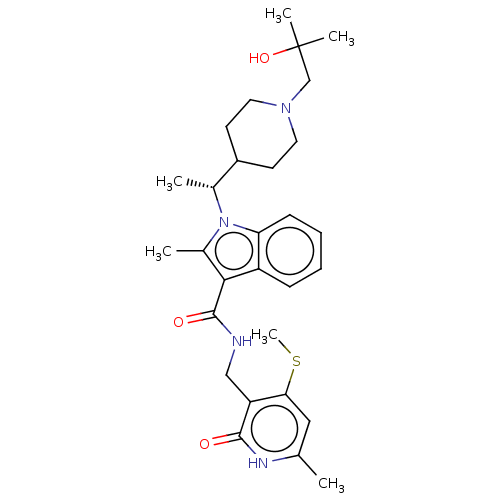 (CHEMBL4635809 | US11459315, Example 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541910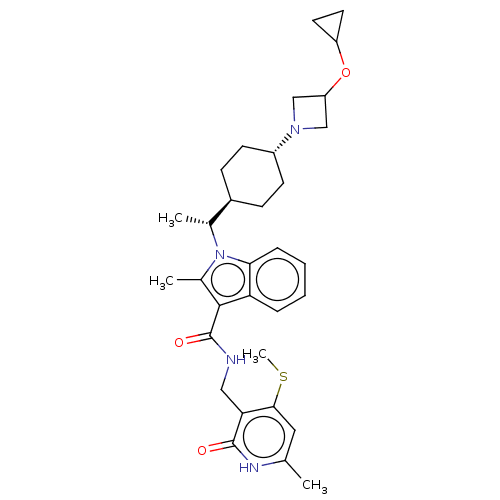 (CHEMBL4635863) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541914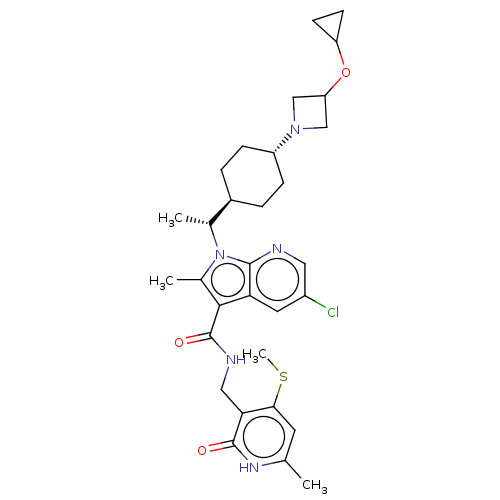 (CHEMBL4635102) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541913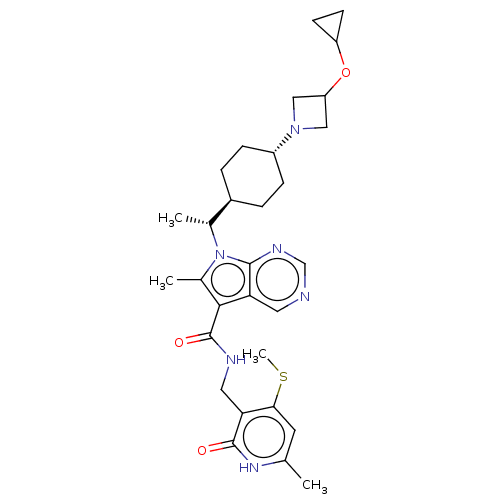 (CHEMBL4633923) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541909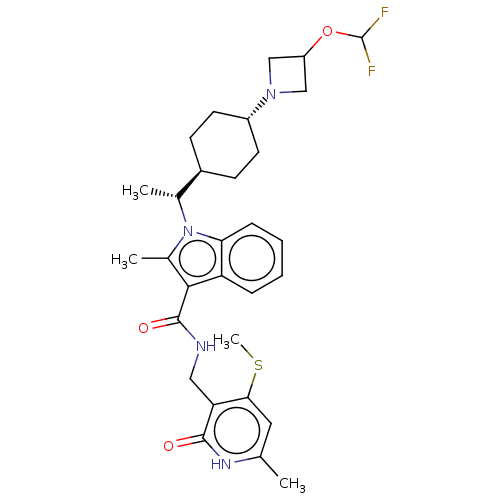 (CHEMBL4639913) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541903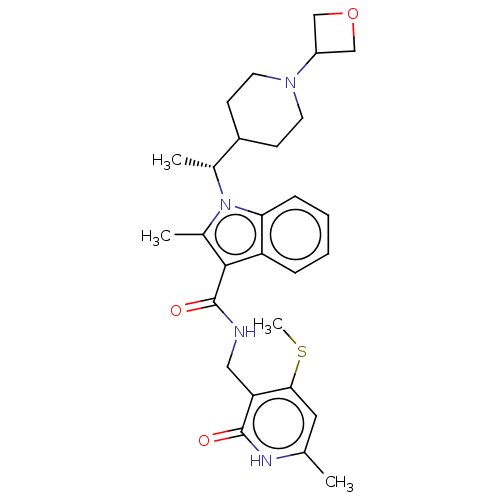 (CHEMBL4633932 | US11459315, Example 133) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541905 (CHEMBL4634677) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541908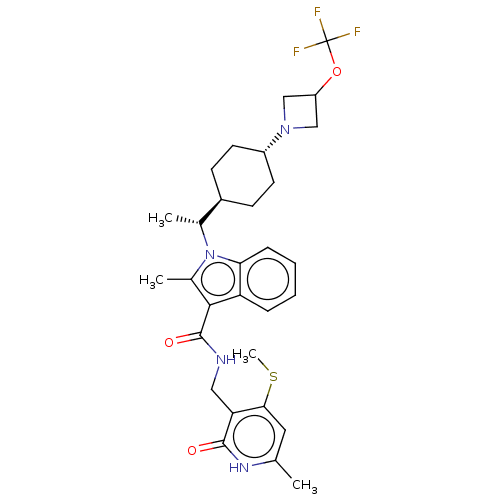 (CHEMBL4637958) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541906 (CHEMBL4649131) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541904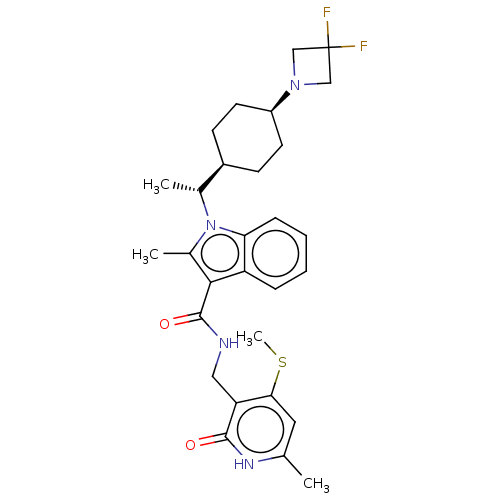 (CHEMBL4639569) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541895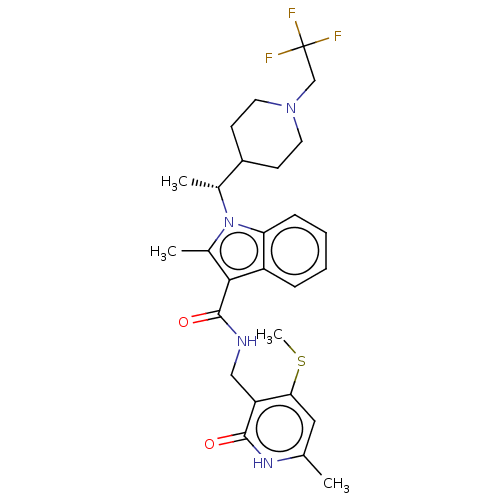 (CHEMBL4639616 | US11459315, Example 12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541918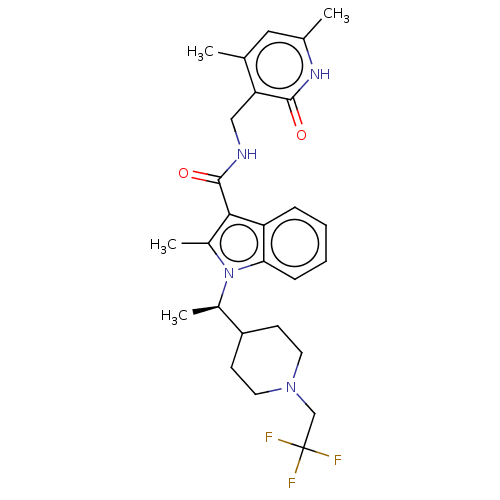 (CHEMBL4641325) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541916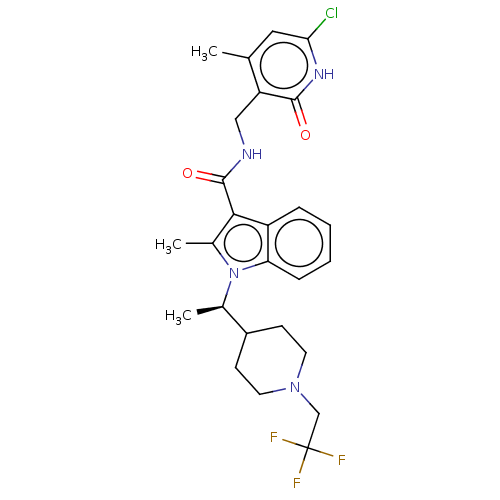 (CHEMBL4633053) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541917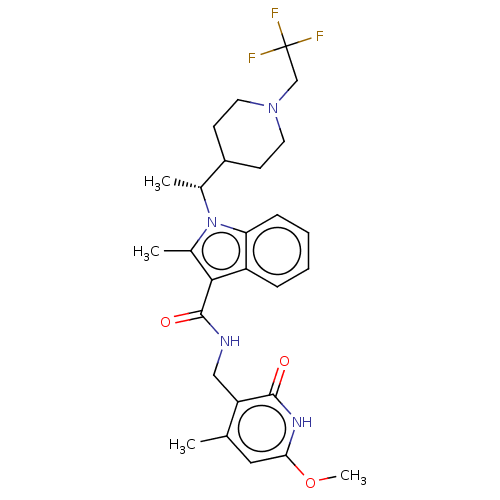 (CHEMBL4644363) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541919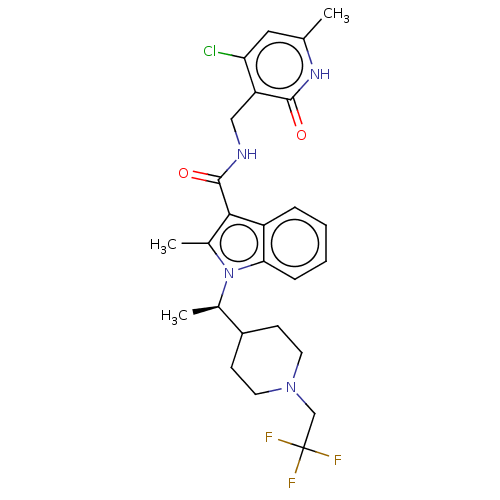 (CHEMBL4632988) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541897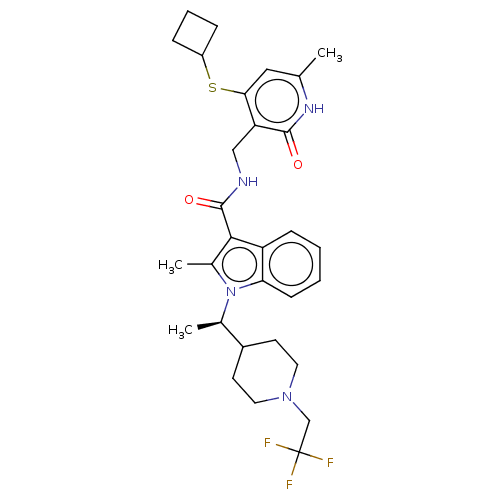 (CHEMBL4634390 | US11459315, Example 109) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of N-terminal FLAG-tagged EZH2 in PRC2 complex (unknown origin) expressed in baculovirus infected Sf9 cells using H2N-RKQLATKAAR(Kme0)SAPA... | ACS Med Chem Lett 11: 1205-1212 (2020) Article DOI: 10.1021/acsmedchemlett.0c00045 BindingDB Entry DOI: 10.7270/Q2Z89GZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574915 (US11459315, Example 60 (isomer 2)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574916 ((R)-1-(1-(4-(tert-butylamino)cyclohexyl)eth-yl)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574917 (2-methyl-N-((6-methyl-4-(methylthio)-2-oxo-1,2-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574919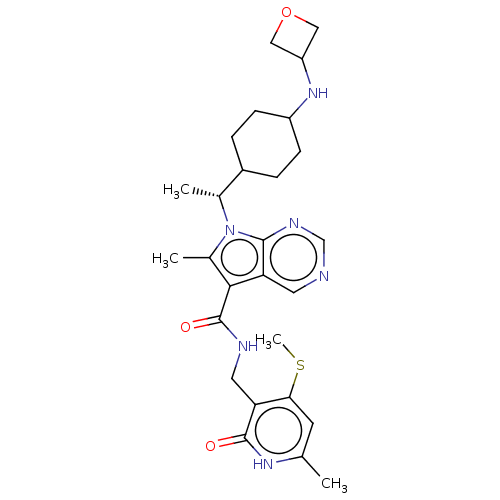 (6-methyl-N-((6-methyl-4-(methylthio)-2-oxo-1,2-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574920 ((R)-1-(1-(1-(3-fluorocyclobutyl)piperidin-4-yl)eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574921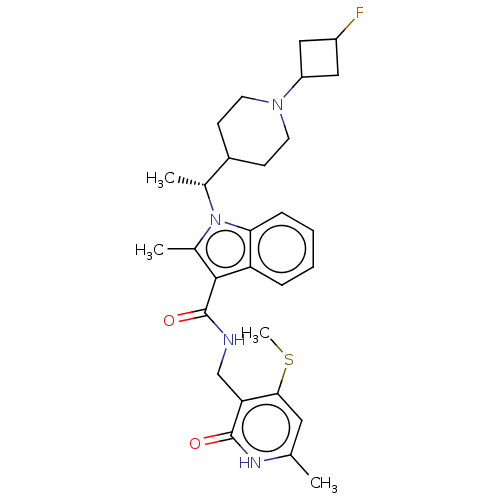 (1-((R)-1-(1-(3-fluorocyclobutyl)piperidin-4-yl)eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574922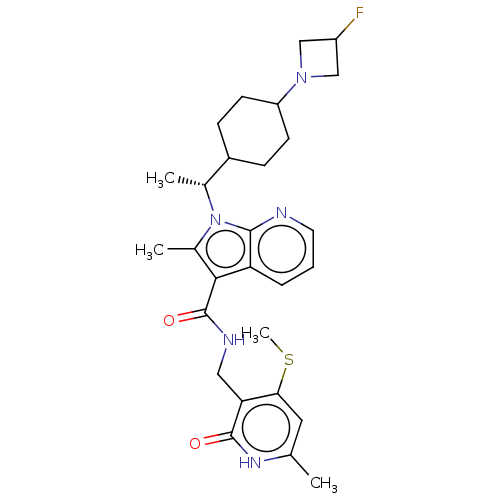 (1-((R)-1-(4-(3-fluoroazetidin-1-yl)cyclohexyl)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574923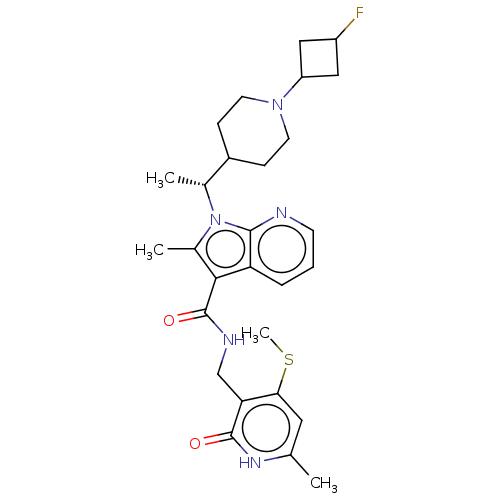 ((R)-1-(1-(1-(3-fluorocyclobutyl)piperidin-4-yl)eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574924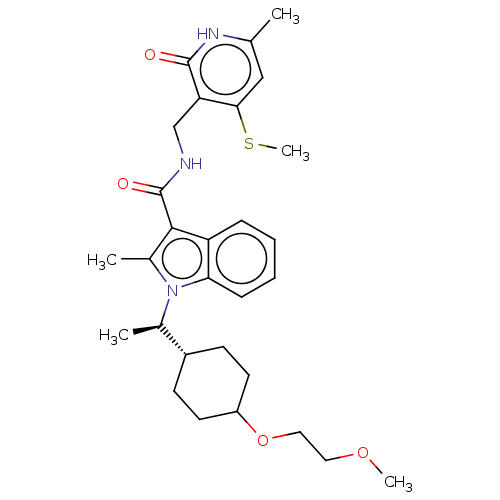 (1-((R)-1-(4-(2-methoxyethoxy)cyclohex-yl)ethyl)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574925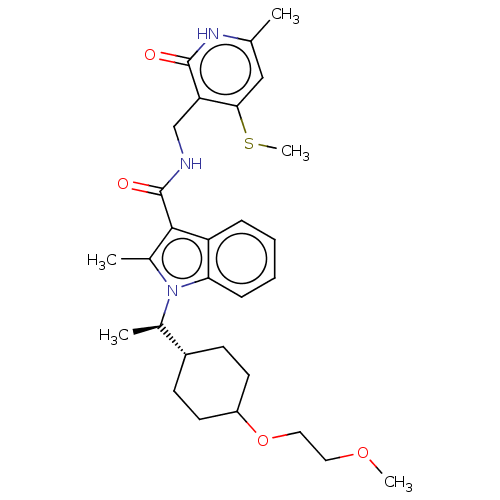 (1-((R)-1-(4-(2-methoxyethoxy)cyclohex-yl)ethyl)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574926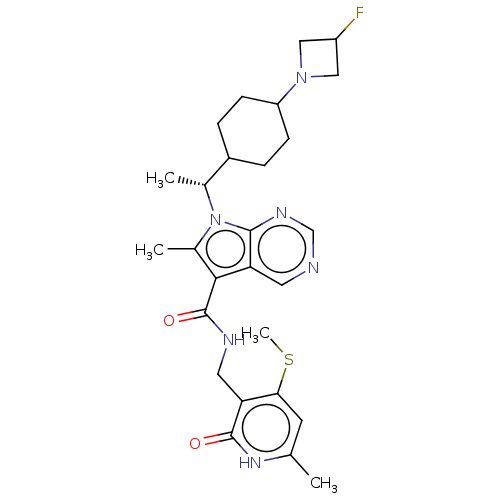 (7-((R)-1-(4-(3-fluoroazetidin-1-yl)cyclohexyl)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574927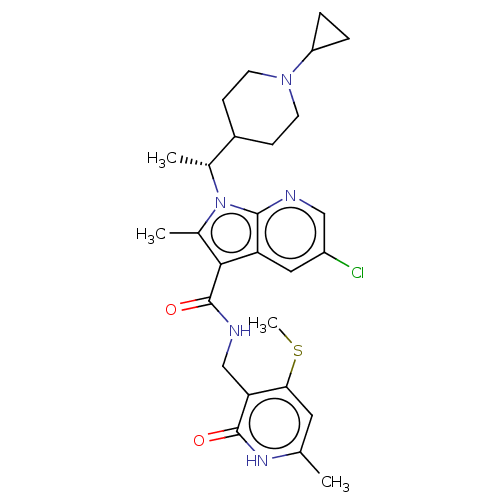 ((R)-5-chloro-1-(1-(1-cyclopropylpiperidin-4-yl)eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574928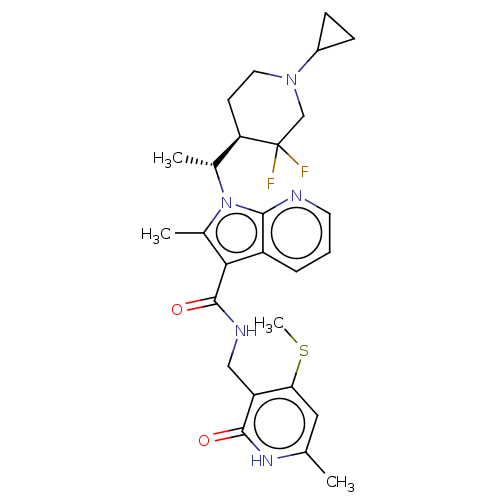 (1-((R)-1-((R)-1-cyclopropyl-3,3-difluoropiperidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574930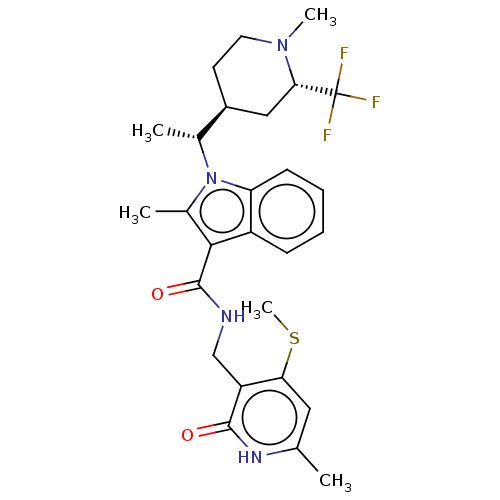 (2-methyl-1-((R)-1-((2S,4S)-1-methyl-2-(trifluorome...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574931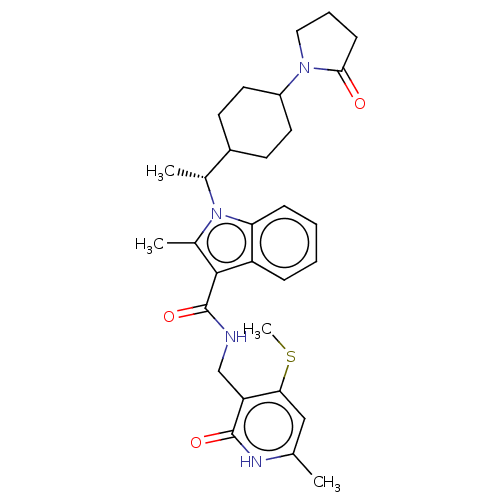 (2-methyl-N-((6-methyl-4-(methylthio)-2-oxo-1,2-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574932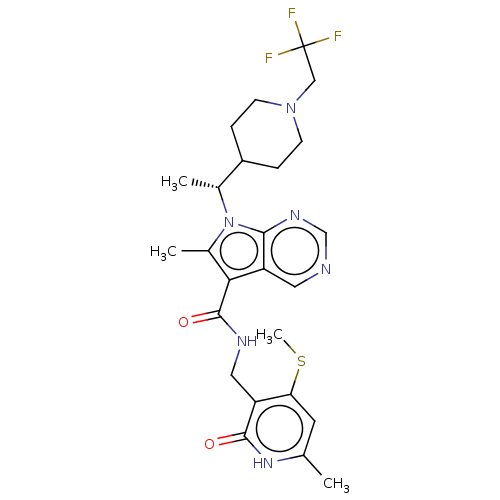 ((R)-6-methyl-N-((6-methyl-4-(methylthio)-2-oxo-1,2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574834 (US11459315, Example 2 (isomer 1)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574837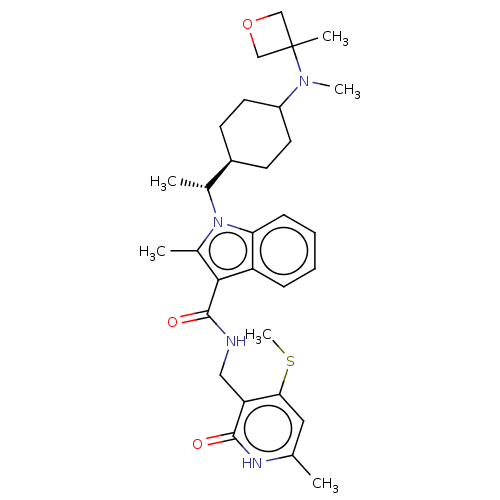 (US11459315, Example 3 (isomer 2)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574838 (US11459315, Example 4 (isomer 1)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574839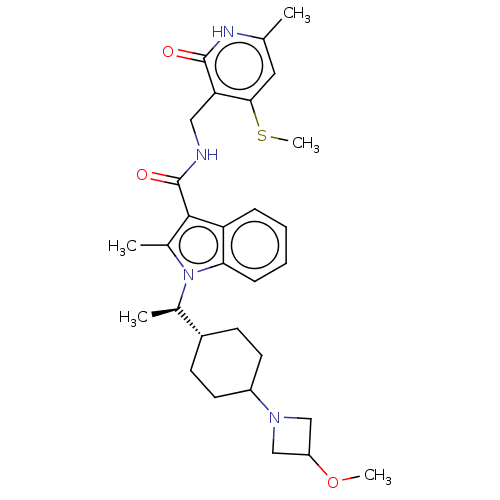 (US11459315, Example 4 (isomer 2)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574841 (US11459315, Example 5 (isomer 2)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574842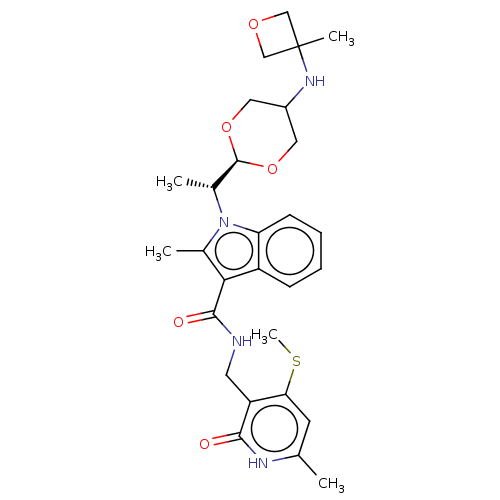 (US11459315, Example 6 (isomer 1)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574845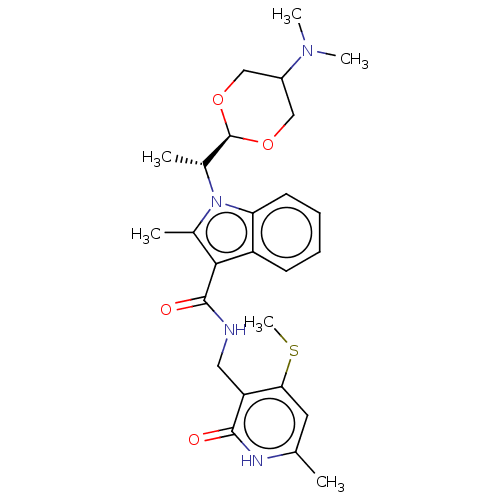 (US11459315, Example 7 (isomer 2)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574846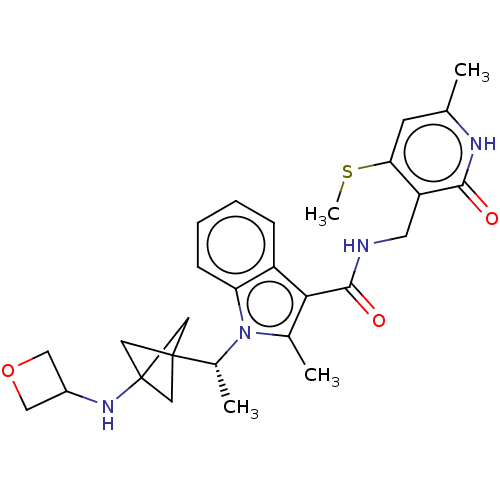 (US11459315, Example 8) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574847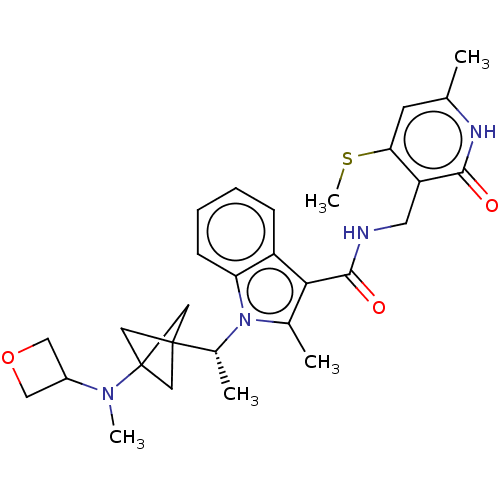 (US11459315, Example 9) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574848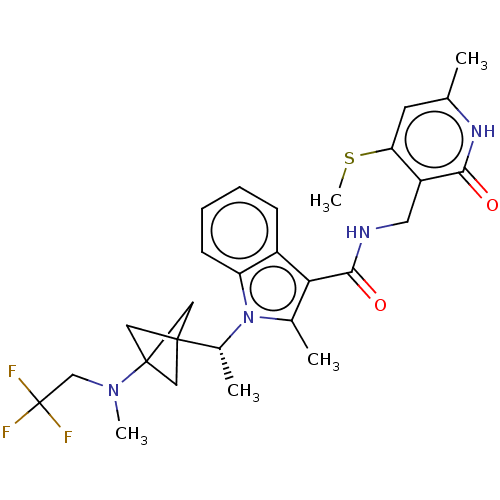 (US11459315, Example 10) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541902 (CHEMBL4635809 | US11459315, Example 11) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50541895 (CHEMBL4639616 | US11459315, Example 12) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574851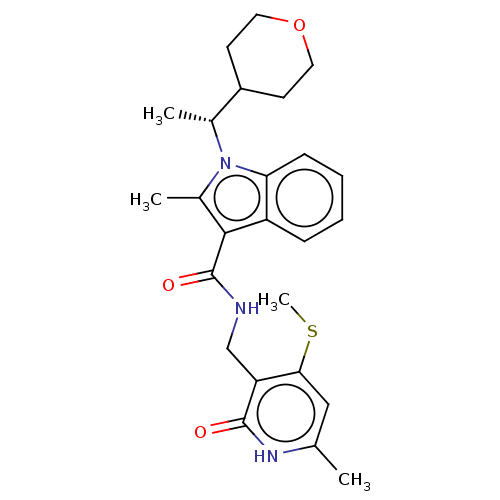 (US11459315, Example 13) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM574852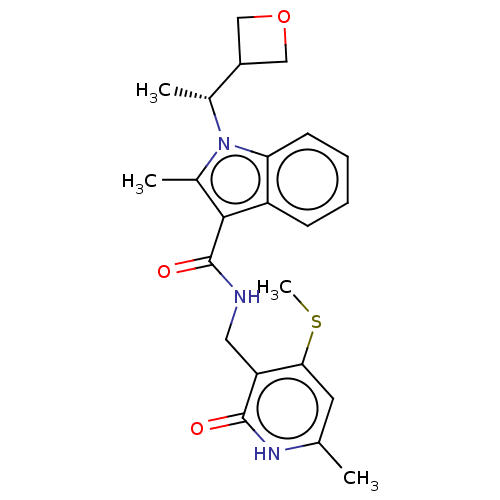 (US11459315, Example 14 (isomer 1)) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound potencies were assessed through incorporation of 3H-SAM into a biotinylated H3 peptide. Specifically, 30 pM PRC2 containing wt EZH2 (pentame... | Citation and Details BindingDB Entry DOI: 10.7270/Q20Z76H4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 855 total ) | Next | Last >> |