 Found 289 hits with Last Name = 'hamon' and Initial = 'j'
Found 289 hits with Last Name = 'hamon' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459
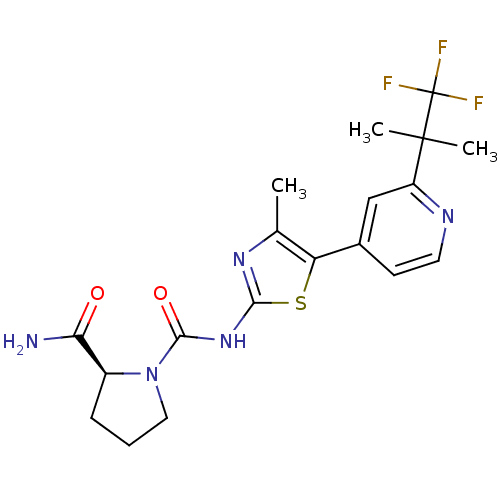
(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448
(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436454
(CHEMBL2397196)Show SMILES CC(C)(c1cc(ccn1)-c1cnc(NC(=O)N2CCC[C@H]2C(N)=O)s1)C(F)(F)F |r| Show InChI InChI=1S/C18H20F3N5O2S/c1-17(2,18(19,20)21)13-8-10(5-6-23-13)12-9-24-15(29-12)25-16(28)26-7-3-4-11(26)14(22)27/h5-6,8-9,11H,3-4,7H2,1-2H3,(H2,22,27)(H,24,25,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436451
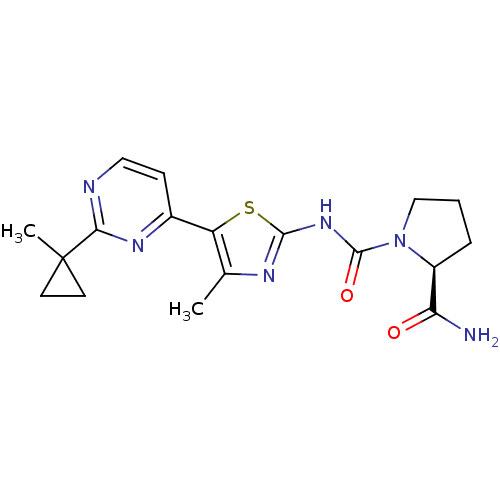
(CHEMBL2397199)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-13(11-5-8-20-15(22-11)18(2)6-7-18)27-16(21-10)23-17(26)24-9-3-4-12(24)14(19)25/h5,8,12H,3-4,6-7,9H2,1-2H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170205
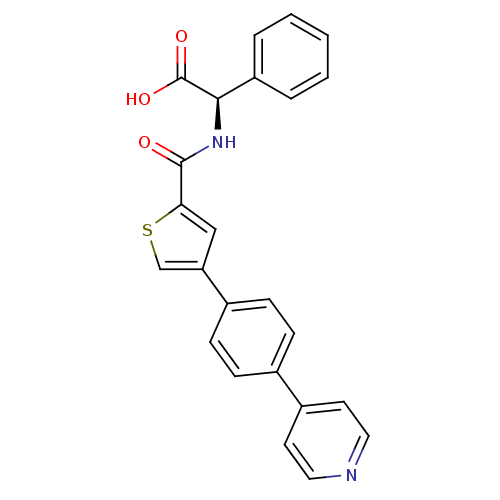
((R)-Phenyl-{[4-(4-pyridin-4-yl-phenyl)-thiophene-2...)Show SMILES OC(=O)[C@H](NC(=O)c1cc(cs1)-c1ccc(cc1)-c1ccncc1)c1ccccc1 Show InChI InChI=1S/C24H18N2O3S/c27-23(26-22(24(28)29)19-4-2-1-3-5-19)21-14-20(15-30-21)17-8-6-16(7-9-17)18-10-12-25-13-11-18/h1-15,22H,(H,26,27)(H,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 in presence of 5 nM acetohydroximate |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436450
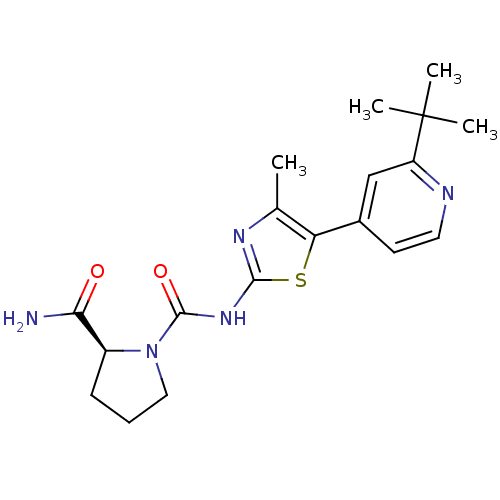
(CHEMBL2397187)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C |r| Show InChI InChI=1S/C19H25N5O2S/c1-11-15(12-7-8-21-14(10-12)19(2,3)4)27-17(22-11)23-18(26)24-9-5-6-13(24)16(20)25/h7-8,10,13H,5-6,9H2,1-4H3,(H2,20,25)(H,22,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436457
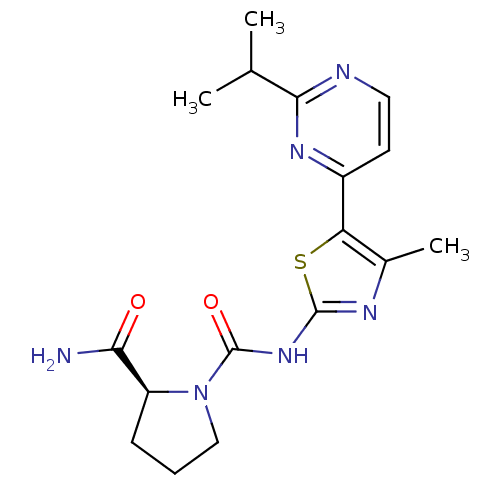
(CHEMBL2397193)Show SMILES CC(C)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9(2)15-19-7-6-11(21-15)13-10(3)20-16(26-13)22-17(25)23-8-4-5-12(23)14(18)24/h6-7,9,12H,4-5,8H2,1-3H3,(H2,18,24)(H,20,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436452
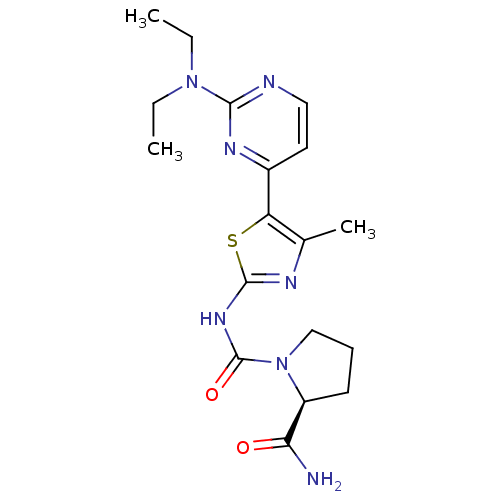
(CHEMBL2397198)Show SMILES CCN(CC)c1nccc(n1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1C |r| Show InChI InChI=1S/C18H25N7O2S/c1-4-24(5-2)16-20-9-8-12(22-16)14-11(3)21-17(28-14)23-18(27)25-10-6-7-13(25)15(19)26/h8-9,13H,4-7,10H2,1-3H3,(H2,19,26)(H,21,23,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436458
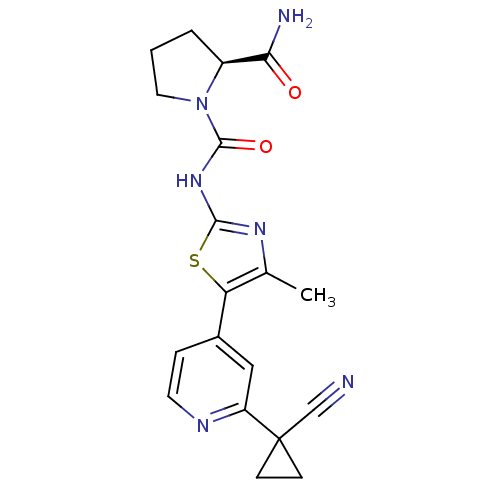
(CHEMBL2397192)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C1(CC1)C#N |r| Show InChI InChI=1S/C19H20N6O2S/c1-11-15(12-4-7-22-14(9-12)19(10-20)5-6-19)28-17(23-11)24-18(27)25-8-2-3-13(25)16(21)26/h4,7,9,13H,2-3,5-6,8H2,1H3,(H2,21,26)(H,23,24,27)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436460
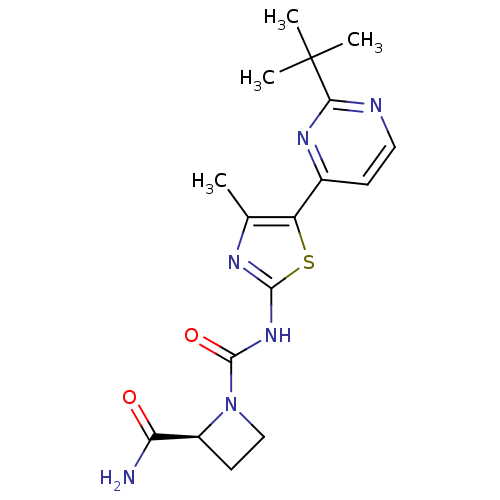
(CHEMBL2397191)Show SMILES Cc1nc(NC(=O)N2CC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C17H22N6O2S/c1-9-12(10-5-7-19-14(21-10)17(2,3)4)26-15(20-9)22-16(25)23-8-6-11(23)13(18)24/h5,7,11H,6,8H2,1-4H3,(H2,18,24)(H,20,22,25)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646438

(US11878973, Example 40)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(F)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646432
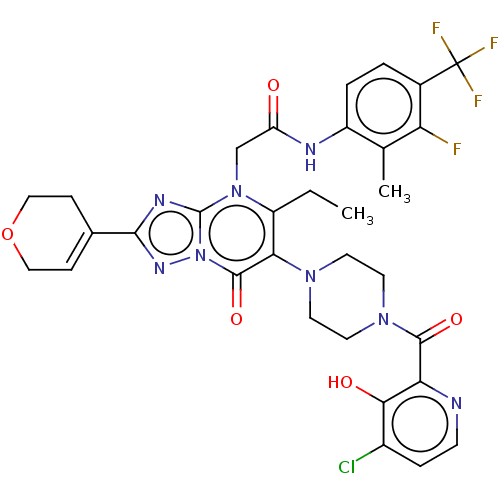
(US11878973, Example 37)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(Cl)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(c(F)c1C)C(F)(F)F)C1=CCOCC1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436456
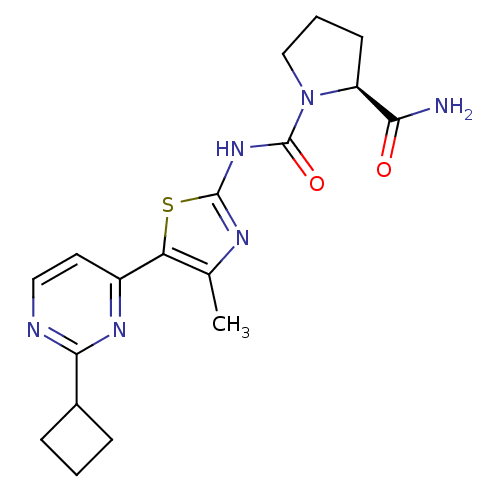
(CHEMBL2397194)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1CCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-10-14(12-7-8-20-16(22-12)11-4-2-5-11)27-17(21-10)23-18(26)24-9-3-6-13(24)15(19)25/h7-8,11,13H,2-6,9H2,1H3,(H2,19,25)(H,21,23,26)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646481
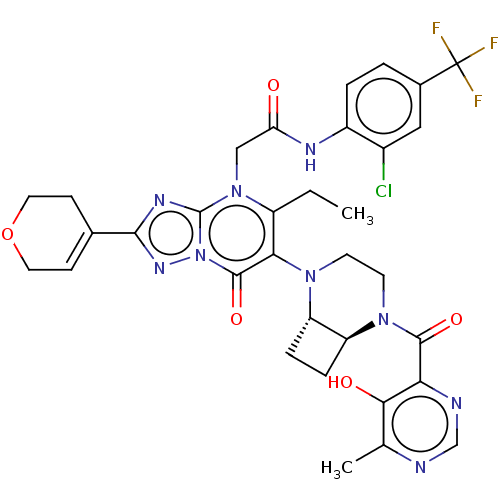
(N-(2-chloro-4-(trifluoromethyl)phenyl)-2-(2-(3,6-d...)Show SMILES CCc1c(N2CCN([C@H]3CC[C@H]23)C(=O)c2ncnc(C)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)C1=CCOCC1 |r,t:51| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646458

(US11878973, Example 50)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncnc(C)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1cc(F)c(cc1C)C(F)(F)F)C1=CCOCC1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646369
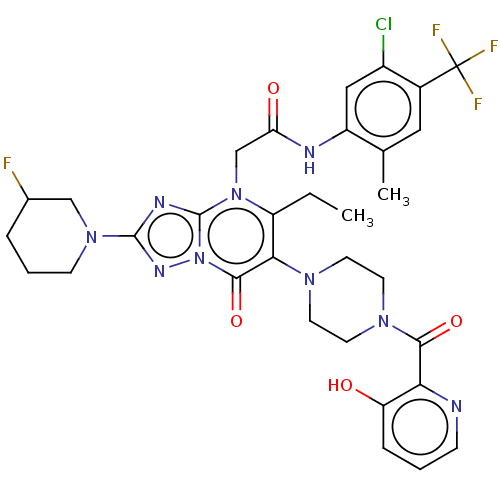
(US11878973, Example 13)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1cc(Cl)c(cc1C)C(F)(F)F)N1CCCC(F)C1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436453
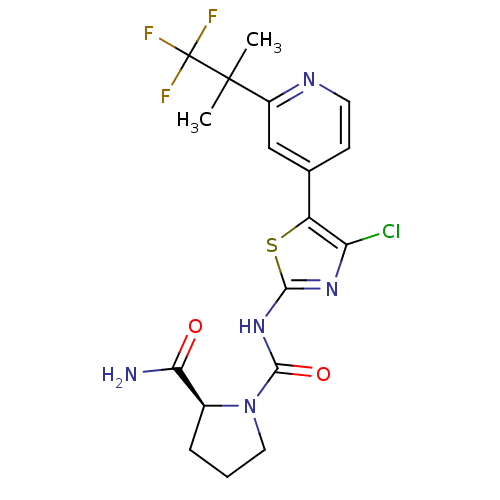
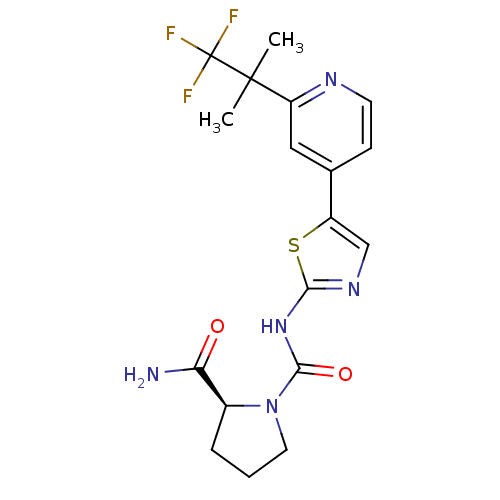
(CHEMBL2397197)Show SMILES CC(C)(c1cc(ccn1)-c1sc(NC(=O)N2CCC[C@H]2C(N)=O)nc1Cl)C(F)(F)F |r| Show InChI InChI=1S/C18H19ClF3N5O2S/c1-17(2,18(20,21)22)11-8-9(5-6-24-11)12-13(19)25-15(30-12)26-16(29)27-7-3-4-10(27)14(23)28/h5-6,8,10H,3-4,7H2,1-2H3,(H2,23,28)(H,25,26,29)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436448

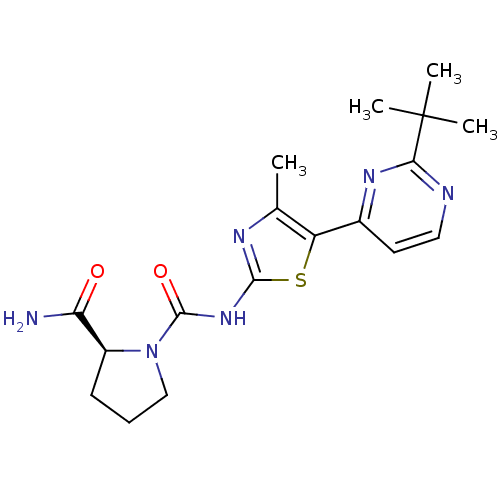
(CHEMBL2397186)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C(C)(C)C |r| Show InChI InChI=1S/C18H24N6O2S/c1-10-13(11-7-8-20-15(22-11)18(2,3)4)27-16(21-10)23-17(26)24-9-5-6-12(24)14(19)25/h7-8,12H,5-6,9H2,1-4H3,(H2,19,25)(H,21,23,26)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISA |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646427
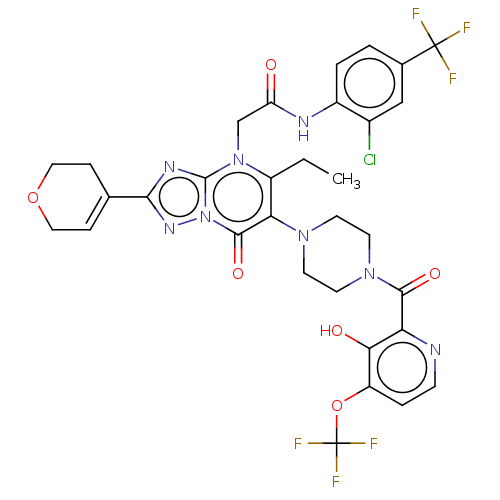
(US11878973, Example 98)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(OC(F)(F)F)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)C1=CCOCC1 |t:52| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646434

(US11878973, Example 38)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(c(F)c1C)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646436

(US11878973, Example 39)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(Cl)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646440
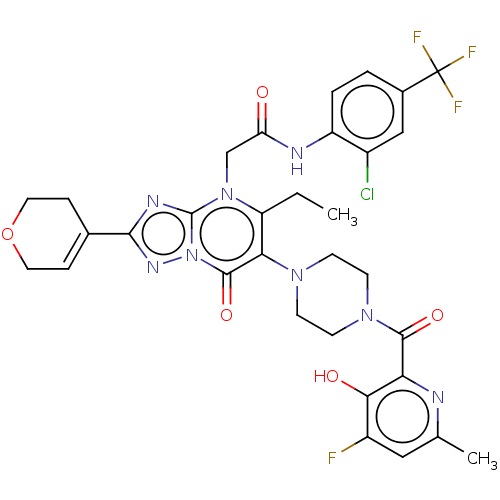
(US11878973, Example 41)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nc(C)cc(F)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)C1=CCOCC1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646457
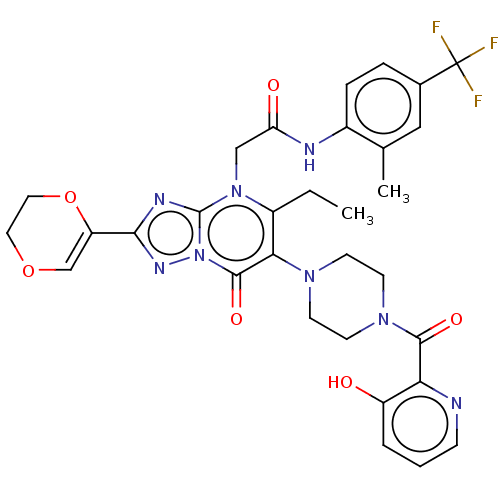
(US11878973, Example 113)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1C)C(F)(F)F)C1=COCCO1 |t:47| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646460

(US11878973, Example 51)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncnc(C)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)S(F)(F)(F)(F)F)C1=CCOCC1 |t:50| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646462

(US11878973, Example 52)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncncc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)S(F)(F)(F)(F)F)C1=CCOCC1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646465
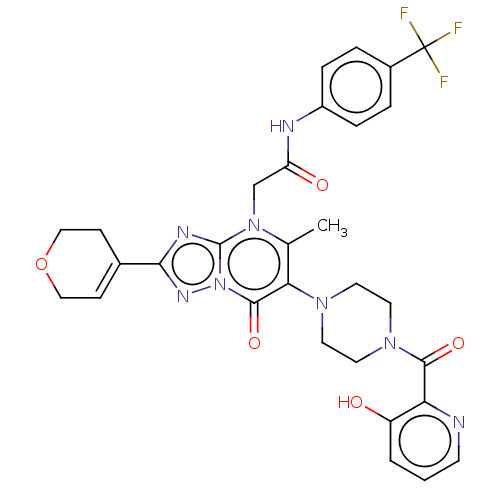
(US11878973, Example 117)Show SMILES Cc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1)C(F)(F)F)C1=CCOCC1 |t:45| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646351
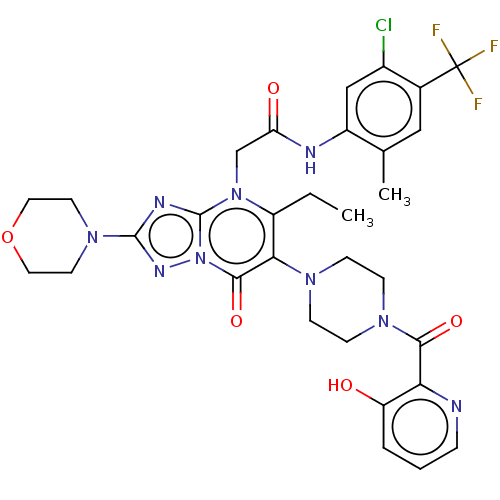
(US11878973, Example 4)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1cc(Cl)c(cc1C)C(F)(F)F)N1CCOCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646356
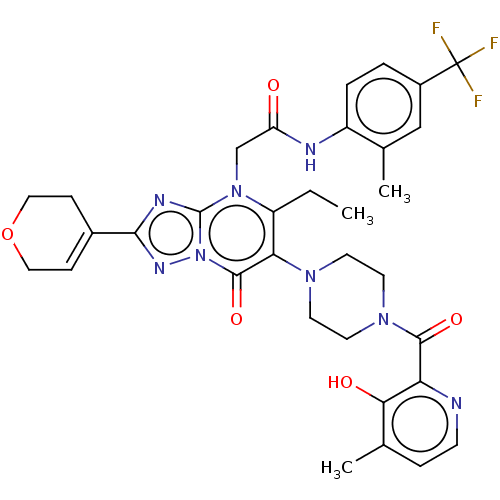
(US11878973, Example 66)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(C)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1C)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646362
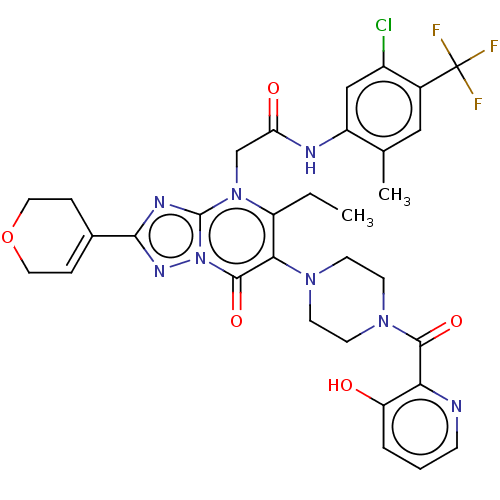
(US11878973, Example 69)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1cc(Cl)c(cc1C)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646383

(US11878973, Example 79)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(Cl)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(c(F)c1C)C(F)(F)F)C1=COCCO1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646391

(US11878973, Example 81a)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1)C(F)(F)F)C1=CC[C@@H](CC1)OC |r,t:46| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646393

(US11878973, Example 81b)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1)C(F)(F)F)C1=CCC(CC1)OC |t:46| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646409
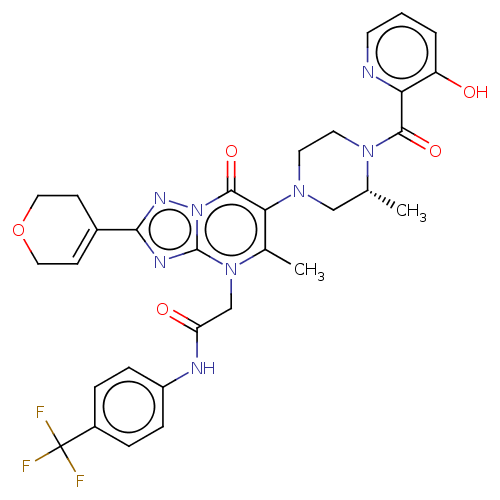
(US11878973, Example 89)Show SMILES C[C@@H]1CN(CCN1C(=O)c1ncccc1O)c1c(C)n(CC(=O)Nc2ccc(cc2)C(F)(F)F)c2nc(nn2c1=O)C1=CCOCC1 |r,t:46| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646419
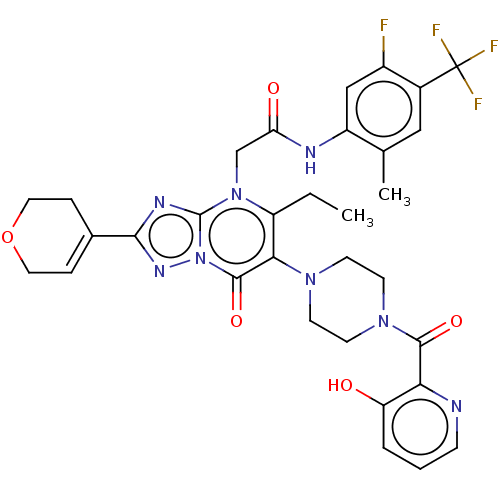
(US11878973, Example 94)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1cc(F)c(cc1C)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646374

(US11878973, Example 75)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(C)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1)C(F)(F)F)C1=CCOCC1 |t:47| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646489
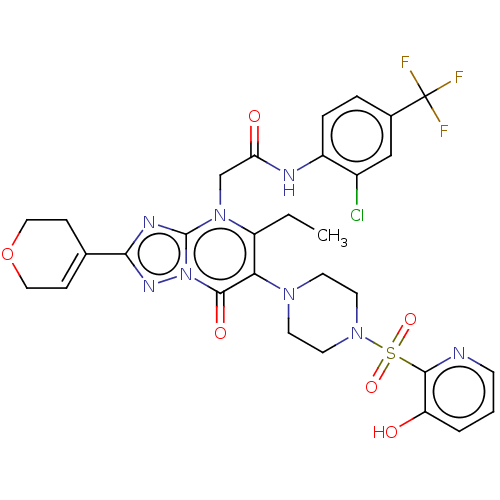
(N-(2-chloro-4-(trifluoromethyl)phenyl)-2-(2-(3,6-d...)Show SMILES CCc1c(N2CCN(CC2)S(=O)(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646345

(US11878973, Example 1)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(Cl)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1Cl)C(F)(F)F)N1CCOCC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646346
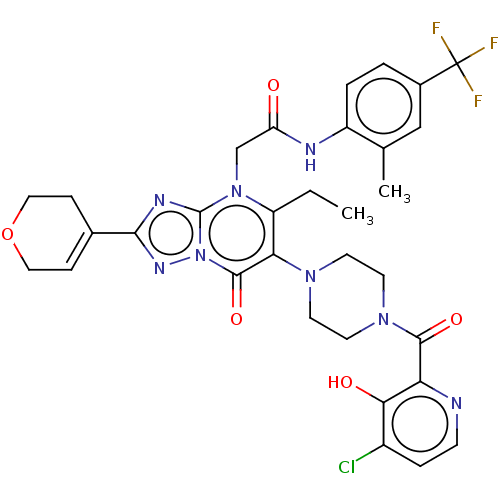
(US11878973, Example 61)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(Cl)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1C)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646399

(US11878973, Example 84)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1)C(F)(F)F)C1=CCCCO1 |t:46| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646445

(US11878973, Example 107)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncnc(C)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1F)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646348
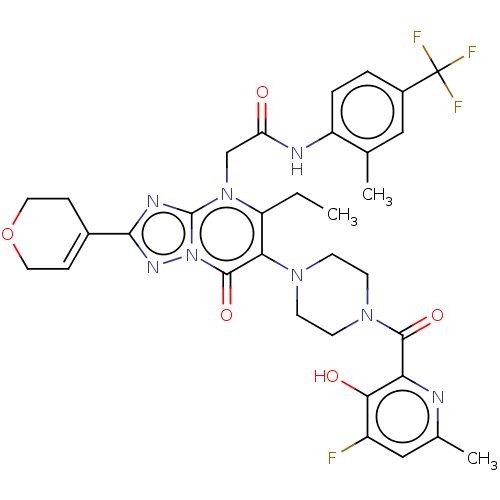
(US11878973, Example 62)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nc(C)cc(F)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1C)C(F)(F)F)C1=CCOCC1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646372

(US11878973, Example 74)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nc(C)ccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1)C(F)(F)F)C1=CCOCC1 |t:47| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646368

(US11878973, Example 72)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1)C(F)(F)F)C1=CCOCC1 |t:46| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646415

(US11878973, Example 92)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1F)C(F)(F)F)C1=CCOCC1 |t:47| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646354
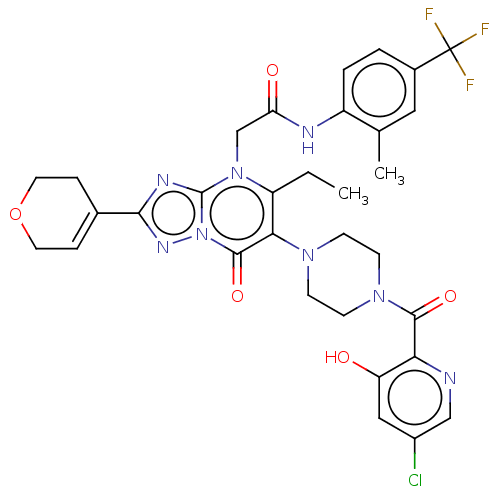
(US11878973, Example 65)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncc(Cl)cc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1C)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646421
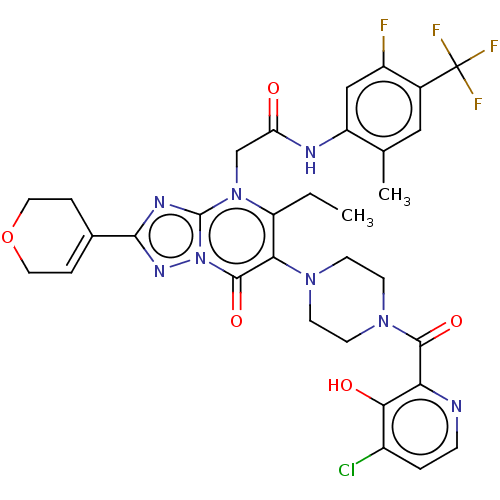
(US11878973, Example 95)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2nccc(Cl)c2O)c(=O)n2nc(nc2n1CC(=O)Nc1cc(F)c(cc1C)C(F)(F)F)C1=CCOCC1 |t:49| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50170206
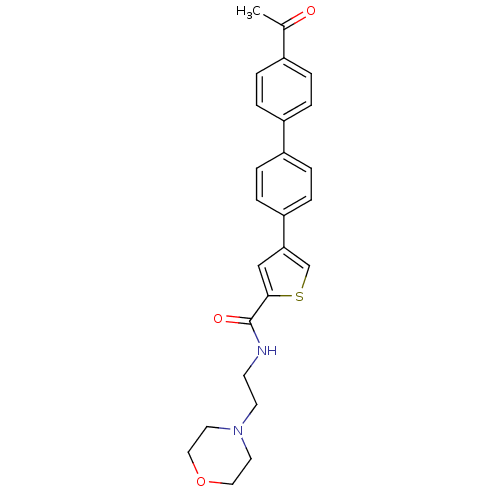
(4-(4'-Acetyl-biphenyl-4-yl)-thiophene-2-carboxylic...)Show SMILES CC(=O)c1ccc(cc1)-c1ccc(cc1)-c1csc(c1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C25H26N2O3S/c1-18(28)19-2-4-20(5-3-19)21-6-8-22(9-7-21)23-16-24(31-17-23)25(29)26-10-11-27-12-14-30-15-13-27/h2-9,16-17H,10-15H2,1H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-12 in presence of 5 nM acetohydroximate |
Bioorg Med Chem Lett 15: 3787-90 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.079
BindingDB Entry DOI: 10.7270/Q2736QFM |
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646483

(US11878973, Example 134)Show SMILES Oc1cccnc1C(=O)N1CCN(CC1)c1c(C2CC2)n(CC(=O)Nc2ccc(cc2)C(F)(F)F)c2nc(nn2c1=O)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646474
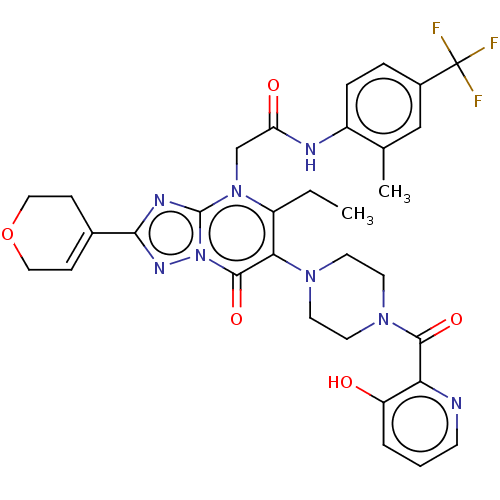
(US11878973, Example 58)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1ccc(cc1C)C(F)(F)F)C1=CCOCC1 |t:47| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional 3'-5' exonuclease/ATP-dependent helicase WRN [517-1238]
(Homo sapiens (Human)) | BDBM646463
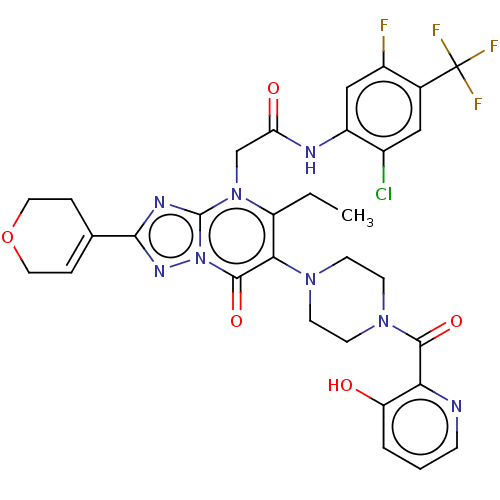
(US11878973, Example 116)Show SMILES CCc1c(N2CCN(CC2)C(=O)c2ncccc2O)c(=O)n2nc(nc2n1CC(=O)Nc1cc(F)c(cc1Cl)C(F)(F)F)C1=CCOCC1 |t:48| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data